Abstract
Offering to provide research results to study participants is gaining increasing support based, in part, on the principle of respect for persons. The frequency and means of this practice is unknown in national and international research communities. All investigators who presented oral abstracts involving human research at the American Society of Hematology Annual Meeting (December 2003) were surveyed. Responses were received from 197 (42%) of 472 eligible investigators. Nonrespondents did not differ in study type or country of origin. Only 30% (n = 48) of those who completed the survey had a formal plan for the return of research results; 40% of these would return both a summary plus individual level results. Of the respondents, 69% (n = 109) supported or strongly supported the practice; only 3% opposed the practice. The most commonly cited reasons for not returning results were: did not consider it (38%), anticipated contact difficulties (32%), and participant difficulty understanding results (26%). Only 11 (7%) indicated that their institutional review board (IRB) mandates the offer to provide results to all participants; this did not vary significantly by country. Given the high level of support in the international research community, evaluation of well-planned interventions for offering to provide research results to participants should be a priority.
Introduction
Respect for persons is widely accepted as the one foundation guiding research with human subjects. The principle of respect for persons is understood by many researchers to encompass a moral obligation to offer research results to research participants upon the completion of a study.1 This practice avoids treating people as merely a means to an end, acknowledges that the person made an important contribution to the research project, and may have direct implications for the health of the participants.2 Additional possible benefits include an improved perception of research by the public and more willing participation in future research.
The potential harm of returning research results to study participants, including emotional distress and impact on insurability, must also be anticipated so that proper measures can be taken to minimize it. Due to these risks, we believe that the offer of disclosure of results should include an informed consent process.2 This process must be designed to include comprehensive discussion and clear opportunity for the participant to decline receipt of results. Such programs must also provide psychological support following dissemination of results and medical follow-up for those in whom health risks are identified.3
A number of concerns have been identified by investigators as deterrents to returning research results.4,5 These include the requirements for financial and other resources that would be needed to commit to this practice, the logistics of retaining contact with participants, and the difficulty in communicating complex scientific or medical results in an understandable manner. The frequency of these and other concerns in the international research community is unknown.
In Canada and the United States, national policies governing research (Tri-council Policy on Research Involving Humans and United States Common Rule)6-8 do not address the return of research results to study participants. The Council for International Organizations of Medical Sciences in Europe does recommend the return of research results but does not provide any operational guidelines. Research ethics boards (REBs) and institutional review boards (IRBs) monitoring human research studies may elect to require provisions for the disclosure of results.9 Large cooperative research groups may also provide guidelines for the return of research results among their member institutions, an initiative that is currently being discussed within the Children's Oncology Group.10
Support for the concept of involving communities in research design and result dissemination is well described.11,12 There is increasing support among both investigators and participants for the return of research results to individual participants,1,4,5,13-15 but the frequency with which results are offered is for the most part unknown. The 2 studies that have addressed this question suggest that it is seldom done on a routine basis,2,4 but the studies have limits to their generalizability in that they surveyed a narrow population of researchers (pediatric oncology and breast cancer trial groups).2,15 The prevalence of providing results to participants in other disciplines and in any discipline internationally is entirely unknown.
We report the frequency with which a broad cross-section of international investigators is offering research results to study participants. We describe the means by which researchers offer results, the role of REBs and IRBs in the return of research results, the reasons for not offering results, and the association between country of origin and the requirements for the return of research results. These results are an important step in addressing barriers to fulfilling an ethical obligation to offer research results to research participants.
Materials and methods
The abstracts of all 888 oral presenters at the American Society of Hematology (ASH) Annual Meeting in San Diego in December 2003 were reviewed separately by 2 investigators for inclusion in the study. The inclusion criteria were defined as all abstracts describing direct human studies including phase 1, phase 2, phase 3, case reports, cohort or case control studies, surveys, descriptive results involving humans, observational studies, and human tissue research in which the specimen was obtained expressly for that study. The exclusion criteria included abstracts that described meta-analyses of human studies, secondary analyses of data, and studies that used established cell lines or tissue banks.
There was an 86% agreement between the 2 investigators in defining eligible abstracts. The disagreements primarily involved discriminating between human tissue and cell line research. All differences were resolved through discussion. Of the 888 abstracts, 478 (54%) met the inclusion criteria. To further ensure proper study classification, an item was included on the questionnaire that had the authors classify their own studies according to the options in the inclusion criteria or opt out of the survey if their study did not fit one of those selections.
E-mail addresses of the primary investigators were obtained from public web sites (primarily PubMed and Google). Of 478 (99%) e-mail addresses, 472 were found for either the first author or senior author of each eligible abstract.
The questionnaire was designed using the Dillman method16 and used selected elements of a previously constructed questionnaire.5 The questionnaire was piloted for length and readability prior to distribution. The questionnaire was designed to gather information about the frequency and means by which researchers offer return of results to research participants, the role of REBs and IRBs in the return of research results, reasons for not disseminating results, and the association between country of origin of the principle investigator and the requirements for the return of research results. Content validity of each question and the overall questionnaire was established with a panel of 5 researchers.
An e-mail survey was sent to the primary or senior authors of abstracts eligible for this study. The questionnaire was completed in an online window and the data automatically downloaded to a secure server. The abstract number from the ASH meeting was indicated on the questionnaire as some investigators presented more than one abstract. Consent was implied by the return of a questionnaire. A tracking code number was assigned for each investigator. Two reminder e-mails were sent. Data analysis was done on the aggregate responses and is reported by descriptive techniques. Chi-square analysis was done defining 6 variables for analysis a priori. Six of the potential participants responded to the survey twice. In 5 cases the most complete data were used for analysis, and in 1 case where the entire survey was completed twice and the responses differed, the second set of data from that investigator was not included in this analysis.
The study was approved by the IWK Health Center Research Ethics Board.
Results
Responses were obtained from 197 (42%) of 472 eligible investigators. Of these, 16 (8%) withdrew after indicating that their abstract did not qualify as human subject research and 23 (12%) did not complete the entire questionnaire. Characteristics of the remaining 158 study participants and their abstracts are shown in Table 1. Of those 158 investigators, 126 (80%) were medical doctors.
The study sample was representative of the entire sample of eligible abstracts according to country of origin and study type. Respondents from the United States represented 48% (n = 76/158) of the respondents compared with 52% (n = 245/472) of the total sample (respondents and nonrespondents); Europeans represented 44% (n = 69/158) of respondents compared with 41% (n = 194/472) of the total sample; Canadians represented 5% (n = 8/158) compared with 4% (n = 19/472) of the total sample; the remaining countries represented 3% (n = 5/158) compared with 3% (n = 14/472) of the total sample. According to our classification of the abstract study types, clinical trials (phase 1, 2, or 3) represented 17% (n = 27/158) of respondent abstracts and 23% (n = 108/472) of the total sample of abstracts; human tissue studies comprised 16% (n = 25/158) of respondent abstracts and 17% (n = 78/472) of the entire sample; other study types (cohort, case control, series, surveys, and case reports) made up the remaining 67% (n = 106/158) of respondent abstracts and 61% (n = 286/472) of the total sample of abstracts. There was a 60% (n = 95/158) agreement between the self-classification of the respondents and our classification of their studies. This disagreement may be explained by the paucity of information from the abstract to allow a full assessment of the study type.
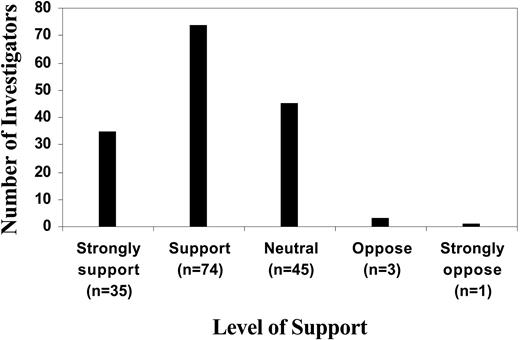
Among the 158 researchers who provided complete responses, only 30% (n = 48) indicated that they had a formal plan for the offer of research results to participants. However, 69% (n = 109) of all responding investigators supported or strongly supported the concept of return of research results, 28% (n = 45) were neutral and only 3% (n = 4) were opposed (Figure 1).
Of the 48 investigators who had a plan, no clear preference for the means of return was indicated, but 73% (n = 35) indicated that they would provide the participant with the choice whether or not to receive the results. Most (60%; n = 29) indicated that they intended to provide an overall summary of results whereas the others (40%; n = 19) intended to provide an overall summary plus individual level results.
Investigator support for the return of research results. Researchers who presented oral abstracts at the American Society of Hematology 2003 annual meeting (n = 158) were asked to indicate their support for the return of research results to research participants, shown on a 5-point Likert scale.
Investigator support for the return of research results. Researchers who presented oral abstracts at the American Society of Hematology 2003 annual meeting (n = 158) were asked to indicate their support for the return of research results to research participants, shown on a 5-point Likert scale.
Of those investigators who did not have a formal plan for the return of research results, the most common reasons identified were: did not consider it (38%), anticipated contact difficulties (32%), and participant difficulty understanding results (26%).
Researchers who had a formal plan for the return of research results tended to have IRBs or REBs that mandated the offer of return of results (P < .05) and tended to support such a practice (P < .05). Pediatric studies were less likely than other studies (adult or mixed) to have a formal plan for the return of results. Returning research results had no significant correlation with being a physician or study type. Further, being a physician researcher did not correspond significantly with support for the practice.
An a priori analysis of support versus study type was not performed but a retrospective analysis was done. We grouped clinical trials, case control studies, and surveys together and found a 77.6% support rate for the practice of offering results. The comparison group comprised case reports, cohorts, case series, and human tissues studies. This group had a 65.8% level of support. Investigators of clinical trials had the highest support (73.5%) and investigators of case series had the lowest support (52.3%).
Only 11 (7%) of the 158 investigators reported that their IRB or REB mandates the offer of return of results to all participants; 110 (70%) reported no mandate; and 37 (23%) reported that they did not know. This policy was not consistently present in any of the 15 countries represented by the responders. Among the researchers from the United States, 8% (n = 6/76) reported having an IRB or REB that mandated the return of results. In Europe, 6% (n = 5/69) of responders indicated that their IRBs or REBs mandated return of results, and in the remaining countries, 0% (n = 0/13) of investigators were under such a policy.
Discussion
There is increasing recognition in the research community that an important expression of respect for persons is offering results to human research participants at the completion of a study. Our study shows that most researchers support the practice of returning research results to participants but that a minority actually do so.
In this study, only 31% of the investigators had a formal plan for the offer of return of research results but 69% supported or strongly supported the practice. This suggests that lack of support for the practice does not explain the infrequency with which it is done. Rather, other barriers are likely to exist that discourage the practice of returning results.
The reasons reported in this study for not returning research results are consistent with the perceived barriers identified by investigators in other studies4,5 and include: did not consider it, anticipation of potential follow up or contact difficulties, and anticipation of participant difficulty understanding results. These difficulties may be compounded in pediatric studies by perceived (and real) difficulties in appropriately communicating with minors or their parents or guardians and in identifying to whom the results should be provided (parent or guardian or maturing child). One consequence of these barriers was identified in a survey of investigators by Partridge et al, who found that 16.2% of responders felt that a policy obligating them to offer results to study participants would make them less willing to engage in research projects that involved enrolling patients.4
To address some of these concerns, implementation of a policy for the return of research results to participants would need to be accompanied by recommendations that minimize potential harms and facilitate patient understanding. Such guidelines need not be strict requirements but rather adaptable strategies that address such issues.1 More studies are needed to identify specific approaches to address the needs and concerns of both researchers and participants as they relate to this process.
Specific limitations exist within this study. As a survey, nonresponse to the questionnaire was anticipated. Our response rate is commensurate with the usual response rates for mailed surveys. However, the respondents do appear to reflect the overall population in proportionate representation of country participation and study type. Another possible limitation was that the respondents were not frank about their current practice, especially if it ran contrary to local REB or IRB requirements. We believe that this is unlikely as the study was conducted in an anonymous way.
Our study has demonstrated that current research involving human subjects is infrequently accompanied by the return of research results to study participants on an international level, despite strong support for the concept. Our study also shows that few researchers are mandated by their IRBs and REBs to offer results to participants. Determining the frequency and conduct of this practice helps define the need for universal policies and is an important step toward the development and incorporation of guidelines into IRB and REB standards of research conduct.
Additional strategies are needed to address researcher concerns and facilitate the offer of return of results including study planning and participant follow-up. Evaluation of well-planned interventions will be needed to examine participant needs and concerns, the economic costs of this kind of a program,17 and the impact the return of results might have on future research initiatives. With this information, appropriate budget structures and policy changes at the IRB and REB regulatory level may be necessary.
Prepublished online as Blood First Edition Paper, May 5, 2005; DOI 10.1182/blood-2005-02-0556.
Supported by the Catherine Margaret Betts Fund and the Hematology/Oncology Endowed Fund, IWK Health Centre Foundation, Halifax, NS, Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.