Abstract
The quantitative assessment of body iron based on measurements of the serum ferritin and transferrin receptor was used to examine iron status in 800 Bolivian mothers and one of their children younger than 5 years. The survey included populations living at altitudes between 156 to 3750 m. Body iron stores in the mothers averaged 3.88 ± 4.31 mg/kg (mean ± 1 SD) and 1.72 ± 4.53 mg/kg in children. No consistent effect of altitude on body iron was detected in children but body iron stores of 2.77 ± 0.70 mg/kg (mean ± 2 standard error [SE]) in women living above 3000 m was reduced by one-third compared with women living at lower altitudes (P < .001). One half of the children younger than 2 years were iron deficient, but iron stores then increased linearly to approach values in their mothers by 4 years of age. When body iron in mothers was compared with that of their children, a striking correlation was observed over the entire spectrum of maternal iron status (r = 0.61, P < .001). This finding could provide the strongest evidence to date of the importance of dietary iron as a determinant of iron status in vulnerable segments of a population. (Blood. 2005;106:1441-1446)
Introduction
The prevalence of anemia continues to be used widely as a surrogate marker of iron deficiency in developing countries. The limitation of this approach is highlighted by estimates of the prevalence of iron deficiency ranging from less than one fifth to more than two thirds of the world's population.1 The problems of relying on isolated hemoglobin measurements to estimate iron deficiency are even greater when surveying populations living at high altitudes. The usual approach has been to apply one of several corrections based on hemoglobin measurements in high-altitude dwellers assumed to have normal iron status.
Quantitative measurements of body iron based on the ratio of serum transferrin receptor to ferritin concentration have recently been described as means of assessing iron status independently of hemoglobin measurements.2 A single normal distribution of body iron stores was observed in US men aged 20 to 65 years (mean, 9.82 mg/kg) and in pregnant Jamaican women (mean, 0.09 mg/kg). Two populations were observed in US women of childbearing age; 93% of women had mean iron stores of 5.5 mg/kg, while the remaining 7% had a mean tissue iron deficit of -3.87 mg/kg.
In the present study, body iron measurements were used to assess the prevalence of iron deficiency in individuals living at altitudes ranging up to 3750 m. To assess segments of the population who are most vulnerable to iron deficiency, measurements were performed in 800 Bolivian mothers and 1 of their preschool children. This approach provided a unique opportunity to compare iron status in high-risk individuals living in the same household.
Materials and methods
Survey design
The survey was designed as a follow-up evaluation of iron status performed originally in 1993-1994 using isolated hemoglobin determinations.3 In the present investigation, multiple survey teams canvassed households in 9 Bolivian provinces at altitudes between 150 to 3750 m. The survey team was composed of a data recorder, a dietician, and a phlebotomist. Mothers who agreed to participate in the survey were given a limited oral questionnaire to determine socioeconomic status, household income, health, and activity status. A 24-hour dietary recall for the household was obtained to evaluate caloric and micronutrient status as estimated from food composition tables. After obtaining anthropometric measurements, the mother and 1 of her preschool children provided blood samples for measurement of the hemoglobin concentration, serum ferritin, serum transferrin receptor (sTfR), and C-reactive protein (CRP). All phlebotomists underwent several days of training prior to the survey to achieve proficiency in the methods for obtaining, processing, labeling, and storing blood specimens. The investigation was approved by the Comité Nacional de Etica Y Bioetica, La Paz, Bolivia, and the Human Subjects Committee, Kansas University Medical Center.
Laboratory methods
Capillary blood was obtained by finger-stick using an automatic lancing device (Becton Dickinson, Rutherford, NJ). A drop of blood was first taken for the measurement of the hemoglobin concentration using a Hemocue analyzer (Hemocue, Lake Forest, CA). A minimum volume of 200 μL of whole blood was then collected directly into a microvette blood sampling device containing ethylenediaminetetraacetic acid (EDTA; Sarstedt, Newton, NC). The sample was placed on ice until centrifuged in a portable microcentrifuge. Plasma was then transferred to a 0.5-mL microfuge tube fitted with a snap-lock cap and kept on ice until transferred to a central laboratory on the same day for storage at -70°C. At the conclusion of the survey, the samples were transported on dry ice to the Kansas University Medical Center by one of the investigators.
Two-site enzyme-linked immunosorbent assays (ELISAs) established with double monoclonal antibodies were used to measure the concentration of serum ferritin4 and sTfR5 as previously described. The serum ferritin assay was calibrated with recrystallized human liver ferritin against World Health Organization (WHO) International Standard 80-578. The sTfR assay was standardized with transferrin-free intact transferrin receptor purified from human placenta at yearly intervals. The performance of both the ferritin and sTfR assay was further monitored by including at least 3 quality control sera that had been stored in aliquots at -70°C. Assays were repeated on microtiter plates in which there was a disparity greater than 10% in more than one control sera from multiple determinations measured prior to freezing.
An assay for CRP was established with a 2-site ELISA identical to that used for serum ferritin and sTfR except for the immunologic reagents and standard. Two monoclonal antibodies were purchased from HyTest (Turku, Finland); C2 antibody was used to coat the microtiter plate (capture antibody) and C6 antibody was conjugated to horseradish peroxidase (indicator antibody). Purified CRP obtained from HyTest was used as the calibrator. The assay, sensitive to 0.1 mg/L, was standardized against the Beckman-Coulter Synchron LX20 Pro (Beckman-Coulter, Brea, CA).
Data analysis
The observed hemoglobin concentrations were adjusted continuously for altitude effects using the exponential equations of Hurtado et al6 as modified from data in Ecuadorian children aged 0-59 months.7 At altitudes of 1000, 2000, 3000, and 4000 m, values of 4, 10, 19, and 34 g/L hemoglobin concentration, respectively, were subtracted from the measured hemoglobin value. The same correction was used in mothers and children.7
Measurements of body iron were based on a study in which repeated phlebotomies were performed in healthy subjects to define the relationship between the sTfR/ferritin ratio and body iron.8 Body iron was calculated as follows2 : Body iron (mg/kg) =- [log(R/F ratio) - 2.8229]/0.1207.
Positive values for body iron represent the amount of storage iron in iron-replete subjects while negative values indicate the deficit in tissue iron in subjects with iron deficiency. To relate anemia prevalence to body iron measurements, it was assumed that the normal hemoglobin concentration must fall by 20 g/L or 4 mg iron/kg body weight before anemia is detected.2 Because of the effect of inflammation on the serum ferritin concentration, calculation of body iron was not performed in subjects with a CRP concentration greater than 20 mg/L.9
Data are presented as arithmetic mean and standard deviation with the exception of serum ferritin values, which were transformed as logarithms. Correlations were calculated using the Pearson correlation coefficient. Differences between groups were examined by analysis of variance (ANOVA) for continuous variables, and post-hoc comparisons were performed with the Scheffé test. A P value less than .05 was considered significant unless otherwise stated. All calculations were performed using the ABSTAT program (AndersonBell Corp, Parker, CO).
Mixed distribution analysis of body iron measurements was performed by an iterative technique to estimate the means and standard deviations of 2 normal distributions using the DISFIT program (Duke University, Durham, NC).10 The program used the likelihood ratio statistic to determine the best fitting model and χ2 analysis to test the goodness-of-fit.
Results
The average age of the mothers was 28.3 ± 8.1 years, with a range from 16 to 52 years (Table 1). The average age of the children was 2.1 ± 1.2 years; 231 were 2 years old, 174 were younger than 2 years, and 295 were 3 or 4 years old. The mean hemoglobin concentration of 139 g/L observed in the mothers decreased to 127 g/L after correction to sea level and a comparable decrease from 123 to 110 g/L occurred in the children. The correction increased the prevalence of anemia in mothers (hemoglobin concentration < 120 g/L) from 14.9% to 26.6% and the prevalence of anemia in children (hemoglobin concentration < 110 g/L) from 23.3% to 45.2%.
Serum ferritin values were 25% lower and sTfR values were 25% higher in children than in their mothers. Consequently, body iron as determined from the receptor-ferritin ratio was about 2-fold higher in the mothers (3.88 ± 4.31 mg/kg) than in children (1.72 ± 4.53 mg/kg). Based on a CRP > 20 mg/L, 52 mothers and 64 children were excluded from the calculation of body iron.
In the 9 provinces where the surveys were conducted, the mean altitude of 2017 ± 1339 m for the total survey ranged from 3753 m in Oruro to 156 m in Beni and Pando (Table 2). In both mothers and children, the mean hemoglobin concentrations in the various provinces were very similar after correcting for altitude with the exception of a small sample of 6 households in Pando, where iron deficiency anemia was prevalent. There was no obvious trend with varying altitude in the concentration of serum ferritin, sTfR, or body iron in either mothers or children.
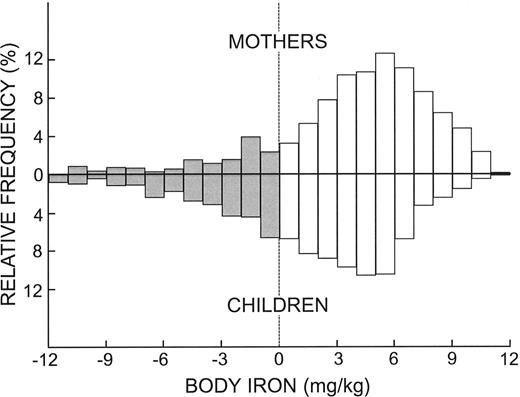
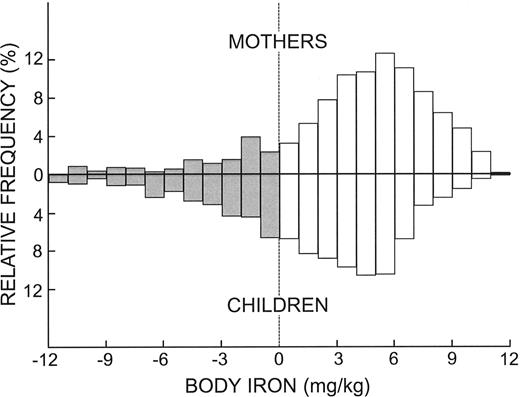
The frequency distributions of body iron in the composite sample of mothers and children were similar except for a shift in children in the direction of lower iron status (Figure 1). Both distributions comprised a large population with adequate iron reserves (positive body iron values) with skewing in the direction of tissue iron deficiency. Iron deficiency defined as a negative value for body iron was present in 15.1% of the women and 30.6% of children. Iron deficiency severe enough to be associated with anemia (body iron < -4 mg/kg2) was observed in 5.7% of mothers and 11.8% of children. In both groups, tissue iron deficiency was severe enough to produce anemia in about one-third of individuals with absent iron stores (body iron < 0 mg/kg).
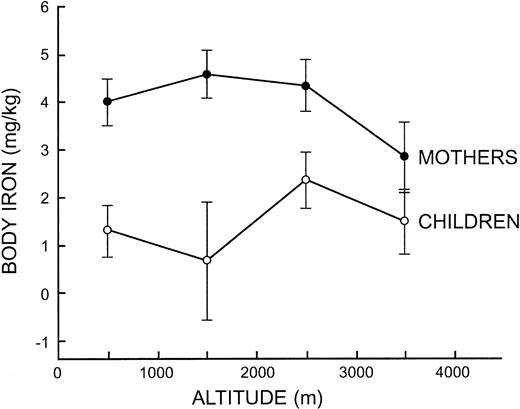
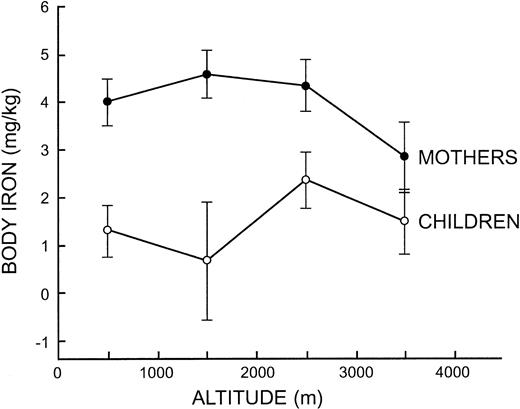
The influence of altitude on iron status measurements was examined further by pooling observations at 1000-m intervals to obtain adequate sample sizes. In children, ANOVA indicated that body iron differed significantly between the 4 altitude intervals (F = 3.17, P = .02) but without a trend to indicate an altitude effect (Figure 2). In mothers, ANOVA was significant for the concentration of serum ferritin (F = 4.63, P = .003), sTfR (F = 6.20, P < .001), and body iron (F = 6.03, P < .001) due to lower iron status in mothers living above 3000 m (Figure 2). In women living below or above 3000 m, the geometric mean serum ferritin concentration was 30.2 μg/L and 23.4 μg/L, the mean sTfR was 6.61 mg/L and 7.97 mg/L, and the mean body iron was 4.32 mg/kg and 2.77 mg/kg, respectively, the latter reflecting a 30% reduction in storage iron.
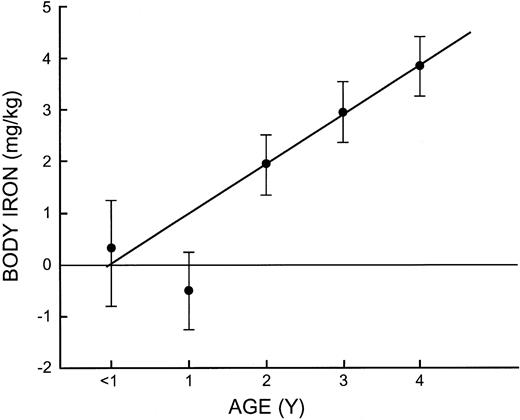
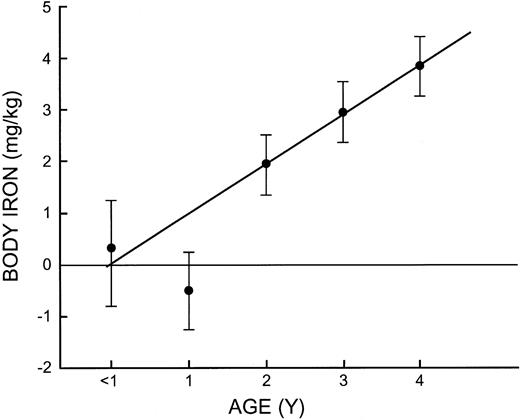
The relationship between age and iron status in children was evaluated by calculating the mean values of iron-related measurements at yearly intervals (Table 3). The mean hemoglobin concentrations of 99 g/L and 100 g/L during the first 2 years increased progressively to reach a mean of 120 g/L in 4-year-olds. The sTfR, serum ferritin, and body iron measurements all indicated a high prevalence of tissue iron deficiency before 2 years of age, but this was followed by a linear increase in body iron over the next 3 years to reach a mean of 3.85 mg/kg at 4 years of age, similar to values in their mothers (Figure 3).
Frequency distributions of body iron in mothers and children. □ and positive values represent storage iron while the negative values and ▦ represent tissue deficiency.
Frequency distributions of body iron in mothers and children. □ and positive values represent storage iron while the negative values and ▦ represent tissue deficiency.
Thirty-three of the 800 mothers were pregnant at the time of the survey. Gestational age was not recorded because of the difficulty in obtaining accurate data. The hemoglobin concentration in pregnant and nonpregnant women averaged 115 ± 15 g/L and 127 ± 17 g/L, respectively, similar to the 10 g/L difference in the WHO criteria of anemia in pregnant (110 g/L) and nonpregnant (120 g/L) women.11 The geometric mean serum ferritin concentration of 15.0 μg/L in pregnant women was significantly lower than the mean of 29.1 μg/L in nonpregnant women (P < .001), whereas the sTfR concentrations of 6.45 ± 2.15 mg/L and 6.98 ± 4.28 mg/L, respectively, indicated similarity in tissue iron status. The mean body iron store of 1.31 ± 3.67 mg/kg in pregnant women was one-third the mean of 3.99 ± 4.30 mg/kg in nonpregnant women (P < .002).
Mean body iron values at 1000 meters intervals from 0 to 4000 m in mothers and children. The vertical bars represent the limits of ± 2 SE.
Mean body iron values at 1000 meters intervals from 0 to 4000 m in mothers and children. The vertical bars represent the limits of ± 2 SE.
The correlations between the age, altitude, and laboratory indices of iron status were calculated in mothers and children separately as well as between the 2 groups for each parameter (Table 4). Only the sTfR correlated significantly with altitude but the relationship was positive in mothers (r = 0.124) and negative in children (r = -0.071). The age of children was highly correlated with all iron status measurements. The hemoglobin concentration was highly correlated with the serum ferritin and sTfR in both mothers and children and the correlation between the hemoglobin concentration in mothers and children was also highly significant (r = 0.190). A significant correlation was observed between the CRP and serum ferritin in both mothers and children reflecting the effect of inflammation. Of interest, an equally strong correlation was observed between the CRP in mothers and children, possibly due to concurrent illness. There was a highly significant inverse correlation between the serum ferritin and sTfR in both mothers and children as expected with these 2 key measurements of iron status. More importantly, remarkably high correlations of 0.578 for the serum ferritin and -0.318 for the sTfR were observed between mothers and children.
Multiple regression analysis indicated that when body iron was analyzed as the dependent variable in mothers, the multiple correlation coefficient was relatively low (r = 0.190), with only the age of their children and altitude significant (Table 5). A much stronger correlation was observed with body iron in children (r = 0.655) due to a highly significant relationship with body iron of the mother (t = 19.91, P < .001), and less so with the age of the child.
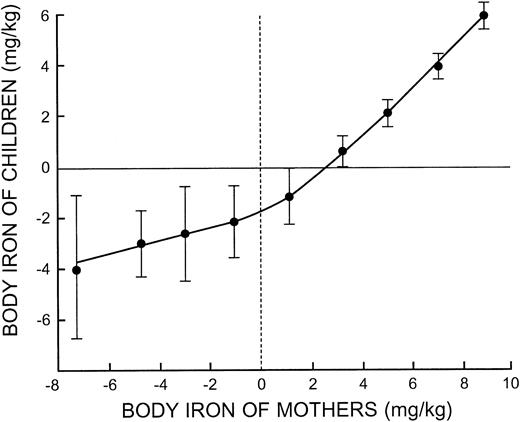
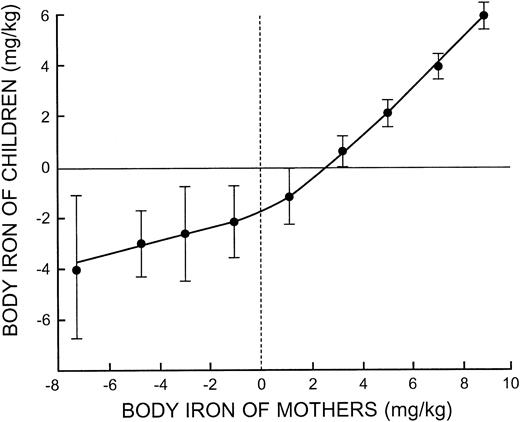
The striking relationship between body iron in mothers and their preschool-aged children observed with correlation and regression analysis is shown in Figure 4. The slope relating the body iron of children to that of their mothers approached unity when mothers had iron stores. With increasing iron deficiency in mothers, the progressive fall in body iron of their children was more gradual. This suggests that one or more factors accounted for increasing iron deficiency in mothers that did not influence the iron status of their children.
Discussion
It is estimated that 20 to 30 million people worldwide live at altitudes above 3000 m commonly defined as high altitude; over half of these individuals live in the Andean region of Latin America.3 It is commonly assumed that the prevalence of iron deficiency is higher in vulnerable segments of these populations because of the added iron requirement imposed by expansion of the red cell mass. However, the results of anemia surveys to identify this iron deficiency are limited by uncertainty in the criteria of anemia at high altitude. In a study of Bolivian women living in Atocha (3600 m) and Santa Barbara (4800 m), Berger and colleagues reported a prevalence of 51.7% and 26.5%, respectively, based on the hemoglobin response to 3 months of iron supplementation.3 However, when the results were compared with mixed distribution analysis, the prevalence was below 10% in both regions. This disparity in estimated prevalence underscores the limitations of hemoglobin measurements for evaluating iron status in the millions of people who reside at high altitude.
In the present study, estimates of iron deficiency based on body iron measurements differed markedly from those based on hemoglobin measurements. After correcting the latter for the effect of high altitude, the prevalence of anemia in women was 26.6%, whereas body iron measurements indicated that only 5.7% had tissue iron deficiency severe enough to produce anemia (body iron < -4 mg/kg). Similarly, 45.2% of children were anemic but only 11.8% had a deficit in tissue iron consistent with anemia. These differences in estimated prevalence of iron deficiency anemia could be due to pitfalls in relating body iron measurements to anemia prevalence, erroneous altitude corrections of the hemoglobin concentration, or causes of anemia other than iron deficiency.
Mean body iron in children at 1-year intervals from 0 to 4 years of age. The vertical bars represent the limits of ± 2 SE. Diagonal line is the regression line for children aged 2 to 4 years.
Mean body iron in children at 1-year intervals from 0 to 4 years of age. The vertical bars represent the limits of ± 2 SE. Diagonal line is the regression line for children aged 2 to 4 years.
The present estimates of iron status in adult Bolivian women are similar to the findings in the only prior population study using body iron measurements.2 In 409 adult US women, mean values for the serum ferritin, sTfR, and body iron were 34 μg/L, 6.3 mg/L, and 4.87 ± 4.14 mg/kg, respectively, as compared to mean values of 30 μg/L, 6.6 mg/L, and 4.23 ± 4.03 mg/kg in 595 Bolivian women living below 3000 m in the present study. In the US study, mixed distribution analysis of body iron values indicated 2 normally distributed populations; the main population representing 93% of women had a mean body iron of 5.5 ± 3.4 mg/kg while the remaining 7% of iron deficient women had a mean of -3.9 ± 3.4 mg/kg. In the present study, mixed distribution analysis also indicated 2 populations in women living below 3000 m; 83% of women had a mean body iron of 5.3 ± 2.6 mg/kg, while the remaining 17% of iron-deficient women had a mean body iron of -2.5 ± 2.6 mg/kg (goodness-of-fit χ2 = 22.9; P = .03). The nature of the second iron-deficient population remains unexplained in both studies.
The present study afforded the first opportunity to assess the effects of living at high altitude on body iron status. The correction at 3500 m to adjust the hemoglobin concentration to sea level is 26 g/L or 88.4 mg iron/L. Based on an average total blood volume in adult women of 67 mL/kg, a 60-kg woman living at 3500 m requires an additional 355 mg iron or 5.9 mg/kg to increase their hemoglobin by 26 g/L. Body iron measurements in women living above 3000 m averaged 2.77 mg/kg compared with 4.31 mg/kg at lower altitudes. The difference of 1.54 mg/kg indicates that women were able to enhance the absorption of dietary iron to acquire three-quarters of the additional iron required for expansion of red cell mass. Studies in small laboratory animals have demonstrated a significant enhancing effect of hypoxia on iron absorption independent of iron status or changes in erythropoiesis, an effect that is apparently mediated by the iron regulatory peptide, hepcidin.12
A possible limitation in estimating body iron at high altitude is the enhancing effect of increased erythropoiesis on the sTfR concentration independent of iron status. However, the potential error appears to be small. The serum ferritin concentration is not influenced by high altitude directly, and the difference in mean values in women living above or below 3000 m (geometric mean ferritin, 21.1 vs 30.2 μg/L, respectively) was greater than the difference in sTfR concentration (7.97 vs 6.61 mg/L). Moreover, if a sTfR value of 6.61 mg/L is used rather than 7.97 mg/L to calculate body iron, iron stores in a woman weighing 60 kg would be 318 mg rather than 277 mg, a relatively minor 10% difference. The higher sTfR concentration in women living above 3000 m appears to be primarily related to diminished iron status rather than enhanced erythropoiesis.
While there was no significant effect of altitude on body iron in children, a small influence could have been masked by the striking effect of age on iron status (Figure 3). One half of infants younger than 1 year had tissue iron deficiency; the modest further decline in 1-year-old infants is presumably due to loss of the iron endowment from the mother that protects the infant during the first few months of life. The rapid increase in body iron after the age of 2 years despite increasing body size is impressive and has not been clearly defined in prior studies. The only data on body iron measurements available for comparison are the observations reported previously in children older than 3 years sampled in a US health and nutrition survey.2 As in the present study, body iron in this age group was the same as in women in their early twenties. Because body iron measurements cannot be calibrated by quantitative phlebotomy in children, the same calculation in adults was used in children; the results are consistent with our current knowledge of iron balance in infants and preschoolers. The age-related changes in body iron observed in the present study indicate the importance of supplying additional iron to infants because of their vulnerability to the neurologic consequences of iron deficiency.13
A potential limitation of body iron measurements is the effect of inflammation on the serum ferritin concentration that occurs independently of iron status.9,14,15 A CRP concentration greater than 20 mg/L9 was used to exclude 6.5% of mothers and 8.0% of children from the calculation of body iron. The iron status of these individuals could not be assessed quantitatively but the percent of individuals with an elevated sTfR greater than 8.5 mg/L, a measure of iron deficiency when inflammation is present,16 was identical in women with a normal and elevated CRP (17%) and similar in children with an elevated and normal CRP (48% and 46%, respectively). An increase in the sTfR concentration is a useful guide to concurrent iron deficiency in those with chronic disease, although the upper normal limit of the sTfR may be higher in those living at altitudes above 3000 m due to enhanced erythropoiesis.
The most important new finding in this investigation is the observation that the iron status of children during the first 5 years of life is highly correlated with the iron status of their mothers. Failure to detect this close association in prior studies of the prevalence of iron deficiency relates to survey methodology. Previous approaches have relied on arbitrary cut-off levels for iron-related laboratory measurements that do not measure the iron status in an individual. In the present investigation, a direct comparison between body iron in a child and his/her mother defined a precise relationship that approached unity when mothers were iron replete (Figure 4). The most likely explanation for this relationship is that diet is a key determinant of iron status in both mothers and their children, at least in regions where iron-fortified infant foods and iron supplements are seldom used. Not surprisingly, data from the limited dietary survey performed on a household basis in this study did not correlate with measurements of body iron in mothers. It is known that although 24-hour dietary recall can be used to evaluate the average iron intake of a population, it is unreliable for assessing individual iron intakes.17 Moreover, it is well established that the iron bioavailability of the diet is more important than total iron intake.18,19
There are other less likely explanations for the close relationship between the iron status of mothers and their children. The iron status of the mother during gestation is known to influence that of her infant.20 However, this does not explain the present findings because the correlation between body iron in mothers and infants younger than 2 years (r = 0.629, n = 245) was similar to that in 2-year-old (r = 0.621, n = 209) and 3- to 4-year-old children (r = 0.551, n = 273). It is conceivable that certain infections associated with blood loss or diminished food intake affected iron status of both the mother and child, but these should have been eliminated by excluding those with an elevated CRP concentration. A genetic determinant of iron status, such as shared iron-regulating genes, could also explain the association. Defining the reason for this maternal-child link in iron status will be an important goal in future investigations.
Body iron in children plotted at intervals of 2 mg/kg body iron in their mothers. The vertical bars represent the limits of ± 2 SE.
Body iron in children plotted at intervals of 2 mg/kg body iron in their mothers. The vertical bars represent the limits of ± 2 SE.
Prepublished online as Blood First Edition Paper, May 3, 2005; DOI 10.1182/blood-2004-12-4782.
Supported by the Micronutrient Initiative, Grant Agreement 10-00029-1-3.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.