Abstract
Production of interferon γ (IFN-γ) is critical for optimal antitumor immunotherapy in several preclinical animal models. Interleukin-12 (IL-12)–induced IFN-γ production is markedly defective after autologous stem cell transplantation. Quantitative deficiency in CD4 T cells, relative increase in CD25+CD4+ T cells, and bias toward T helper 2 (Th2) differentiation are not the primary mechanisms of defective IFN-γ production. IL-12 receptor β1 (IL-12Rβ1) and IL-12Rβ2 are expressed at equivalent or higher levels on posttransplantation patient peripheral blood mononuclear cells (PBMCs) as compared with control PBMCs. IL-12–induced tyrosine phosphorylation of signal transducer and activator of transcription 4 (STAT4) was undetectable or barely detectable in posttransplantation patient PBMCs, whereas IL-4–induced tyrosine phosphorylation of STAT6 did not differ in posttransplantation patient and control PBMCs. Levels of STAT4 protein were decreased by 97% in posttransplantation patient PBMCs. Levels of STAT4 mRNA were also significantly decreased in posttransplantation patient PBMCs. Incubation with IL-12 and IL-18 in combination partially reversed the defective IFN-γ production by posttransplantation patient PBMCs. IFN-γ production in response to IL-12 plus IL-18 did not require increased expression of STAT4 but was dependent on the activity of p38 mitogen-activated protein kinase (MAPK). These results indicate that defective IFN-γ production is due to an intrinsic deficiency in STAT4 expression by posttransplantation patient lymphocytes and suggest strategies for circumventing this deficiency in cancer immunotherapy.
Introduction
Non-Hodgkin lymphoma is the most common of the hematologic malignancies.1 The incidence of non-Hodgkin lymphoma has been rising steadily for the past 50 years, and approximately 60 000 new cases are currently diagnosed annually in the United States. Because of this increased incidence, non-Hodgkin lymphoma is one of the few cancers for which mortality has increased in the past 20 years.2 High-dose therapy and autologous hematopoietic stem cell transplantation is the treatment of choice for eligible patients with non-Hodgkin or Hodgkin lymphoma that is refractory to or has relapsed after conventional chemotherapy.3-6 Nevertheless, greater than 50% to 60% of patients with relapsed or refractory lymphoma will ultimately develop progressive disease after autologous stem cell transplantation. Novel strategies are needed to reduce the risk of recurrent lymphoma after high-dose therapy. One approach is posttransplantation immunotherapy to eliminate chemotherapy-resistant tumor cells.7 Immunotherapeutic approaches under investigation include posttransplantation administration of monoclonal antibodies, immunostimulatory cytokines, and cancer vaccines.8-10
In attempts to stimulate effective antitumor immune responses, we have investigated the use of interleukin 12 (IL-12) after autologous stem cell transplantation. IL-12 plays an important role in the regulation of innate and adaptive immune responses.11 In preclinical tumor models, IL-12 therapy induces regression of established primary tumors, inhibits the formation of distant metastases, and prolongs the survival of tumor-bearing mice.12-15 In several animal models production of interferon γ (IFN-γ) in vivo has been shown to be necessary, but not sufficient, for the antitumor effects of IL-12.13,14,16,17 IL-12 mediates its immunologic effects by binding to a specific dimeric cell surface receptor complex, composed of β1 and β2 chains, which results in activation of the Janus kinases Jak2 and Tyk2.18,19 The subsequent activation of signal transducer and activator of transcription 4 (STAT4) is required for normal responses to IL-12.18,20,21
Our previous studies have shown that IL-12 can be safely given in biologically active doses after autologous stem cell transplantation.22 However, serum IFN-γ levels during IL-12 therapy after transplantation were found to be an order of magnitude lower than those seen in patients with solid tumors not receiving a transplant who received the same doses of IL-12.22,23 Moreover, posttransplantation patient peripheral blood mononuclear cells (PBMCs) were shown to be intrinsically defective in their ability to produce IFN-γ after direct in vitro stimulation with IL-12. We have investigated the mechanisms responsible for defective IL-12–induced IFN-γ production by PBMCs of patients with lymphoma who have undergone autologous stem cell transplantation.
Patients, materials, and methods
Patient and control subject samples
The study was approved by the Institutional Review Board at Indiana University Medical Center, and written informed consent was obtained from each subject prior to collection of blood samples. Blood samples were obtained from patients with non-Hodgkin and Hodgkin lymphoma who were scheduled to undergo high-dose chemotherapy and autologous stem cell transplantation. Procedures for stem cell collection, administration of high-dose therapy, and autologous stem cell transplantation were as previously described.24 Blood samples were obtained prior to collection of peripheral blood stem cells, prior to the initiation of high-dose chemotherapy, and after hematologic engraftment following autologous stem cell transplantation. Serum samples, obtained after allowing whole blood to clot at room temperature, were stored in aliquots at –70°C. PBMCs were isolated on a Ficoll-diatrizoate gradient from venous blood samples. Control PBMCs were obtained from healthy volunteer donors. Freshly isolated PBMCs were used for immunofluorescence studies. Aliquots of PBMCs were cryopreserved in liquid nitrogen for subsequent in vitro studies.
Reagents
Recombinant human IL-2 was obtained from Chiron (Emeryville, CA), IL-4 from Genzyme (Cambridge, MA), IL-12 from Genetics Institute (Cambridge, MA), and IL-18 from R&D Systems (Minneapolis, MN). Phorbol 12-myristate 13-acetate (PMA) was obtained from Sigma Chemical (St Louis, MO). Ionomycin, p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 (4-(4-fluorophenyl)-2-(4-methylsufinylphenyl)-5-(4-pyridyl)-1H-imidazole), and control reagent SB202474 (4-ethyl-2-(p-methoxyphenyl)-5-(4-pyridyl)-1H-imidazole) were obtained from Calbiochem (San Diego, CA). Fluorochrome-conjugated monoclonal antibodies (mAbs) specific for CD3 and CD4 were purchased from Beckman-Coulter (Brea, CA) and for CD56, IL-12 receptor β1 (IL-12Rβ1), IL-12Rβ2, IFN-γ, and tyrosine-phosphorylated STAT6 from BD Pharmingen (San Diego, CA). MAbs specific for STAT4 were obtained from BD Transduction Laboratories (San Diego, CA) and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Biodesign International (Saco, ME). Polyclonal rabbit antisera specific for STAT3 and Jak2 (Upstate, Lake Placid, NY), STAT4 and Tyk2 (Santa Cruz Biotechnology, Santa Cruz, CA), and tyrosine phosphorylated (Y693)–STAT4 (Zymed Laboratories, South San Francisco, CA) were used according to the manufacturer's instructions. Horseradish peroxidase–conjugated goat anti–rabbit immunoglobulin G was obtained from Bio-Rad (Hercules, CA). Enzyme-linked immunosorbent assay (ELISA) kits specific for human IL-4, IL-12, and IFN-γ were purchased from R&D Systems. Culture medium was prepared as previously described,25 except that 15% fetal calf serum was substituted for 15% human AB serum.
Immunophenotypic analysis
PBMCs were stained directly with fluorochrome-conjugated mAb, washed, fixed in 1% formaldehyde, and analyzed by flow cytometry as previously described25 using an FACScan (fluorescence-activated cell scanner) or FACS Calibur instrument from Becton Dickinson (San Diego, CA). For intracellular staining of IFN-γ and phospho-STAT6, PBMCs were incubated with fluorochrome-conjugated mAb to stain cell-surface antigens, washed, and fixed in 2% formaldehyde. After permeabilization in 90% methanol, cells were washed, incubated with fluorochrome-conjugated anti–IFN-γ, anti–phospho-STAT6, or control mAb, and analyzed by flow cytometry. During analysis, forward and side scattering properties were used to create a lymphocyte gate. Thresholds for discriminating levels of staining above background were established by analysis of PBMCs stained with fluorescein isothiocyanate–, phycoerythrin (PE)–, and allophycocyanin-conjugated control mAb.
Detection of antigens expressed by T-cell and natural killer (NK) cell subsets of PBMCs was performed by electronic gating on CD3+ cells or CD3–CD56+ cells, respectively. The absolute number of various lymphocyte subsets was calculated by multiplying the total lymphocyte count (derived from a routine complete blood count performed at the same time a blood sample was obtained for flow cytometry studies) by the percentage of cells in a sample expressing the relevant phenotype (derived from flow cytometric analysis).
Cytokine levels
Patient and control PBMCs were thawed, washed in medium, and cultured at 50 000 or 250 000 cells per well in U-bottomed 96-well microplates in medium alone or medium containing stimuli as indicated. After 3 to 5 day of incubation at 37°C, cell-free supernatants were collected, and IFN-γ or IL-4 levels were measured using specific ELISA kits according to the manufacturer's instructions. Serum samples obtained from patients after autologous stem cell transplantation were thawed, and IL-12 levels were measured by using specific ELISA kits.
Immunoblotting
Patient and control PBMCs were thawed, washed in medium, and cultured in medium alone or medium containing cytokines as indicated. After stimulation, cells were washed with 10 mL phosphate-buffered saline, lysed in 50 μL protein lysis buffer as previously described,26 followed by centrifugation at 4°C for 5 minutes. Cleared total protein extracts (50 μg) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto Nytran SuperCharge and Nytran N membranes (Schleicher & Schuell BioScience, Keene, NH). The blots were blocked in 3% dry nonfat milk in phosphate-buffered saline for 1 hour at room temperature, probed with rabbit polyclonal antibodies for 2 hours at room temperature, and subsequently incubated with horseradish peroxidase–conjugated goat anti–rabbit immunoglobulin G (1:5000) for 1 hour at room temperature. The final reaction was developed with a Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer, Wellesley, MA). The blots were stripped and reprobed with polyclonal rabbit antisera or murine mAb recognizing STAT3, STAT4, Jak2, or Tyk2. A mAb to GAPDH was used as the loading control. For lysates of both control subject and patient PBMCs, immunoblots using the polyclonal antisera to STAT4 usually resulted in a large nonspecific band of faster electrophoretic mobility than the specific STAT4 band. Confirmation that the upper band represents authentic STAT4 was done by stripping and reprobing selected blots with a specific STAT4 mAb and by comparison with immunoblots using the polyclonal antisera to test lysates from cultured wild-type and STAT4-deficient murine lymphocytes. Densitometric analysis of the STAT4 band was performed on immunoblots probed with the STAT4 mAb.
Polymerase chain reaction (PCR) analysis
Total RNA was extracted from PBMCs using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Total RNA (1 μg) was used for first-strand cDNA synthesis using the Cloned AMV First-Strand cDNA synthesis kit (Invitrogen). cDNA (7 μL) was used to amplify the STAT4 sequence by PCR as previously described.26 The GAPDH sequence was amplified from 0.5 μL cDNA using the sense primer 5′-ACCACAGTCCATGCCATCAC-3′ and the antisense primer 5′-TCCACCACCCTGTTGCTGTA-3′. Amplified product (5 μL) was resolved on a 2.0% agarose gel stained with ethidium bromide. Intensity of the amplified bands was analyzed by densitometry.
Statistical analysis
The lower limit of detection for the IFN-γ ELISA is 8 pg/mL. For data analysis, samples with IFN-γ levels less than 8 pg/mL were assigned a value of 8 pg/mL. Means, SEs, and t test comparisons were calculated using STATview software (Abacus Concepts, Piscataway, NJ).
Results
Defective IL-12–induced IFN-γ production by PBMCs from patients with lymphoma obtained after autologous stem cell transplantation
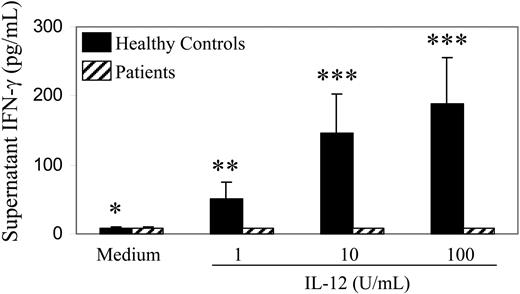
We have previously found that PBMCs obtained from patients with hematologic malignancies who have undergone autologous stem cell transplantation are deficient in IL-12–induced IFN-γ production. We have extended this observation by analyzing a larger number of PBMC samples obtained from healthy control subjects and from patients with lymphoma after autologous stem cell transplantation. IFN-γ was not detectable in the supernatants of posttransplantation patient PBMCs after stimulation with IL-12 in concentrations as high as 100 U/mL (Figure 1). By contrast, IFN-γ was readily detectable in the supernatants of control PBMCs stimulated with IL-12 in concentrations as low as 1 U/mL. Statistically significant differences in supernatant IFN-γ levels were seen for all of the IL-12 concentrations tested in vitro.
The total absolute lymphocyte count at the time that blood samples were collected from patients after transplantation was 1.960 ± 0.359 × 109/L (1960 ± 359 cells per μL; mean ± SE; n = 10), which is within the normal range. Nevertheless, the distribution of lymphocyte subsets in the peripheral blood of patients after transplantation is known to differ significantly from that of healthy control subjects. Whereas the absolute numbers of B cells and NK cells are similar to control subjects by about 2 to 3 months after transplantation, the number of CD4 T cells is subnormal for up to a year or more after transplantation.27-29 Accordingly, the proportion of CD4 T cells (10% ± 1%) in the posttransplantation patient samples used for the IFN-γ assays in Figure 1 was significantly less than that (47% ± 7%) in the control samples (P ≤ .001). The relative paucity of CD4 T cells might contribute to defective IFN-γ production by posttransplantation PBMCs. To address this possibility, we incubated PBMCs obtained from 8 patients with lymphoma in IFN-γ assays using 250 000 cells per well. The number of CD4 T cells per well in these assays (31 250 ± 4532) did not differ significantly from the number of CD4 T cells per well (23 834 ± 1188) in assays of control subject PBMCs plated at 50 000 cells per well. Nevertheless, the amount of IFN-γ detected in the supernatants of patient PBMCs plated at 250 000 cells per well was less than 38 pg/mL for all but 1 of the 8 patients tested; it was an order of magnitude less than that detected in the supernatants of control subject PBMCs plated at 50 000 cells per well (data not shown).
IL-12–induced production of IFN-γ by PBMCs. PBMCs (50 000 cells per well) obtained from 10 patients with lymphoma at a median of 3 months (range, 1-6 months) after autologous stem cell transplantation (▨) or from 10 healthy control subjects (▪) were incubated in vitro in medium alone or medium containing different concentrations of IL-12 as indicated. IFN-γ levels in supernatants were measured by ELISA. Values are means; error bars indicate SEs. Absence of visible error bars indicates SE ≤ 1 pg/mL. The P values for comparison of patient versus control subject results are as follows: > .4 (*), ≤ .05 (**), ≤ .025 (***).
IL-12–induced production of IFN-γ by PBMCs. PBMCs (50 000 cells per well) obtained from 10 patients with lymphoma at a median of 3 months (range, 1-6 months) after autologous stem cell transplantation (▨) or from 10 healthy control subjects (▪) were incubated in vitro in medium alone or medium containing different concentrations of IL-12 as indicated. IFN-γ levels in supernatants were measured by ELISA. Values are means; error bars indicate SEs. Absence of visible error bars indicates SE ≤ 1 pg/mL. The P values for comparison of patient versus control subject results are as follows: > .4 (*), ≤ .05 (**), ≤ .025 (***).
Evaluation of CD25+ regulatory T cells after autologous stem cell transplantation
CD25+ regulatory T cells (T reg cells) are a subset of CD4 T cells that inhibit the activation and proliferation of conventional CD4 and CD8 T cells.30,31 CD25+CD4+ T reg cells have been found to suppress IFN-γ production in vitro and IFN-γ–dependent antitumor immune responses in vivo.32,33 Therefore, we examined whether CD25+CD4+ T reg cells were relatively increased in PBMCs obtained from patients after autologous stem cell transplantation. The proportion of CD4 T cells that coexpressed CD25 in posttransplantation patient PBMCs (30% ± 8%) was not significantly different from the proportion (26% ± 5%) in control subject PBMCs (P > .1). Moreover, because of the decreased absolute number of CD4 T cells after transplantation, the absolute number of CD25+CD4+ T cells in posttransplantation patient PBMCs was significantly lower than that in control subject PBMCs (P ≤ .005).
Evaluation of Th1 and Th2 subsets after autologous stem cell transplantation
T helper 2 (Th2) cells are helper effector cells that selectively produce type 2 cytokines (eg, IL-4, IL-5, IL-13) after activation.34-36 Th2 cells inhibit the differentiation of Th1 cells, which selectively produce IFN-γ. Bias toward Th2 differentiation of CD4 T cells after transplantation could account for defective IFN-γ production by posttransplantation patient PBMCs. Human Th1 cells selectively express the chemokine receptors CXC chemokine receptor 3 (CXCR3) and CC chemokine receptor 5 (CCR5), whereas human Th2 cells selectively express CCR3 and CCR4.37-39 We therefore examined the expression of CXCR3 and CCR3 on CD4 T cells after autologous stem cell transplantation. The proportion of CD4 T cells that coexpressed CXCR3 or CCR3 in posttransplantation PBMCs did not differ significantly (P > .1) from control subject PBMCs (data not shown).
As the use of chemokine receptor expression to identify human Th1 and Th2 subsets is not uniformly accepted,40 we also investigated the production of type 1 versus type 2 cytokines by PBMCs exposed to strong nonspecific stimulation in vitro. Control subject and patient PBMCs produced high levels of IFN-γ, but no detectable IL-4, under these experimental conditions (Table 1). Taken together with the chemokine receptor expression data, these results argue against any bias toward Th2 differentiation in the posttransplantation setting. Moreover, the levels of IFN-γ secreted by posttransplantation patient PBMCs did not differ significantly from control subject PBMCs after stimulation with PMA and ionomycin (Table 1). Production of IFN-γ by both T cells and NK cells was detected by flow cytometric analysis of PBMCs stimulated with PMA and ionomycin. The percentage of T and NK cells expressing intracellular IFN-γ did not differ in control subject as compared with posttransplantation patient PBMCs after stimulation with PMA and ionomycin (data not shown).
Expression of IL-12–receptor subunits by PBMCs obtained from patients with lymphoma after autologous stem cell transplantation
As CD4 lymphopenia, expansion of CD25+CD4+ T reg cells, or Th2 bias did not appear to account for defective IL-12–induced IFN-γ production after autologous stem cell transplantation, we investigated whether posttransplantation patient PBMCs were deficient in expression of IL-12 receptors. Both IL-12Rβ1 and IL-12Rβ2 are required for high-affinity binding of IL-12 and intracellular signal transduction.19 Failure of posttransplantation patient PBMCs to produce IFN-γ after direct stimulation with IL-12 in vitro could therefore be due to defective expression of either IL-12 receptor subunit. Unexpectedly, we found that a significantly higher proportion of posttransplantation patient PBMCs expressed IL-12Rβ1, as compared with control subject PBMCs. IL-12Rβ1 was expressed by 65% ± 6% of posttransplantation patient PBMCs and 24% ± 5% of control subject PBMCs (P ≤ .001). This was largely due to increased expression of IL-12Rβ1 on posttransplantation patient peripheral blood T cells. IL-12Rβ1 was expressed by 85% ± 4% of posttransplantation patient T cells but only 14% ± 4% of control subject T cells (Table 2). IL-12Rβ1 was expressed by most peripheral blood NK cells in samples obtained from both patients after transplantation and control subjects. Nevertheless, a significantly higher percentage of posttransplantation patient NK cells expressed IL-12Rβ1 as compared with control subject NK cells. Comparing control subject and patient samples, no significant differences were observed in IL-12Rβ2 expression by total PBMCs (data not shown) or T cells (Table 2). However, a significantly higher percentage of posttransplantation patient NK cells expressed IL-12Rβ2 as compared with control subject NK cells (Table 2).
Defective IL-12–induced STAT4 phosphorylation by posttransplantation patient PBMCs
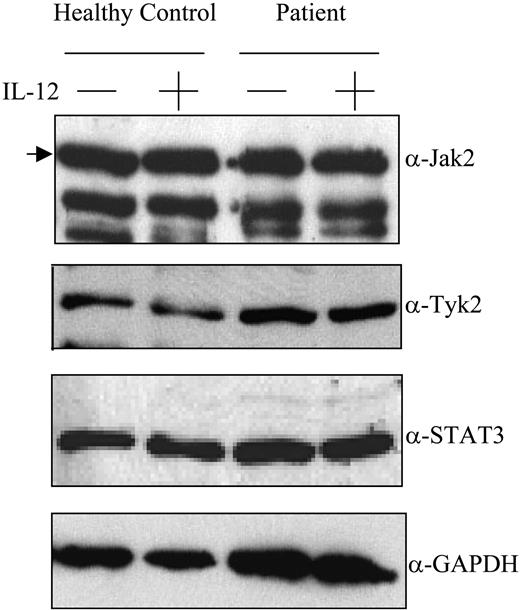
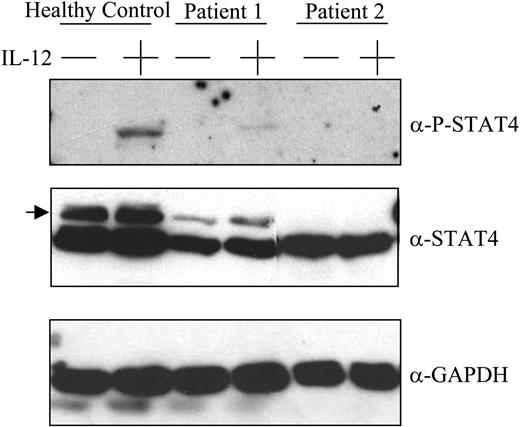
The data indicate that the mechanism of defective IL-12–induced IFN-γ production by posttransplantation PBMCs involves signaling events distal to the interaction of IL-12 with its cell-surface receptor. We therefore evaluated the integrity of IL-12 receptor–related signaling pathways in posttransplantation patient PBMCs. Total cellular levels of Jak2 and Tyk2 were comparable in control subject and patient PBMCs (Figure 2). Furthermore, as expected, IL-12 induced the tyrosine phosphorylation of STAT4 proteins in control subject PBMCs (Figure 3). In contrast, STAT4 tyrosine phosphorylation was undetectable or barely detectable in posttransplantation patient PBMCs incubated with IL-12. This defect in IL-12–induced STAT4 phosphorylation appears to be due to a deficiency in STAT4 protein levels in patient PBMCs as compared with control subject PBMCs (Figure 3). By densitometric analysis, the relative amount of STAT4 protein in posttransplantation patient PBMCs was 315 ± 27 (mean ± SE) as compared with 9720 ± 1309 in control subject PBMCs (P = .02). In contrast, the levels of STAT3 protein were not significantly different when comparing posttransplantation patient with control subject PBMCs (Figure 2).
Expression of Jak2, Tyk2, and STAT3 by PBMCs. PBMCs obtained from a healthy control subject (lanes 1-2) and a patient with lymphoma 3 months after transplantation (lanes 3-4) were incubated with or without IL-12 100 U/mL for 1 hour as indicated, lysed, resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted using antibodies specific for Jak2, Tyk2, STAT3, or GAPDH as indicated. Location of the specific band for Jak2 is indicated by the arrow. Results are representative of those seen with 4 control samples (4 for Jak2, 2 for Tyk2 and STAT3) and 4 patient samples (4 for Jak2, 2 for Tyk2, 3 for STAT3) that were tested.
Expression of Jak2, Tyk2, and STAT3 by PBMCs. PBMCs obtained from a healthy control subject (lanes 1-2) and a patient with lymphoma 3 months after transplantation (lanes 3-4) were incubated with or without IL-12 100 U/mL for 1 hour as indicated, lysed, resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted using antibodies specific for Jak2, Tyk2, STAT3, or GAPDH as indicated. Location of the specific band for Jak2 is indicated by the arrow. Results are representative of those seen with 4 control samples (4 for Jak2, 2 for Tyk2 and STAT3) and 4 patient samples (4 for Jak2, 2 for Tyk2, 3 for STAT3) that were tested.
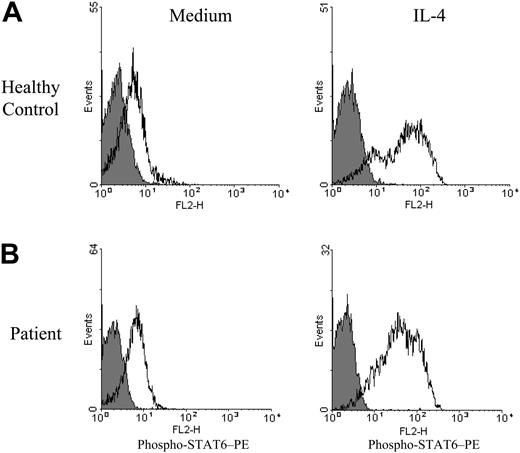
In contrast to their defective STAT4 tyrosine phosphorylation in response to IL-12, posttransplantation patient PBMCs exhibit normal STAT6 tyrosine phosphorylation in response to IL-4 (Figure 4). After stimulation with IL-4, the percentage of posttransplantation patient T cells (59% ± 8%) and NK cells (79% ± 6%) that coexpressed phospho-STAT6 did not differ significantly (P > .1) from that of control subject T cells (64% ± 8%) and NK cells (85% ± 5%).
IL-12 levels in the serum of patients with lymphoma after autologous stem cell transplantation
Prolonged exposure to IL-12 in vitro causes STAT4 protein levels to decline markedly in human NK cells and activated T cells.41,42 Given the enhanced expression of the IL-12 receptor complex on posttransplantation patient PBMCs, high endogenous levels of IL-12 after transplantation could lead to internalization of IL-12 by PBMCs in vivo and subsequent STAT4 protein deficiency. We therefore measured serum IL-12 levels in 10 patients with lymphoma after autologous stem cell transplantation. IL-12 was undetectable in the serum of 6 patients; IL-12 levels of 21, 30, 68, and 69 pg/mL were detected in the serum of 4 patients, respectively. Thus, high serum concentrations of IL-12 do not appear to account for most cases of STAT4 deficiency after transplantation.
Tyrosine phosphorylation of STAT4 in response to IL-12. PBMCs obtained from a healthy control subject (lanes 1-2) and 3 months after transplantation from 2 patients with lymphoma (lanes 3-6) were incubated with or without IL-12 100 U/mL for 1 hour as indicated, lysed, resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted using antibodies specific for tyrosine phosphorylated STAT4, total STAT4, or GAPDH as indicated. Location of the specific band for total STAT4 is indicated by the arrow. The identity of the STAT4 band was confirmed as described in “Patients, materials, and methods.” Results are representative of those seen with 6 control samples and 6 patient samples that were tested.
Tyrosine phosphorylation of STAT4 in response to IL-12. PBMCs obtained from a healthy control subject (lanes 1-2) and 3 months after transplantation from 2 patients with lymphoma (lanes 3-6) were incubated with or without IL-12 100 U/mL for 1 hour as indicated, lysed, resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted using antibodies specific for tyrosine phosphorylated STAT4, total STAT4, or GAPDH as indicated. Location of the specific band for total STAT4 is indicated by the arrow. The identity of the STAT4 band was confirmed as described in “Patients, materials, and methods.” Results are representative of those seen with 6 control samples and 6 patient samples that were tested.
Tyrosine phosphorylation of STAT6 in response to IL-4. PBMCs obtained from a healthy control subject (A) and from a patient with lymphoma 3 months after transplantation (B) were incubated for 1 hour in medium alone or medium containing 10 ng/mL IL-4 as indicated and analyzed for intracellular expression of phospho-STAT6 by flow cytometry as described in “Patients, materials, and methods.” Logarithm of red fluorescence is displayed on the abscissa and relative cell number on the ordinate. Staining with PE-conjugated anti–phospho-STAT6 mAb is indicated by open histograms and staining with PE-conjugated control mAb by shaded histograms. Expression of phospho-STAT6 after IL-4 stimulation did not differ significantly (P > .4) when comparing control PBMCs (n = 3) with posttransplantation patient PBMCs (n = 6).
Tyrosine phosphorylation of STAT6 in response to IL-4. PBMCs obtained from a healthy control subject (A) and from a patient with lymphoma 3 months after transplantation (B) were incubated for 1 hour in medium alone or medium containing 10 ng/mL IL-4 as indicated and analyzed for intracellular expression of phospho-STAT6 by flow cytometry as described in “Patients, materials, and methods.” Logarithm of red fluorescence is displayed on the abscissa and relative cell number on the ordinate. Staining with PE-conjugated anti–phospho-STAT6 mAb is indicated by open histograms and staining with PE-conjugated control mAb by shaded histograms. Expression of phospho-STAT6 after IL-4 stimulation did not differ significantly (P > .4) when comparing control PBMCs (n = 3) with posttransplantation patient PBMCs (n = 6).
Expression of STAT4 mRNA after autologous stem cell transplantation
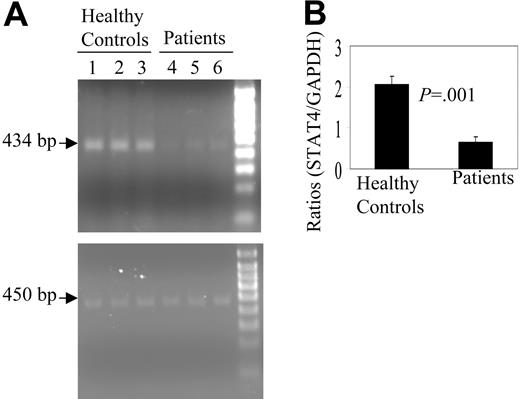
Inhibition of STAT4 gene expression and/or mRNA stability could contribute to STAT4 protein deficiency after transplantation. We therefore evaluated STAT4 mRNA levels using the PCR technique. STAT4 mRNA levels were significantly lower after transplantation as compared with control subject PBMCs, using GAPDH mRNA levels as a control for equal amounts of RNA (Figure 5A). Densitometric analysis confirmed that the approximate 69% reduction in STAT4 mRNA levels seen in posttransplantation samples compared with control samples was statistically significant (Figure 5B).
IL-18 augments IL-12–induced IFN-γ production by posttransplantation patient PBMCs
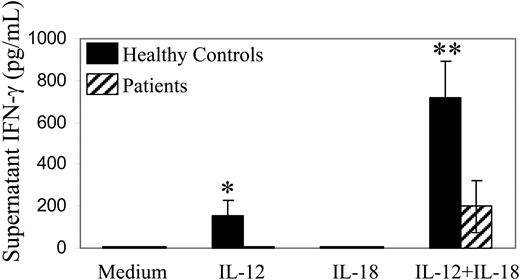
IL-18 can act synergistically with IL-12 to promote IFN-γ production by normal PBMCs. IL-18 by itself (in concentrations as high as 100 ng/mL) did not stimulate significant IFN-γ production by either control subject or patient PBMCs (Figure 6 and data not shown). However, IL-12 and IL-18 in combination induced substantial IFN-γ production by both control and patient PBMCs. The level of IFN-γ secreted in response to IL-12 plus IL-18 was higher for control subject as compared with patient PBMCs (Figure 6). Nevertheless, patient PBMCs stimulated with IL-12 plus IL-18 produced IFN-γ in amounts that were not significantly different (P > .375) than those produced by control subject PBMCs stimulated with IL-12 alone. IFN-γ can also be detected in supernatants of posttransplantation patient PBMCs incubated with IL-2 and IL-12 in combination.22 However, IFN-γ levels (433 ± 168 pg/mL) produced by posttransplantation patient PBMCs stimulated with IL-12 plus 100 pM IL-2 were significantly less (P < .025) than IFN-γ levels (> 1000 pg/mL) produced by the same PBMCs stimulated with IL-12 plus 10 ng/mL IL-18.
Expression of STAT4 mRNA by PBMCs. (A) Total RNA was extracted from PBMCs of 3 healthy control subjects (lanes 1-3) or 3 patients with lymphoma after transplantation (lanes 4-6), reverse transcribed to cDNA, subjected to 25 cycles of PCR amplification, and resolved by electrophoresis as described in “Patients, materials, and methods.” The specific bands corresponding to the amplified STAT4 cDNA (upper) or amplified GAPDH cDNA (lower) are indicated by arrows. (B) Intensities of the STAT4 and GAPDH bands were measured by densitometry. Values shown are mean ± SD of the ratio of the intensity of STAT4 to GAPDH bands.
Expression of STAT4 mRNA by PBMCs. (A) Total RNA was extracted from PBMCs of 3 healthy control subjects (lanes 1-3) or 3 patients with lymphoma after transplantation (lanes 4-6), reverse transcribed to cDNA, subjected to 25 cycles of PCR amplification, and resolved by electrophoresis as described in “Patients, materials, and methods.” The specific bands corresponding to the amplified STAT4 cDNA (upper) or amplified GAPDH cDNA (lower) are indicated by arrows. (B) Intensities of the STAT4 and GAPDH bands were measured by densitometry. Values shown are mean ± SD of the ratio of the intensity of STAT4 to GAPDH bands.
Mechanism of augmented IFN-γ production by PBMCs stimulated with IL-12 and IL-18
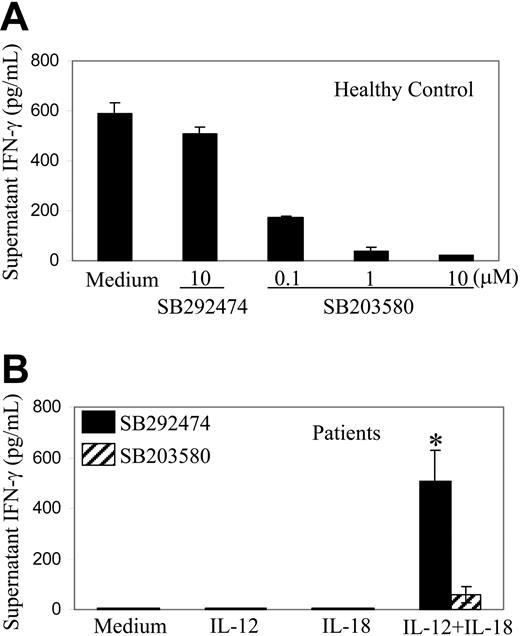
As IL-12 plus IL-18 could restore IFN-γ production by posttransplantation patient PBMCs, we examined whether IL-12 plus IL-18 could reverse defective STAT4 expression in these cells. In 4 samples tested, we failed to detect increased STAT4 protein levels or cytokine-induced STAT4 tyrosine phosphorylation after incubation of posttransplantation patient PBMCs with IL-12 plus IL-18 (data not shown). These results suggest that enhanced IFN-γ production under our experimental conditions occurs predominantly by mechanisms other than recovery of STAT4 protein levels. Activation of p38 MAPK has been reported to participate in both STAT4-dependent and STAT4-independent production of IFN-γ in response to IL-12.43-45 We therefore tested the effects of a specific p38 MAPK inhibitor on the production of IFN-γ by PBMCs stimulated with IL-12 and/or IL-18. Using PBMCs obtained from healthy control subjects, we confirmed that the p38 MAPK inhibitor (SB203580) inhibited, in a dose-dependent manner, production of IFN-γ in response to IL-12 and IL-18; the control reagent (SB202474) had no major effect (Figure 7A). Similarly, the p38 MAPK inhibitor specifically inhibited (by ∼ 90%) production of IFN-γ induced by IL-12 and IL-18 stimulation of PBMCs obtained from patients after autologous stem cell transplantation (Figure 7B).
Production of IFN-γ by PBMCs stimulated with IL-12 and IL-18. PBMCs (50 000 cells per well) obtained from 5 patients with lymphoma after autologous stem cell transplantation (▨) or from 5 healthy control subjects (▪) were incubated in vitro in medium alone or medium containing IL-12 10 U/mL, IL-18 10 ng/mL, or both cytokines as indicated. IFN-γ levels in supernatants were measured by ELISA. Values are means; error bars indicate SE. Absence of visible error bars indicates SE ≤ 1 pg/mL. The P values for comparison of patient versus control subject results are as follows: ≤ .05 (*), ≤ .025 (**).
Production of IFN-γ by PBMCs stimulated with IL-12 and IL-18. PBMCs (50 000 cells per well) obtained from 5 patients with lymphoma after autologous stem cell transplantation (▨) or from 5 healthy control subjects (▪) were incubated in vitro in medium alone or medium containing IL-12 10 U/mL, IL-18 10 ng/mL, or both cytokines as indicated. IFN-γ levels in supernatants were measured by ELISA. Values are means; error bars indicate SE. Absence of visible error bars indicates SE ≤ 1 pg/mL. The P values for comparison of patient versus control subject results are as follows: ≤ .05 (*), ≤ .025 (**).
Effect of p38 MAPK inhibitor on production of IFN-γ in response to IL-12 and IL-18. (A) PBMCs (50 000 cells per well) obtained from a healthy control subject were incubated in medium containing IL-12 100 U/mL and IL-18 10 ng/mL; a specific inhibitor of p38 MAPK (SB203580) or control reagent (SB202474) were added at the concentrations indicated. Values are mean ± SD of IFN-γ levels in supernatants of duplicate wells as measured by ELISA. The experiment was repeated once with similar results. (B) PBMCs (50 000 cells per well) obtained 3 months after transplantation from 3 patients with lymphoma were incubated with cytokines and 10 μM p38 MAPK inhibitor (▨) or 10 μM control reagent (▪) as indicated. Values are mean ± SE of supernatant IFN-γ levels. The P value for comparison of p38 MAPK inhibitor versus control reagent results is ≤ .025 (*).
Effect of p38 MAPK inhibitor on production of IFN-γ in response to IL-12 and IL-18. (A) PBMCs (50 000 cells per well) obtained from a healthy control subject were incubated in medium containing IL-12 100 U/mL and IL-18 10 ng/mL; a specific inhibitor of p38 MAPK (SB203580) or control reagent (SB202474) were added at the concentrations indicated. Values are mean ± SD of IFN-γ levels in supernatants of duplicate wells as measured by ELISA. The experiment was repeated once with similar results. (B) PBMCs (50 000 cells per well) obtained 3 months after transplantation from 3 patients with lymphoma were incubated with cytokines and 10 μM p38 MAPK inhibitor (▨) or 10 μM control reagent (▪) as indicated. Values are mean ± SE of supernatant IFN-γ levels. The P value for comparison of p38 MAPK inhibitor versus control reagent results is ≤ .025 (*).
Discussion
Production of IFN-γ in vivo is required for the efficacy of IL-12–based immunotherapy in many preclinical tumor models.13,14,16,46,47 IFN-γ production after vaccination has also been found to be critical for the efficacy of peptide, dendritic cell, and cytokine-transduced tumor cell vaccines.33,48,49 Furthermore, by activating cells bearing the receptor for the constant region of immunoglobulin (Fc), IFN-γ may potentiate the beneficial effects of monoclonal antibody therapies for cancer.50 Failure to adequately produce IFN-γ in vivo is therefore likely to have broad and profound implications for cancer immunotherapy. Our previous work has shown that, compared with patients with a solid tumor not receiving transplants, patients with hematologic malignancies who have undergone autologous stem cell transplantation produce an order of magnitude less IFN-γ in vivo during systemic IL-12 therapy.22 Furthermore, posttransplantation patient PBMCs are intrinsically defective in their ability to produce IFN-γ after direct in vitro stimulation with IL-12. Successful cytokine-, vaccine-, or antibody-based immunotherapy after autologous stem cell transplantation may therefore require strategies to circumvent defective IFN-γ production in this setting.
We have investigated the mechanisms underlying defective IFN-γ production after transplantation. Our results clearly indicate that, with respect to IL-12–induced IFN-γ production, the deficiency must occur distal to the interaction of IL-12 with its cell-surface receptor. Furthermore, we have found that posttransplantation patient PBMCs are profoundly deficient in the expression of STAT4. Signaling through STAT4 has previously been shown to be critical for many biologic activities of IL-12, including IFN-γ production.20,21 The low levels of STAT4 expression that we observed in posttransplantation patient PBMCs were not due to a general decrease in members of JAK/STAT signaling pathways; levels of Jak2, Tyk2, and STAT3 did not differ significantly between control subject and posttransplantation patient PBMCs. Moreover, IL-4–induced tyrosine phosphorylation of STAT6 was similar in posttransplantation patient versus control subject PBMCs. It therefore appears that a selective deficiency in STAT4 protein levels, and consequent inadequate tyrosine phosphorylation of STAT4, is the major mechanism responsible for defective IL-12–induced IFN-γ production by PBMCs obtained from patients with lymphoma after transplantation. This state of STAT4 deficiency may be relatively prolonged after autologous stem cell transplantation. Although most of the PBMC samples used in our studies were obtained 3 months after transplantation, a similar degree of STAT4 deficiency was seen in PBMC samples obtained 5 to 6 months after transplantation (n = 4; data not shown). Thus, simply delaying immunotherapy until 6 months after stem cell transplantation is not likely to be a successful strategy for circumventing defective IFN-γ production. Indeed, such a delay could potentially inhibit the efficacy of immunotherapy by allowing the tumor burden to increase.
The mechanisms underlying deficient STAT4 protein levels in posttransplantation patient PBMCs have not been fully elucidated and will be the subject of future investigation. Inhibition of STAT4 gene expression, decrease in STAT4 mRNA stability, or accelerated degradation of STAT4 protein could contribute to STAT4 deficiency. The reduction in STAT4 protein levels (97%) was more profound than the reduction in STAT4 mRNA levels (69%). Although direct comparison of RNA and protein levels is not appropriate given the different methodologies used, enhanced STAT4 protein degradation as well as decreased STAT4 mRNA levels could be involved in posttransplantation STAT4 deficiency. The relative contributions of high-dose chemotherapy, pretransplantation conventional chemotherapy, and disease status (histologic subtype of lymphoma, tumor burden) to STAT4 deficiency also require further study. Our preliminary data indicate that varying degrees of STAT4 deficiency can be seen in lymphoma patient PBMCs obtained prior to autologous stem cell transplantation; however, the STAT4 deficiency before transplantation is not as profound or consistent as that seen after autologous stem cell transplantation (M.J.R. and M.H.K., unpublished data, 2004). In contrast, we did not observe decreased STAT4 protein levels in PBMCs obtained from 2 patients with lymphoma who had not been treated with chemotherapy (M.J.R. and M.H.K., unpublished data, 2004). One plausible hypothesis is that STAT4 deficiency is a consequence of cytotoxic chemotherapy and that its severity is directly related to the duration or intensity of such chemotherapy. Further investigation is needed to test this hypothesis.
After stimulation in vitro with PMA and ionomycin, comparable IFN-γ levels were detected in the supernatants of posttransplantation patient and control subject PBMCs. This indicates that posttransplantation patient PBMCs are capable of producing normal levels of IFN-γ when sufficiently activated. We therefore wanted to identify more physiologic and clinically feasible stimuli that could promote IFN-γ production after stem cell transplantation. We found that IL-12 and IL-18 in combination could partly reverse defective IFN-γ production by posttransplantation patient PBMCs. IFN-γ levels produced by posttransplantation patient PBMCs after stimulation with IL-12 plus IL-18 were comparable to IFN-γ levels produced by control subject PBMCs after stimulation with IL-12 alone. In preliminary intracellular-staining experiments, we have found that NK cells are the major producers of IFN-γ after stimulation of posttransplantation patient PBMCs with IL-12 and IL-18 (M.H.K., unpublished data, 2004). These results are in agreement with previous studies using normal PBMCs.51,52 The rare CD56bright subset of NK cells appears to be particularly efficient at producing IFN-γ after stimulation with cytokines.51,53 Nevertheless, we have observed significant production of IFN-γ by both CD56bright and CD56dim NK cells after stimulation of posttransplantation patient PBMCs by IL-12 and IL-18 in combination (M.J.R., unpublished data, 2004). Moreover, we cannot exclude a contribution by rare T-cell subsets to IFN-γ production by posttransplantation patient PBMCs. A subset of CD8 T cells, characterized by high expression of the lymphocyte function-associated antigen 1 (LFA-1) adhesion molecule, can produce substantial amounts of IFN-γ after stimulation with IL-12 plus IL-2.54 Further studies will be required to define the cell subsets that contribute to IFN-γ production after cytokine stimulation of posttransplantation patient PBMCs.
IFN-γ production after stimulation with IL-12 plus IL-18 required p38 MAPK activity and was not due to enhanced expression of STAT4. Synergistic production of IFN-γ in response to IL-12 plus IL-18 has previously been shown to require p38 MAPK activity.45 Moreover, p38 MAPK can promote IFN-γ production by activated T cells in a STAT4-independent manner.44 IL-12 and IL-18 can augment IFN-γ production in STAT4-deficient cells, albeit to a lesser extent than in wild-type cells.55 Additional studies are in progress to determine methods of enhancing STAT4 expression in posttransplantation patient PBMCs with a goal of further augmenting cytokine-induced IFN-γ production.
IL-12 and IL-2 in combination can also augment IFN-γ production by patient PBMCs,22 although not as effectively as IL-12 plus IL-18. IL-2 therapy is feasible after autologous stem cell transplantation for hematologic malignancies.56,57 Furthermore, combined IL-2 and IL-12 treatment is tolerable in patients with solid tumors and can reverse the attenuation of serum IFN-γ levels seen during prolonged therapy with IL-12 alone.58 IL-18 can also be given safely in biologically active doses to patients with advanced cancer.59,60 Thus, administration of IL-12 together with IL-2 or IL-18 might circumvent defective IFN-γ production and enhance antitumor immune responses. Further investigation of cytokine-based immunotherapy after autologous stem cell transplantation is warranted.
Prepublished online as Blood First Edition Paper, April 7, 2005; DOI 10.1182/blood-2005-01-0201.
Supported in part by grants from the National Institutes of Health (M01 RR00750-27S3) (M.J.R.), (AI45515) (M.H.K.), (T32DK007519) (H.C.C.), and (MO1 RR750); by the Indiana Genomics Initiative of Indiana University (partially supported by the Lilly Endowment) (M.H.K.), and by an Immunology and Hematopoiesis Program grant (M.J.R.) from the Indiana University Cancer Center (P30CA82709).
M.J.R. and M.H.K. designed the research, analyzed the data, and wrote the paper; H.-C.C. and D.P. performed the research and analyzed the data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lisa Wood, RN, Jill Weisenbach, RN, and the nursing staff in the General Clinical Research Center and Adult Bone Marrow Transplant Clinic at Indiana University Medial Center for their assistance with these studies.