Abstract
Viral heterogeneity is a major hurdle for potential therapeutic use of RNA interference (RNAi) against HIV-1. To determine the extent of RNAi tolerance to mutations, we tested 3 viral target sites with differing propensity for mutations: a highly variable rev sequence, a gag sequence conserved only among clade B isolates, and a vif sequence highly conserved across clades. Lentiviral expression of all 3 shRNAs inhibited replication of the homologous HIVIIIB strain. However, they differed in their ability to protect primary CD4 T cells against multiple isolates within and across HIV clades. The least conserved rev sequence inhibited only 2 of 5 clade B isolates. The gag sequence (conserved within clade B) protected 5 of 5 clade B isolates but not other clade viruses with 2 or 3 mutations in the central region. In contrast, the vif sequence, which was conserved across clades except for single mutations at positions 14 and 17, inhibited viruses from 5 different clades. Moreover, siRNAs with introduced mutations at sites of gag sequence polymorphisms showed reduced antiviral activity, whereas mutations in vif siRNA only modestly decreased silencing. Thus, although 1 or 2 mutations at peripheral sites are tolerated, mutations in the central target cleavage region abolish RNAi activity.
Introduction
Despite the success of highly active antiretroviral therapy (HAART) in ameliorating HIV disease, alternative therapeutic strategies are being explored because of the practical problems associated with lifelong therapy, including toxicity, drug resistance, and patient adherence.1 The recent discovery that RNA interference (RNAi) can induce sequence-specific degradation of target mRNA in mammalian cells has opened a new molecular strategy for potential therapeutic intervention against HIV.2-4 The key for specific gene silencing by RNAi in mammalian cells is the use of short interfering RNAs (siRNAs), of fewer than 30 bp, to bypass the induction of an interferon (IFN) response that can nonspecifically shut down gene expression.5 In plants and invertebrates such short 21 to 23 nucleotide (nt) duplex RNAs with 2 nt 3′ overhangs are the natural cleavage products generated from long dsRNAs by the RNase III–type enzyme Dicer during the initiation step of RNAi (reviewed by Lieberman et al,2 Dykxhoorn et al,6 Denli and Hannon,7 and Cerutti8 ).
A major limitation of synthetic siRNA for potential therapeutic applications is the transient nature of gene silencing because of dilution with cell division. However, stable suppression of gene expression has now been achieved by endogenously expressing siRNA precursors in target cells using nonviral and viral vectors containing transcription units that encode short hairpin RNAs (shRNAs) under the control of the RNA polymerase III (pol III) small nuclear RNA U6, RNase P RNA H1 promoters, or the RNA polymerase II (pol II) cytomegalovirus (CMV) promoter.6,9-12 Lentiviral vectors are particularly attractive because they make it possible to introduce such shRNA cassettes into hard-to-transfect primary cells and facilitate sustained gene expression by integrating into the host genome.13,14
A number of studies have demonstrated the potential of RNAi targeting cellular receptor/coreceptors and/or viral genes to inhibit HIV replication in cell lines and in primary cells susceptible to the virus.11-26 However, the published studies thus far have used sequences from laboratory (lab) strains of HIV-1. It is unclear whether these sequences can inhibit diverse strains of the virus in a clinical setting. Based on genomic nucleotide sequence comparisons, HIV-1 is divided into multiple genetic subtypes or clades (clades A, B, C, D, E, F1, F2, G, H, J, and K).27 Moreover, intersubtype recombinant forms occur, resulting in considerable viral sequence diversity within each clade. Further compounding the complexity, multiple viral quasispecies are present at any given time within a single host, the number of which increases with disease progression.28 Thus, it is important to test the efficacy of RNAi-based approaches in the context of multiple strains of the virus.
The vulnerability of RNAi to viral mutations is evident from studies demonstrating that mismatches in the siRNA sequence abrogate RNAi-mediated silencing of HIV-1 in culture. Two recent studies looking at long-term silencing of viral replication in vitro found that nucleotide changes within the siRNA recognition site produced escape variants resistant to silencing by the original siRNA.11,29 These nucleotide changes occurred predominately in the center of the siRNA-mRNA recognition site. However, while there are stringent homology requirements for the critical central residues, peripheral nucleotide changes are better tolerated. Moreover, tolerance to sequence mismatches may depend on the specific siRNA under evaluation.30,31
The HIV genome contains highly conserved regions where structural or functional constraints restrict mutation.32 These regions may be desirable targets for RNAi because of the reduced likelihood of emergence of viral escape mutations. To determine how effectively RNAi can protect against a broad spectrum of HIV-1 isolates, shRNAs targeting viral rev, gag, and vif sequences, conserved to variable degrees within or across different clades of HIV-1, were expressed from lentiviral vectors. Evaluating viral inhibition by these small RNAs enabled us to assess the extent to and positions at which nucleotide changes are tolerated and to determine whether the approach of targeting conserved sequences can protect against the diversity of HIV viruses. We compared the ability of the 3 shRNAs to confer protection against a panel of lab-adapted and primary HIV isolates from multiple clades in stably transduced primary CD4+ T cells. Our data suggest that for broad-spectrum RNAi activity, high fidelity of the target sequence is critical within the central region surrounding the siRNA-mRNA recognition site. shRNAs designed to target sequences that are highly conserved within this central region can efficiently inhibit multiple strains of HIV-1. In particular, RNAi targeting the well-conserved vif sequence studied here inhibits the replication of both primary isolates and lab-adapted virus from all HIV-1 clades tested.
Materials and methods
Cell culture
293 T cells were maintained in Dulbecco modified Eagle medium (DMEM; Invitrogen, Rockville, MD) supplemented with 10% fetal calf serum (FCS), 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mM l-glutamine. HeLa-CD4 cells were cultured under similar conditions but in the presence of 500 μg/mL G418. Cells were plated at about 70% confluence 1 day before experimental manipulation. Primary CD4+ T-cell blasts were generated from immunomagnetically isolated CD4+ T cells from peripheral blood mononuclear cells (PBMCs) of healthy donors by culturing for 3 to 4 days in RPMI 1640 medium containing 15% FCS and 4 μg/mL phytohemagglutinin (PHA). All cultures and experimental manipulations of CD4+ T cells after 4 days were done in medium supplemented with 60 IU/mL recombinant interleukin-2 (rIL-2) (Chiron, Emeryville, CA).
RNAi target sequences
Short interfering RNAs were designed to target sequences in rev, gag, and vif of the HXB2 molecular clone of HIV. Sequence selection was based on the rules for RNAi susceptibility proposed by Tuschl's group5 using the RNAi prediction program (siRNA Target Finder) from Ambion (Austin, TX). The sense strand sequence of the siRNAs correspond to the following: rev 5′-GGCACTTATCTGGGACGAT-3′ (HXB2, nt 8485-8501), gag 5′-GATTGTACTGAGAGACAGG-3′ (HXB2, nt 2062-2080), and vif 5′-GTTCAGAAGTACACATCCC-3′ (HXB2, nt 5195-5213). The control siRNA sequence targeting green fluorescent protein (GFP) has been described previously.15 The prevalence of sequence mutations in the targeted regions was determined by analysis of the Los Alamos HIV Sequence Database (http://hiv-web.lanl.gov/content/hiv-db/mainpage.html). The sequence variation at each residue within the RNAi target sequence was calculated separately for each clade and expressed as percentage mutation: ([number of isolates exhibiting sequence change]/[total number of sequenced isolates in database] × 100).
Generation of short hairpin RNA (shRNA)–expressing lentiviruses
Oligonucleotides targeting viral gag, rev, and vif were synthesized as 21 nt inverse repeats separated by a 9-nt loop sequence and cloned into the U6 promoter–expressing lentiviral vector lentilox pLL3.7 shRNA essentially as reported by Rubinson et al.33 A control vector targeting the luciferase gene was also similarly generated using a published sequence (nt 155-173).9 The cloning strategy results in expression of shRNAs in transduced cells, which are processed into siRNAs. For lentivirus generation, 293 T cells were plated to semiconfluency in a 10-mm dish 1 day before transfection. The plasmid carrying the shRNA expression cassette was packaged into lentiviruses by cotransfection with helper plasmids pCMV-VSV-G (envelope) and pHR′8.9ΔVPR (core protein) in 293 T cells by calcium phosphate precipitation (Invitrogen, Rockville, MD). Viral supernatants were harvested 48 hours after transfection. To determine viral titers, HeLa-CD4 cells were transduced with serial dilutions of the lentiviral stocks, and 48 hours later GFP expression was assessed by flow cytometry. Although the gag and rev RNAi sequences' target sites are also present in the helper plasmids, there were no deleterious consequences on lentivirus generation, because the viral titers were comparable to that obtained with the luciferase and vif shRNA constructs. The viral titers generally ranged from 5 × 105/mL to 10 × 105/mL for all shRNA constructs. Modified Northern blot analyses of total RNA isolated from 293 T cells transduced 3 days earlier with each of the lentiviral constructs verified successful production of the hairpin precursor as well as the siRNA species (data not shown).
Transduction of cell lines or primary cells with shRNA lentiviruses
Cells were spin-infected with lentivirus for 2 hours at 1200g on 2 consecutive days (multiplicity of infection [MOI], 1 to 5) in DMEM containing 10% FCS and 8 μg/mL polybrene. After 2 hours of further incubation at 37°C, fresh medium was added to the cells. After 2 to 3 days of culture, the transduction efficiency was ascertained on the basis of GFP expression (more than 90% in HeLa-CD4 cells and 25% to 40% in CD4+ T cells). Homogeneous GFP-positive populations, obtained by flow cytometric sorting, were used for all experiments.
Viruses
The following HIV-1 viruses were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIAID, NIH): HIV89.6, HIVBaL, HIVIIIB, 92UG29 (UG29), 98IN017 (W17), 97ZA012 (ZA12), 92UG024 (UG24), 93TH64 (TH64), 93TH65 (TH65). To generate primary isolates from HIV-infected donors, immunomagnetically (Miltenyi Biotec, Auburn, CA) selected CD4+ T cells were stimulated with PHA (4 μg/mL) in RPMI 1640 supplemented with 15% FCS and 60 IU/mL rIL-2 (Chiron) to promote virus production. Culture supernatants were tested for p24 production using the HIV-1 p24 Antigen EIA kit (Beckman Coulter, Fullerton CA). In some cases, virus production was amplified by coculture with PHA blasts from HIV-seronegative subjects. To determine HIVIIIB viral titer, HeLa-CD4 cells infected with various dilutions of stock virus were analyzed for percentage of p24-expressing cells by flow cytometry. Chinese B′ virus (RL42) and Chinese C/B′ (CN54) viruses (a gift from Y. Shao, National Center for AIDS Prevention and Control, Beijing, China) were generated by transfecting H9 cells with the respective molecular clones as described.34
HIV-1 infection
GFP-sorted HeLa-CD4 cells or CD4+ T-cell blasts expressing control or test shRNAs were plated at 1 × 105 cells per well in 24-well plates. Cells were infected with the indicated HIV-1 isolates using 100 ng p24 gag antigen equivalents per well (MOI, 0.5). After viral challenge, the cells were analyzed for p24 expression by flow cytometry. Cell-free viral production was measured by p24 antigen enzyme-linked immunosorbent assay (ELISA) using the HIV-1 p24 Antigen EIA kit (Beckman Coulter).
Northern blot analysis of HIV gene expression
For Northern Blot analysis, 5 to 10 μg total cellular RNA, purified by the RNeasy mini kit (Qiagen, Valencia, CA), was run on a 1% denaturing agarose gel, transferred to a positively charged nylon membrane (BrightStar-plus; Ambion), and probed using the Northern Max protocol (Ambion). The p24 probe was polymerase chain reaction (PCR) amplified from the HXB2 plasmid using the p24-forward 5′-CCAGGGGCAAATGGTACATCAGGCCATA-3′ and p24-reverse 5′-CCTCCTGTGAAGCTTGCTCGGCTCTTA-3′ primers and gel purified using the Qiaquick gel extraction kit (Qiagen). The DECA template β-actin probe (Ambion) was used as a loading control (Ambion). The gel-purified PCR products (about 25 to 30 ng) and the β-actin control template were labeled with α-[32P]deoxyadenosine triphosphate (α-[32P]dATP) using the DECA prime II random prime labeling kit (Ambion), purified by NucAway spin columns (Ambion), and used as probes.
Intracellular p24 staining
Uninfected cells or cells infected with various HIV-1 strains were washed with Hanks balanced salt solution (HBSS), fixed and permeabilized using the Caltag (Burlingame, CA) Fix and Perm kit, stained for p24-RD1 (Beckman Coulter) as described,20 and analyzed on a FACSCalibur with Cell Quest software (Becton Dickinson, Franklin Lakes, NJ). The percentage of HIV-1 inhibition was calculated relative to the control luc shRNA-transduced cells as 100 – ([% of p24-positive cells transduced with viral target shRNA /% of p24-positive cells transduced with luc shRNA] × 100).
Proviral DNA sequencing
For proviral HIV-1 sequencing, DNA was extracted from infected CD4+ T cells as described earlier.35 Nested PCR was performed to amplify the gag, rev, and vif genes. Primers for gag amplification were as follows: gag outer (5′-GCAGGACTCGGCTTGCTGAAGCGC-3′, nt 691-714, and 5′-TACTGTATCATCTGCTCCTGTATC-3′, nt 2325-2351) and gag inner (5′-GCGGCGACTGGTGAGTACGCC-3′, nt 734-754, and 5′-TCCTTTAGTTGCCCCCCTATC-3′, nt 2294-2314); for rev amplification: rev outer (5′-AAAAGAGCAGTGGGAATAGGAGC-3′, nt 7752-7775, and 5′-CCTGTCTTATTCTTCTAGGTATGTGG-3′, nt 8772-8747) and rev inner (5′-CAGCAGGAAGCACTATGGGCGC-3′, nt 7798-7818, and 5′-ATAACCCTATCTGTCCCCTCAGCTAC-3′, nt 8713-8688); for vif amplification: vif outer (5′-CAAAAATTTTCGGGTTTATTACAGGGAC-3′, nt 4890-4916, and 5′-CTGCTATGTTGACACCCAATTCTG-3′, nt 5775-5798) and vif inner (5′-GGAAAGGTGAAGGGGCAGTAG-3′, nt 4957-4977, and 5′-TCCATTCATTGTGTGGCTCCC-3′, nt 5611-5595). The conditions for the first PCR reaction were 1 cycle (95°C for 9 minutes); 30 cycles (94°C for 1 minute); 55°C for 1 minute, and 72°C for 1.5 minutes and a final cycle (72°C for 10 minutes). For the second round of PCR, 5 μL of the primary reaction product was amplified under the same conditions. The amplified products were sequenced on an automated sequencer (Applied Biosystems, Foster City, CA) using the internal primers.
siRNA transfection
HeLa-CD4 cells were trypsinized and seeded in 6-well plates at 1 × 105 per well for 12 to 16 hours before transfection. Cationic lipid complexes, prepared by incubating 200 nM of indicated siRNA with 3 μL oligofectamine (Invitrogen) in 100 μL DMEM (Invitrogen) for 20 minutes, were added to the wells in a final volume of 1 mL. After overnight incubation, cells were washed, resuspended in DMEM media containing 10% FCS, and infected with HIV.
Results
HeLa-CD4 cells transduced with antiviral shRNAs resist HIVIIIB challenge
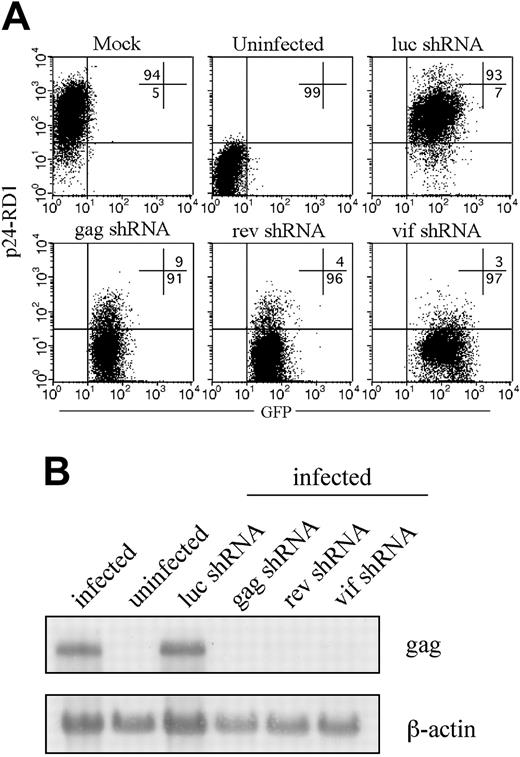
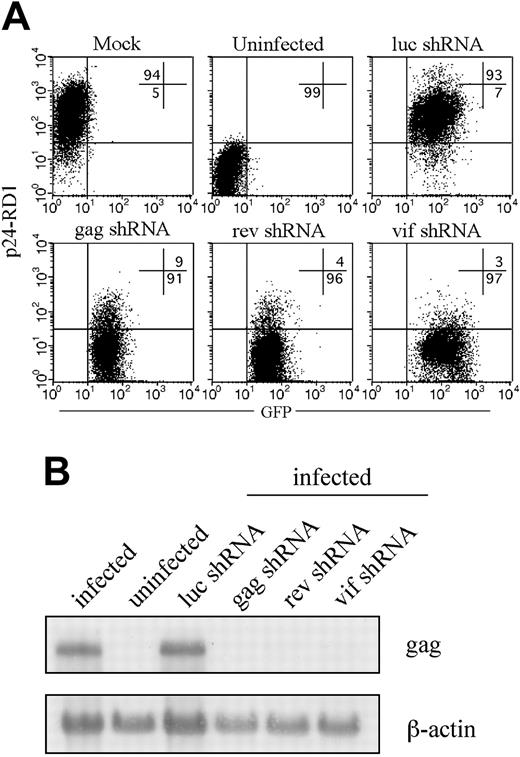
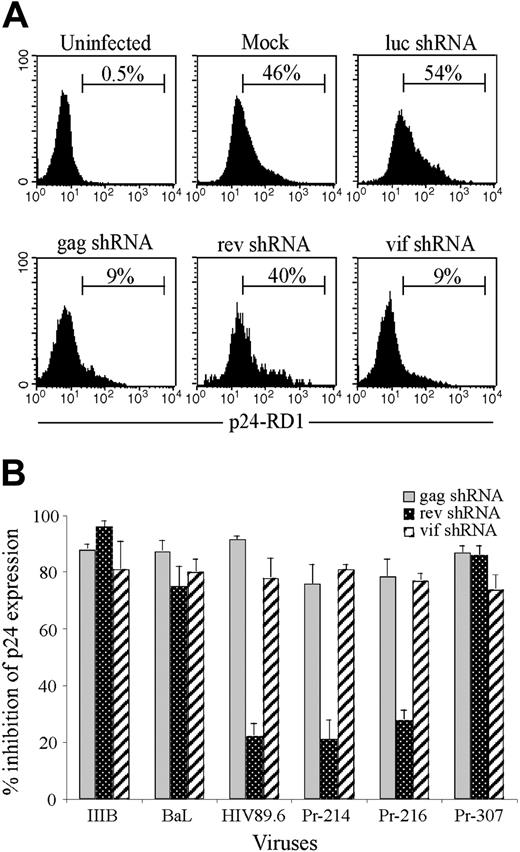
In initial experiments, we evaluated the antiviral activity of shRNAs targeting rev, gag, and vif to homologous HIVIIIB challenge. HeLa-CD4 cells were spin-infected with the lentiviruses, and after 3 to 4 days GFP-positive cells were sorted by fluorescence-activated cell sorting (FACS) to generate stable shRNA-expressing cell lines capable of targeted disruption of each one of the 3 viral or the control luciferase genes. These cell lines were maintained in culture for up to 12 weeks. Five days after challenge with 100 ng p24 equivalent of HIVIIIB, viral inhibition was assessed by measuring intracellular p24 expression by flow cytometry. As shown in Figure 1A, more than 90% of HeLa-CD4 cells expressing luc shRNA or no shRNA became infected with HIV. In contrast, in cells expressing shRNAs targeting gag, rev, or vif, viral replication was efficiently inhibited, with only 3% to 9% of cells staining positive for p24. Northern blot analysis verified that the steady state level of full-length RNA was reduced in the presence of specific shRNAs (Figure 1B). In cells transduced with the specific shRNAs, reduction in gag protein levels was accompanied by a marked reduction in expression of the 9.3-kb full-length HIV RNA. shRNA-expressing cells maintained in culture for up to 3 months showed no diminution in their efficiency of silencing (data not shown).
shRNAs targeting viral RNA protect against HIVIIIB infection in primary CD4+ T cells
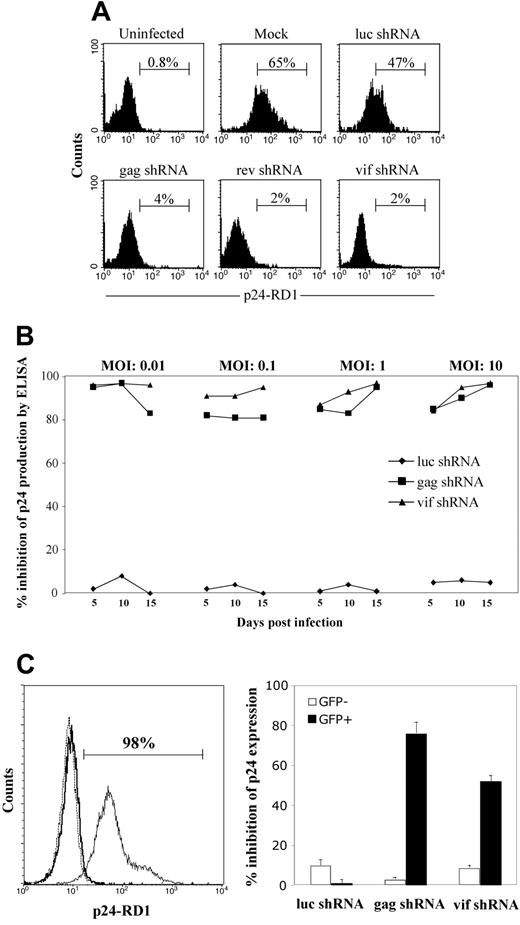
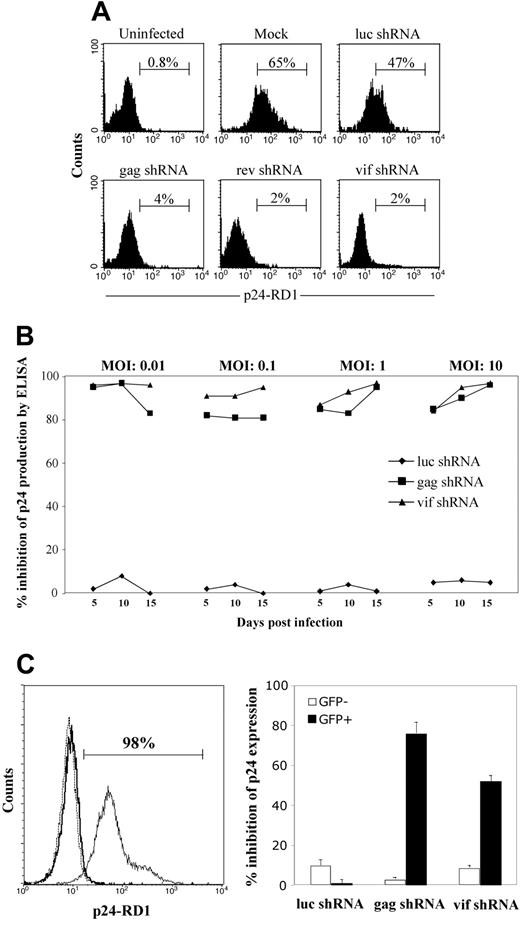
To confirm the results in a more physiologically relevant setting, we also tested the ability of the shRNAs to inhibit the same HIVIIIB clade B isolate in primary CD4+ T cells, which are the major targets of HIV-1 infection in vivo. After transduction with the shRNA-expressing lentiviruses, CD4+ T cells were FACS sorted on the basis of GFP expression and maintained in culture for 5 to 7 days. After ascertaining the expression of GFP (90% to 95% of the sorted cells), the cells were infected with HIVIIIB (MOI, 0.5) and p24 expression was evaluated 6 days later. Effective inhibition of virus replication was observed in CD4+ T cells transduced with lentiviruses expressing gag, rev, or vif shRNA. Only 2% to 4% of cells stained positive for p24 while 45% to 70% of cells transduced with luc or no shRNA were positive for p24 expression (Figure 2A). Thus, all 3 anti-HIV shRNAs reduced the p24 level by more than 90%, demonstrating their ability to protect primary CD4+ T cells from homologous virus.
Lentiviral delivery of shRNAs targeting viral genes inhibits HIVIIIB replication. HeLa-CD4 cells stably expressing shRNAs targeting luc, gag, rev, or vif generated by sorting GFP-positive cells 3 to 5 days after lentiviral transduction were infected with HIVIIIB and analyzed 5 days later. (A) The level of infection was determined by flow cytometry after staining for intracellular p24 expression. Numbers indicate percentage of cells in the respective quadrants. (B) The 9.3-kb HIV RNA was detected on Northern blots using a gag RNA probe.
Lentiviral delivery of shRNAs targeting viral genes inhibits HIVIIIB replication. HeLa-CD4 cells stably expressing shRNAs targeting luc, gag, rev, or vif generated by sorting GFP-positive cells 3 to 5 days after lentiviral transduction were infected with HIVIIIB and analyzed 5 days later. (A) The level of infection was determined by flow cytometry after staining for intracellular p24 expression. Numbers indicate percentage of cells in the respective quadrants. (B) The 9.3-kb HIV RNA was detected on Northern blots using a gag RNA probe.
To further validate the data, the shRNA-transduced CD4+ T cells were challenged with HIVIIIB at a range of MOIs (0.01 to 10), and culture supernatants collected at different times were assayed for p24 production by ELISA (Figure 2B). At all MOIs tested, a robust inhibition of viral replication relative to the mock control was observed with shRNAs targeting the viral gag and vif genes but not with the irrelevant luc shRNA. Further, viral inhibition with the specific shRNAs was sustained for as long as 15 days after infection.
shRNAs targeting viral genes inhibit HIVIIIB replication in primary CD4 T cells. (A) FACS-sorted GFP-positive CD4 T cells stably expressing luc, gag, rev, or vif shRNAs were infected with HIVIIIB (MOI, 0.5) and evaluated 6 days later for intracellular p24 expression by flow cytometry. Horizontal bars identify the percentage of p24 antigen–positive cells. (B) Inhibitory effect of shRNAs in CD4+ T cells challenged with increasing amounts of HIV-1. Mock- and shRNA-transduced CD4+ T cells were infected with HIVIIIB at MOIs of 0.01 to 10, and the culture supernatants harvested at various time points were tested for p24 levels by ELISA. The extent of viral inhibition in culture supernatants from cells expressing each of the shRNAs was calculated as the reduction in p24 production with shRNA compared with corresponding cultures with no shRNA. The p24 levels in mock-transduced culture supernatants were 468, 576, 729, and 1610 ng/mL, respectively, at 0.01, 0.1, 1.0, and 10 MOI of HIVIIIB on day 15 after infection. (C) shRNAs inhibit HIV replication in previously infected CD4+ T cells. (Left panel) HIVIIIB-infected CD4+ T cells are shown by intracellular p24 staining (bold line, uninfected cultures; thin solid line, HIV-infected cells; dotted line, infected cells stained with control isotype-matched antibody). Horizontal bars identify the percentage of p24 antigen–positive cells. These infected cells were transduced with shRNA-expressing lentiviruses, and 2 weeks later p24 expression in GFP-positive (GFP+) and GFP-negative (GFP–) cells was assessed by FACS (right panel). The extent of viral inhibition was calculated as the reduction in the proportion of p24-positive cells in GFP-positive or GFP-negative cultures relative to the mock-transduced culture. Error bars denote the standard deviation from 3 independent experiments.
shRNAs targeting viral genes inhibit HIVIIIB replication in primary CD4 T cells. (A) FACS-sorted GFP-positive CD4 T cells stably expressing luc, gag, rev, or vif shRNAs were infected with HIVIIIB (MOI, 0.5) and evaluated 6 days later for intracellular p24 expression by flow cytometry. Horizontal bars identify the percentage of p24 antigen–positive cells. (B) Inhibitory effect of shRNAs in CD4+ T cells challenged with increasing amounts of HIV-1. Mock- and shRNA-transduced CD4+ T cells were infected with HIVIIIB at MOIs of 0.01 to 10, and the culture supernatants harvested at various time points were tested for p24 levels by ELISA. The extent of viral inhibition in culture supernatants from cells expressing each of the shRNAs was calculated as the reduction in p24 production with shRNA compared with corresponding cultures with no shRNA. The p24 levels in mock-transduced culture supernatants were 468, 576, 729, and 1610 ng/mL, respectively, at 0.01, 0.1, 1.0, and 10 MOI of HIVIIIB on day 15 after infection. (C) shRNAs inhibit HIV replication in previously infected CD4+ T cells. (Left panel) HIVIIIB-infected CD4+ T cells are shown by intracellular p24 staining (bold line, uninfected cultures; thin solid line, HIV-infected cells; dotted line, infected cells stained with control isotype-matched antibody). Horizontal bars identify the percentage of p24 antigen–positive cells. These infected cells were transduced with shRNA-expressing lentiviruses, and 2 weeks later p24 expression in GFP-positive (GFP+) and GFP-negative (GFP–) cells was assessed by FACS (right panel). The extent of viral inhibition was calculated as the reduction in the proportion of p24-positive cells in GFP-positive or GFP-negative cultures relative to the mock-transduced culture. Error bars denote the standard deviation from 3 independent experiments.
To determine if these shRNAs select for RNAi escape mutants, the viruses from luc, gag, and vif shRNA-transduced cultures infected at MOIs of 1 and 10 were sequenced by reverse transcriptase (RT)–PCR on day 15 after infection. In the absence of selection pressure in the luc shRNA-transduced culture, the vif and gag target sequences were identical to the input virus at both MOIs (not shown). In the vif shRNA-transduced cultures, no change in the target sequence was seen, suggesting that no escape mutants were being generated (not shown). In contrast, in the gag shRNA-transduced cultures infected at an MOI of 1, 1 of 10 clones exhibited a point mutation at position 6 (T>C) in the target sequence. Although the mutant was not dominant (representing only 10% of virus population) when tested on day 15 after infection, it could potentially represent a genuine escape variant, which could become dominant after extended selection pressure. Unfortunately, long-term follow-up of the cultures was not possible, because of poor viability of primary CD4+ T cells upon prolonged culture.
We also tested the ability of shRNAs to reduce viral replication in an established infection (Figure 2C). HIVIIIB-infected CD4 PHA blasts from a healthy donor were cultured until more than 95% of the cells became p24 positive (Figure 2C, left panel) and then transduced with gag, vif, or control luc shRNA-expressing lentiviruses. Although the initial transduction efficiency was similar in all cultures on day 3 after transduction, after 2 weeks the relative frequency of GFP-positive cells increased 3-fold in the vif and gag shRNA-transduced cultures but was unchanged in the control luc shRNA-transduced culture (data not shown). The proportion of GFP-positive cells replicating virus, as indicated by intracellular p24 expression, was specifically reduced by 52% and 76%, respectively, relative to the luc shRNA-transduced GFP-positive cells, whereas p24 expression was comparable in the corresponding GFP-negative cells (Figure 2C, right panel). Therefore, lentiviral delivery of shRNA can inhibit HIV replication even when administered after infection.
Analysis of natural HIV variants in RNAi target sequences
To determine the extent of natural sequence variation in the 19 nt sequence targeted by each of the 3 shRNAs used in the study, we analyzed the available sequences in the Los Alamos HIV Sequence Database (Figure 4). The rev target region (HXB2, 8485-8501) was highly variable at many residues in all clades examined, including clade B from which the consensus sequence was derived. The gag (HXB2, 2062-2080) sequence was well conserved within isolates from clade B but contained double or triple mutations in clades A, C, D, and E. The common variants in clade A were either a triple mutation at positions 3, 6, and 12 (49%; n = 41) or a double mutation at positions 3 and 6 (32%; n = 41). In clade C isolates, most variants had double mutations at positions 3 and 15 (43%; n = 66) or triple mutations at positions 3, 12, and 15 (19%; n = 66). Preferential mutations at certain positions were even more pronounced for clade D and E isolates. Most clade D strains in the database contained double mutations at positions 6 and 12 (79%; n = 33), and all clade E strains contained double mutations at position at 3 and 6 (100%; n = 9). The vif sequence (HXB2, 5195-5213), which was the best conserved of the 3 targeted regions, also showed natural single nucleotide variations at position 14 in a majority of clade E strains (62%; n = 9) and at position 17 in a minority of clade C isolates (30%, mainly present in Zaire; n = 66) but remained invariant among clade A, B, and D isolates.
HIV sequence variations in regions targeted by the shRNAs. HIV sequences in the Los Alamos HIV Sequence Database were analyzed for sequence variation at each of the 19 nt residues in the rev-, gag-, and vif-targeted regions. The number of isolates within each clade (A, B, C, D, or E) that contain mutations relative to the total number of isolates were determined and expressed as a percentage. For each sequence, 41 to 45 clade A isolates, 31 clade B isolates, 61 to 66 clade C isolates, 33 clade D isolates, and 9 clade E isolates were available.
HIV sequence variations in regions targeted by the shRNAs. HIV sequences in the Los Alamos HIV Sequence Database were analyzed for sequence variation at each of the 19 nt residues in the rev-, gag-, and vif-targeted regions. The number of isolates within each clade (A, B, C, D, or E) that contain mutations relative to the total number of isolates were determined and expressed as a percentage. For each sequence, 41 to 45 clade A isolates, 31 clade B isolates, 61 to 66 clade C isolates, 33 clade D isolates, and 9 clade E isolates were available.
shRNAs targeting gag, rev, and vif protect primary CD4+ T cells from multiple clade B HIV isolates
To understand whether the natural intraclade and interclade sequence variations interfered with the ability of the 3 shRNAs to inhibit HIV-1 replication, we first challenged stably transduced CD4+ T cells with multiple lab-adapted and primary clade B viruses. In parallel, we sequenced the viral isolates around the target regions. Viral clade was assigned based on gag sequence using the genotyping tool from the National Center for Biotechnology Information (NCBI). Figure 4A depicts a FACS analysis of p24 expression after infection with a representative primary isolate, Pr-216, and Figure 4B and Table 1 show the inhibition observed with each of the viral isolates. The gag and vif shRNAs uniformly protected CD4 T cells from all clade B strains tested, including 3 lab-adapted strains of HIV (IIIB, BaL, and 89.6) and 3 primary clinical isolates (Pr-214, Pr-216, and Pr-307), whereas protection with the rev shRNA was variable. This was in concordance with the sequencing data, which showed that in all the isolates tested, the gag and vif target sequences were completely homologous with the consensus HIVIIIB sequence (Table 1). However, the rev shRNA target sequence was identical to the consensus sequence only in the lab-adapted HIVIIIB, HIVBaL, and patient sample Pr-307, which were efficiently inhibited. On the other hand, in all instances where rev shRNA failed to inhibit viral replication (lab strain virus 89.6 and clade B primary viruses Pr-214 and Pr-216), sequence analysis demonstrated multiple sequence alterations in the targeted region. These data underscore the importance of targeting sequences with a high degree of conservation to maximize silencing of multiple viral strains.
shRNA targeting vif provides cross-clade protection in primary CD4+ T cells
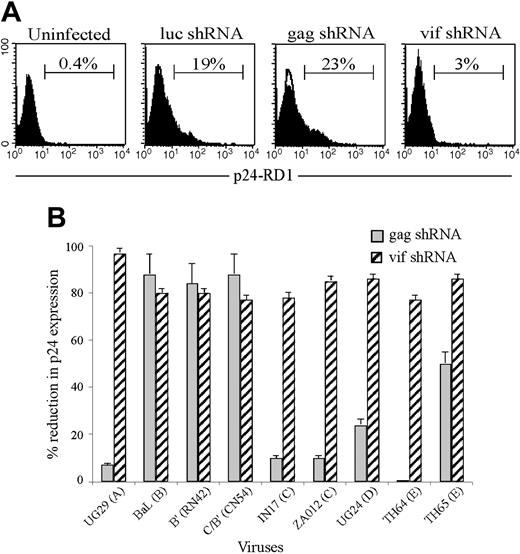
To determine whether gag and vif shRNAs, which target HIV sequences that are highly conserved within clade B strains, are also effective against non–clade B viruses, we challenged GFP-positive CD4+ T cells with representative isolates from multiple clades, including those containing common variants for the targeted sequences. A control shRNA expression vector targeting luciferase was included as a negative control. The rev shRNA was not used for the analysis because of the low degree of conservation of the target sequence predicted from the database analysis and its inability to silence many of the clade B virus isolates. The gag-, vif-, and control shRNA-expressing cells were analyzed for p24 expression by flow cytometry 6 days after HIV infection (Figure 5A). When challenged with the non–clade B viruses, the gag shRNA that was effective against all clade B strains could only protect against the 2 Chinese isolates, B′ and C/B′ (Figure 5B and Table 2) One of the Chinese viruses is highly related to Thai B′ clade virus (RL42), and the other is a recombinant virus (CN54) containing sequences derived from the Thai B′ virus and the Indian clade C virus.36,37 Although the C/B′ and B′ virus sequences only share overall homologies of 90% and 92%, respectively, with HIVIIIBgag, the RNAi target sequences are identical. The gag shRNA-mediated protection was abrogated to various extents, from a 50% loss in protection to no protection at all against the remainder of the non–clade B viruses tested, all of which contained one or more commonly present variations in the gag sequence. Silencing was most affected by mutations that included position 12 in the target gene: triple mutations at positions 3, 6, and 12 (TH64, clade E) or 3, 12, and 15 (IN17, clade C); double mutations at positions 3 and 12 (UG29, clade A); as well as a single mutation at position 12 (UG24, clade D). Mutations at positions 3 and 6 in a clade E virus (TH65) resulted in only a partial loss of protection. Therefore, a change in homology at position 12 is all that is required to interfere with silencing, while changes at nt 3 and 6 are less relevant. Of note, unlike the IN17 and ZA012 isolates that we tested, most of the clade C viruses in the Los Alamos Sequence Database are not mutated at position 12. Remarkably, the vif shRNA efficiently inhibited all the viruses from multiple clades of HIV-1 that were tested, reducing infection by as much as 75% to 95% (Figure 5B and Table 2). These included viruses containing the most prevalent vif sequence mutations at positions 14 and 17. The vif shRNA inhibited p24 expression by 80% and 85%, respectively, in CD4+ T cells challenged with TH64 virus (clade E) containing a single C>T alteration at position 14 or the ZA012 isolate containing a C>A sequence change at position 17. Thus, combined results from analysis with the gag and vif shRNAs suggest that viral silencing requires homology in the central region around the mRNA cleavage site (after residue 10 in the target mRNA) but tolerates incomplete homology in the 5′ end of the target sequence from positions 1 to 6 and in the 3′ end from positions 14 to 19.
Antiviral activity of shRNAs against clade B primary viruses. Sorted GFP-positive CD4+ T cells expressing luc, gag, rev, or vif shRNAs were infected with the IIIB, 89.6, or BaL lab-adapted strains or with primary HIV isolates from donors 214, 216, and 307 and analyzed by flow cytometry for p24 expression 6 days later. (A) Representative flow cytometry analysis of lentivirus-transduced CD4 T cells infected with primary virus from donor 216. (B) The extent of viral inhibition in cells expressing each of the 3 shRNAs was calculated as the reduction in the percent of p24-positive cells in cultures selected for HIV shRNA expression compared with cultures expressing luc shRNA. Values represent averages of 2 independent experiments, with the range indicated. The target sequences of the various viruses used are depicted in Table 2.
Antiviral activity of shRNAs against clade B primary viruses. Sorted GFP-positive CD4+ T cells expressing luc, gag, rev, or vif shRNAs were infected with the IIIB, 89.6, or BaL lab-adapted strains or with primary HIV isolates from donors 214, 216, and 307 and analyzed by flow cytometry for p24 expression 6 days later. (A) Representative flow cytometry analysis of lentivirus-transduced CD4 T cells infected with primary virus from donor 216. (B) The extent of viral inhibition in cells expressing each of the 3 shRNAs was calculated as the reduction in the percent of p24-positive cells in cultures selected for HIV shRNA expression compared with cultures expressing luc shRNA. Values represent averages of 2 independent experiments, with the range indicated. The target sequences of the various viruses used are depicted in Table 2.
Analysis of mismatch tolerance using mutant siRNAs corresponding to common HIV variants in targeted gag and vif regions of HIV-1
For RNAi targeting the more conserved gag and vif target regions, we also confirmed our data on their differential tolerance to viral mutations with mutant siRNAs corresponding to the common single, double, or triple nucleotide changes in the target regions. HeLa-CD4 cells were transfected with siRNAs corresponding to wild-type or mutant sequences, and virus replication was evaluated after challenge with the consensus HIVIIIB strain both by intracellular staining for p24 antigen and by p24 ELISA (Figure 6 and Table 3). Lack of homology at positions 3 and 15 (clade C) did not adversely affect the inhibitory ability of the gag siRNA, once again indicating that these residues are outside the region important for silencing. However, siRNAs harboring multiple mutations corresponding to positions 3, 6, and 12 (clade A); 6 and 12 (clade D); or 3 and 6 (clade E) of the gag target sequence totally abrogated the protective effects of the gag shRNA, with HIVIIIB replication similar to that in cells expressing the control GFP siRNA. These results closely mirror the results obtained using HXB2 shRNAs to inhibit viral isolates containing these mutations (Figure 5B and Table 2). Although lack of homology only at position 6 (gag-p6) partially inhibits silencing, adding an additional mutation at position 3 (clade E) completely abrogates silencing. Taken together, these results suggest that homology at position 12 of the gag target sequence is critical, whereas mutations at position 6 are important and can become critical in the context of mutations elsewhere. Thus, although this RNAi gag target sequence has the potential to inhibit replication of clade B isolates, it is not a good target for non–clade B viruses where mutations at positions 6 and 12 are frequently present. Similar analysis with variant vif siRNAs demonstrated that nucleotide changes at positions 14 and 17 (corresponding to natural variations in clades C and E) did not significantly compromise the antiviral effect of the vif siRNAs. This suggests that the vif siRNA used here could serve as an optimal target for RNAi-mediated protection against multiple HIV isolates, including non–clade B strains that predominate in geographic regions other than the Western Hemisphere.
Antiviral effect of gag or vif shRNAs against viruses from diverse clades. GFP-positive CD4+ T cells expressing luc, gag, or vif shRNAs were infected with primary viruses representative of multiple HIV clades and analyzed for intracellular p24 expression by flow cytometry 6 days later. (A) Representative histogram of CD4 cells transduced with indicated shRNA and infected with clade C, Indian virus IN17. (B) The reduction in p24 expression in HIV shRNA-transduced cells relative to luc shRNA-transduced cultures is graphed. Data represent means and SD of 3 independent experiments. The target sequences of the various viruses used are depicted in Table 3.
Antiviral effect of gag or vif shRNAs against viruses from diverse clades. GFP-positive CD4+ T cells expressing luc, gag, or vif shRNAs were infected with primary viruses representative of multiple HIV clades and analyzed for intracellular p24 expression by flow cytometry 6 days later. (A) Representative histogram of CD4 cells transduced with indicated shRNA and infected with clade C, Indian virus IN17. (B) The reduction in p24 expression in HIV shRNA-transduced cells relative to luc shRNA-transduced cultures is graphed. Data represent means and SD of 3 independent experiments. The target sequences of the various viruses used are depicted in Table 3.
Antiviral activity of mutated siRNAs corresponding to common variants in gag and vif targets. siRNAs with single, double, or triple mutations were designed based on the common gag and vif sequence variants present in the Los Alamos HIV Sequence Database. Cells transfected with 200 nM of the indicated synthetic siRNA were challenged with HIVIIIB virus on day 1 after transfection. Three days later, supernatants were collected for evaluation of p24 production by ELISA (the p24 level was 10 ng/mL for the control mock-treated cells and was inhibited to 0.5 ng/mL by wild-type [wt] gag or vif siRNA). Five days after infection, cells were stained for intracellular p24 and the antiviral activity of mutant siRNAs was assessed by comparing the percent of p24-positive cells in cultures treated with the mutated siRNA with that in wt siRNA-treated HeLa-CD4 cells (35% to 40% of mock-treated cells and 3% to 5% of wt siRNA-transfected cells stained for p24). Values represent means and SD of 2 independent experiments. The bars and circles represent the levels of inhibition obtained by intracellular staining for p24 and ELISA, respectively. The sequences of the mutant siRNAs are shown in Table 1.
Antiviral activity of mutated siRNAs corresponding to common variants in gag and vif targets. siRNAs with single, double, or triple mutations were designed based on the common gag and vif sequence variants present in the Los Alamos HIV Sequence Database. Cells transfected with 200 nM of the indicated synthetic siRNA were challenged with HIVIIIB virus on day 1 after transfection. Three days later, supernatants were collected for evaluation of p24 production by ELISA (the p24 level was 10 ng/mL for the control mock-treated cells and was inhibited to 0.5 ng/mL by wild-type [wt] gag or vif siRNA). Five days after infection, cells were stained for intracellular p24 and the antiviral activity of mutant siRNAs was assessed by comparing the percent of p24-positive cells in cultures treated with the mutated siRNA with that in wt siRNA-treated HeLa-CD4 cells (35% to 40% of mock-treated cells and 3% to 5% of wt siRNA-transfected cells stained for p24). Values represent means and SD of 2 independent experiments. The bars and circles represent the levels of inhibition obtained by intracellular staining for p24 and ELISA, respectively. The sequences of the mutant siRNAs are shown in Table 1.
Discussion
The sequence specificity of RNAi, which limits inadvertent silencing of partially homologous genes, is a major hurdle for its clinical application against a highly mutable virus such as HIV-1. Despite the importance of defining highly conserved target sites in the virus, previous studies examining RNAi-mediated silencing of HIV-1 with siRNAs against a variety of structural or regulatory genes including gag, pol, nef, vif, tat, env, and the HIV long terminal repeat (LTR) have limited their scope to the inhibition of a few common lab-adapted viral strains.11,12,15-26,32,38,39 We therefore examined whether siRNAs targeting viral sequences characterized by differing degrees of sequence variability could effectively silence a broad spectrum of clinical isolates. Our aim was to identify conserved viral targets for RNAi-mediated inhibition of a wide range of HIV strains and to define sequence variations tolerated by RNAi using natural viral variants in the targeted sequences. Three siRNAs chosen to target consensus clade B sequences in rev, vif, and gag afforded similar levels of protection against homologous HIVIIIB challenge, but their effectiveness differed greatly in response to a variety of viral isolates from the same and different clades. Overall, the siRNA directed at the vif sequence was the most broadly potent, providing protection against all tested viruses from multiple clades, followed by gag, which could protect against all tested clade B viruses but not other clades, whereas the rev sequence was the least effective, failing to protect even against many of the clade B strains. The shRNA targeting vif efficiently inhibited viral replication against clade B lab-adapted strains, clade B primary patient viruses, and primary viral isolates from clades A, C, D, and E. Although there are 2 common viral variants in positions 14 and 17 in the vif target region, they did not interfere with gene silencing. While additional in vivo studies are required, these studies demonstrate that this highly conserved vif target sequence may be a suitable potential target site for development of an RNAi-based HIV therapy.
Target sequence homology is an important determinant for RNAi. Initial studies on siRNA-mediated silencing demonstrated a remarkably high level of sequence specificity,40 and several studies have shown allele-specific silencing of sequences that differ only at single nucleotide positions.41,42 Similarly, RNAi-resistant polio virus isolates that emerged in siRNA-treated cultures contained single nucleotide mutations corresponding to nucleotide position 6 or 9 from the 5′ end of the target sequence.43 However, sequence mismatches at certain positions are tolerated without compromising gene silencing.16,44,45 Sequence homology around the central region of the siRNA surrounding the cleavage site of the target mRNA has been shown to be important in several studies. One study indicated that base pairing of the central 13 nt of the siRNA was required for activity, but mismatches at the 4 nt residues at either end may be tolerated.30 Other studies have suggested that siRNAs mutated at the 3′ end of the sense strand (which corresponds to the 3′ end of the target mRNA) adversely affect mRNA cleavage, but mismatches at the 5′ end were less important.31,45 Our data indicating that HIV silencing is maximally affected by a mismatch at residue 12 in the gag target gene are in agreement with the view that homology at the central region is critical. At the periphery, single mismatches in the gag target sequence (positions 3, 6, and 15 in the target mRNA) or at the 3′ end of the vif target sequence (positions 14 and 17) were relatively well tolerated. On the other hand, introduction of double mutations at positions 3 and 6 in the gag sequence severely compromised viral inhibition. Therefore, for the sequences studied here, we find that mismatches in the central region can abrogate silencing, while single mismatches at the 3′ end of the target sequence are well tolerated. These seemingly conflicting results about tolerance for 3′ mismatches suggest that the impact of specific residue mismatches may vary for individual siRNA-mRNA pairings. Differences in target mRNA secondary structure might also impose limits on accessibility46 that differentially affect RNAi mismatch tolerance. Further, it is important to note that the effects that nucleotide substitutions introduced into the siRNA have on gene silencing do not necessarily mirror the effects seen with mutations in the target mRNA because introducing mutations in the siRNA duplex can have a profound impact on the crucial initial steps of duplex unwinding, selective strand processing, and incorporation into RNA-induced silencing complex (RISC).47,48
The effect of HIV sequence variability on RNAi has been addressed in a few studies. One of the earliest studies on the antiviral potential of RNAi found that mutating siRNAs targeting vif and the HIV-1 LTR had variable effects on the silencing of a cotransfected molecular clone HIV-1NL-GFP.16 A single nucleotide change at position 10 in the siRNA targeting LTR showed no loss of silencing, while a single nucleotide change at position 11 in the siRNA targeting the vif region resulted in a partial loss of silencing. The introduction of 4 mismatches in the central region compared with the HIV-1 sequence in the vif siRNA completely abrogated silencing. In another study, mutational scanning of an anti-HIV shRNA targeting gag expressed from an adenoviral vector showed that changes at many residues significantly inhibited effectiveness.49 As more studies analyze the effects of mismatches targeting a variety of sequences, it will become clear whether general rules independent of specific sequence can be formulated to predict which mismatches will interfere with silencing.
The prospect of HIV mutations to escape from RNAi has already been demonstrated in vitro. In 2 long-term culture studies, HIV viruses resistant to RNAi outgrew wild-type virus. In one report, stable expression of an shRNA targeting tat led to a sharp increase in viral replication after 25 days in culture due to the selection for viral escape variants that contained a single nucleotide mutation at position 9.11 Similarly, another study examining the durability of RNAi-mediated silencing in a cell line expressing shRNA targeting nef reported the emergence of a series of siRNA escape variants, including RNAi-resistant variants that contained single nucleotide changes in positions 10 and 13 in the target mRNA, as well as deletions encompassing part or all of the nef gene, including regions outside of the RNAi recognition site.29 Because nef is dispensable for HIV-1 culture in vitro,50 it is unclear whether all of the sequence changes noted in the study could be attributed directly to selection of RNAi escape mutants. In the present study, while the shRNA targeting the highly conserved vif sequence did not generate escape variants, the use of gag shRNA did lead to the emergence of at least one escape mutant, suggesting that mutations in vif but not gag sequence may come at a high cost for viral fitness.
What these studies and ours suggest is that viral sequence diversity and mutation must be considered when designing siRNAs for broad antiviral activity. It will be important to avoid targeting viral sequences in which mutations can occur in and around the central nucleotide cleavage site, while some sequence heterogeneity in the peripheral regions may be less critical. Identifying conserved sequences for targeting, such as the vif sequence used here, will provide the best chance for inhibiting a diversity of HIV isolates and for protecting against the emergence of viral escape. By careful siRNA design and testing against a panel of viral isolates, the inherent vulnerability of RNAi to viral mutations can be minimized. However, the best safeguard against the viral propensity for escape may be to target multiple conserved viral sequences in combination with host genes such as the viral coreceptor CCR5. The number of siRNAs that can be accommodated at one time without saturating the cellular RNAi machinery is still unknown. The optimal delivery vehicle for RNAi is also an important practical consideration that will have to be addressed prior to adapting RNAi technology for human therapeutics.
Prepublished online as Blood First Edition Paper, April 14, 2005; DOI 10.1182/blood-2004-10-3959.
Supported by National Institutes of Health (NIH) grant AI49792 (P.S.) and American Foundation for AIDS Research (amfAR) Scholar Award 70589-32RF (S.-K.L.). S.-K.L. and D.M.D. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Luk Van Parijs and Christopher Dillon for providing the lentiviral vector and for advice on generating the shRNA constructs.
Dr Sang-Kyung Lee is now at the Department of Bioengineering, Han-Yang University, Seoul, Korea, 133-791.


![Figure 6. Antiviral activity of mutated siRNAs corresponding to common variants in gag and vif targets. siRNAs with single, double, or triple mutations were designed based on the common gag and vif sequence variants present in the Los Alamos HIV Sequence Database. Cells transfected with 200 nM of the indicated synthetic siRNA were challenged with HIVIIIB virus on day 1 after transfection. Three days later, supernatants were collected for evaluation of p24 production by ELISA (the p24 level was 10 ng/mL for the control mock-treated cells and was inhibited to 0.5 ng/mL by wild-type [wt] gag or vif siRNA). Five days after infection, cells were stained for intracellular p24 and the antiviral activity of mutant siRNAs was assessed by comparing the percent of p24-positive cells in cultures treated with the mutated siRNA with that in wt siRNA-treated HeLa-CD4 cells (35% to 40% of mock-treated cells and 3% to 5% of wt siRNA-transfected cells stained for p24). Values represent means and SD of 2 independent experiments. The bars and circles represent the levels of inhibition obtained by intracellular staining for p24 and ELISA, respectively. The sequences of the mutant siRNAs are shown in Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/3/10.1182_blood-2004-10-3959/6/m_zh80150582090006.jpeg?Expires=1767774629&Signature=wtC-jPgFz9K2xSklFkB6-WfV7xLT8M0vncTczKaFY4KFJmOUjRPLUvxPUinbS4XuHdgsaVdYhbj52suJ6Nn45jHybBNK-YU6JgorUVUyJ~8ublkGmEdN1tAUmptqAnrny0KcsfTvsU5dOMO~212LoKuoDVjUqFk4Xur9AiRnyjkV9oxPgWwdvyMdDWupR525OaogzTOVSQCmL66dv6q-RTDxlGdfPn1zxsPVrQVHAc202vwf6bnMXK~WGLGYX~IrTs93CdO6bhaz-sLP9xTD-eU7hrFfVilLcZsOp-vwYPyynUFM1I9byIYQW5TC1H4MQrf3abKGtKiEq7HEF7F8Ew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)