Abstract
The occurrence of delayed neutropenia following rituximab is poorly defined and of unknown cause. We hypothesized it may be related to perturbations of stromal derived factor-1 (SDF-1) and granulocyte homeostasis. Late-onset neutropenia (LON) was investigated in 130 patients with untreated aggressive B-cell lymphoma receiving DA-EPOCH (dose-adjusted etoposide, prednisone, Oncovin [vincristine], cyclophosphamide, and hydroxydaunorubicin) chemotherapy with or without rituximab. All patients were in remission and had no known causes for neutropenia. LON occurred in 6 (8%) of 76 patients receiving rituximab and 0 of 54 patients not receiving rituximab (P = .04). The median onset was 175 days (range, 77-204 days) after treatment with a median duration of 14 days (range, 11-16 days). In a subset of 24 patients, a significant correlation was found between rapid B-cell recovery and granulocyte decline over the 6-month recovery period (R = –0.53; P = .04). Rapid B-cell recovery directly correlated with prerecovery SDF-1 levels (R = 0.65; P = .015) and SDF-1 decline (R = –0.67; P = .013) after recovery. Our results suggest that early B-cell lymphopoiesis is important for B-cell recovery following rituximab, and that perturbation of SDF-1 during B-cell recovery retards neutrophil egress from the bone marrow. These findings illustrate the dual role of SDF-1 in human B-cell and granulocyte homeostasis.
Introduction
Rituximab is a chimeric antibody that targets the CD20 B-cell antigen expressed on normal and neoplastic B cells.1,2 To date, the biologic functions of CD20 remain uncertain, although incubation of B cells with anti-CD20 antibody has variable effects on cell cycle progression and signaling and depletes normal circulating B cells.3,4 Clinically, rituximab is widely used for the treatment of B-cell hematologic disorders because of its broad efficacy and attractive toxicity profile.5-9 In particular, rituximab has not been associated with acute myelosuppression that is commonly seen following cytotoxic agents.
Late-onset neutropenia (LON) occurring at least 4 weeks after treatment has recently been reported following rituximab-based chemotherapy for hematologic disorders.10-12 In these reports, LON was attributed to rituximab, but the results are difficult to interpret because of variable treatments including stem cell transplantation and bone marrow compromise. Genentech, the manufacturer of rituximab, has also received rare reports of LON following rituximab with a postmarketing reporting rate of 0.02% in more than 300 000 patients.13 Due to the absence of controlled studies, however, the natural history and incidence of LON have yet to be adequately defined. Its mechanism is also unknown, although investigators have hypothesized production of antineutrophil antibodies, suppression of neutrophils by large granular lymphocytes, and immune dysregulation during B-cell recovery as potential etiologies.10,11
To characterize the natural history of LON, we retrospectively evaluated 2 patient cohorts with newly diagnosed aggressive B-cell lymphoma treated with doxorubicin-based (DA-EPOCH [dose-adjusted etoposide, prednisone, Oncovin {vincristine}, cyclophosphamide, and hydroxydaunorubicin]) chemotherapy with or without rituximab.14 Based on a hypothesis that LON is caused by perturbations of granulocyte homeostasis, we investigated the relationship between B-cell recovery and granulocyte dynamics, and the role of stromal derived factor-1 (SDF-1)/CXC ligand 12 (CXCL12), a chemokine required for early B-cell development and retention of B-lineage and granulocytic precursors in the bone marrow.15-19
Patients and methods
Study design
Data were reviewed from 153 consecutive patients with untreated aggressive B-cell lymphoma enrolled on DA-EPOCH–based protocols at the National Cancer Institute between May 1993 and August 2002. Histologies included diffuse large B-cell lymphoma, Burkitt lymphoma, and mantle cell lymphoma. Patients with human immunodeficiency virus infection were included. To control for confounding causes of neutropenia and ensure adequate follow-up, the analysis was restricted to 130 patients in complete remission who had hematopoietic recovery with an absolute neutrophil count (ANC) higher than 1.0 × 109/L after treatment and were observed for at least 12 months. Patients had no other identifiable causes of neutropenia and had no recent changes to medications before or during the LON. Patients were routinely evaluated with complete blood counts (CBCs) and computerized tomography scans every 3 months for the first year. LON was defined as a neutrophil count lower than 0.5 × 109/L occurring at least 60 days after the last treatment.
All patients received DA-EPOCH–based treatment (50 mg/m2 continuous intravenous etoposide per day for days 1-4; 60 mg/m2 prednisone twice a day by mouth for days 1-5; 0.4 mg/m2 continuous intravenous Oncovin [vincristine] per day for days 1-4; 750 mg/m2 intravenous cyclophosphamide for day 5; and 10 mg/m2 continuous intravenous hydroxydaunorubicin [doxorubicin] per day for days 1-4). Filgrastim was administered from day 6 until neutrophil recovery. Drug doses were adjusted as previously described and treatment was administered for 3 to 8 cycles depending on the protocol.14,20 Patients were treated on 1 of 5 DA-EPOCH–containing regimens, 3 of which also contained rituximab. Rituximab was administered at 375 mg/m2 on day 1 in the protocols for diffuse large B-cell lymphoma and mantle cell lymphoma, and on days 1 and 5 in the protocol for AIDS-related lymphoma. All protocols were approved by the National Cancer Institute institutional review board, and patients gave written informed consent, per the Declaration of Helsinki.
Patients with mantle cell lymphoma also received 5 idiotype vaccine treatments over a 6-month period, beginning no sooner than 12 weeks after completing DA-EPOCH with rituximab (DA-EPOCH-R).21 The first 4 idiotype vaccines were administered 4 weeks apart, and the fifth vaccine was administered 8 weeks later. To control for potential effects of the idiotype vaccine on the incidence of LON and the analysis of biologic end points, all measurements of SDF-1, granulocytes, and B cells were conducted on blood specimens obtained either before or at least 4 weeks after the administration of the idiotype vaccine. Specifically, measurements at the 3-month time points were obtained prior to any idiotype vaccine, and most measurements at the 9-month time points were obtained 8 weeks after the last idiotype vaccine.
Flow cytometry
In 24 mantle cell lymphoma patients, peripheral blood B cells and granulocytes were determined by flow cytometry. Blood samples were obtained before treatment and approximately 0, 3, 9, 12, and 18 months after completing DA-EPOCH-R. Flow cytometry was performed in Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories. The absolute numbers of B cells (CD3– and CD19+) per microliter of blood were calculated from the percent of these cells in the lymphocyte gate, the percent of lymphocytes (CD45bright and low side scatter), and the complete blood counts. Leukemic mantle cell lymphoma cells were defined as CD5+ with light chain restriction, and granulocytes were defined as CD45dull and high side scatter.
Analysis of stromal derived factor-1
Circulating levels of SDF-1 were measured in 19 patients with mantle cell lymphoma who received DA-EPOCH-R and in 17 patients with diffuse large B-cell lymphoma who received DA-EPOCH. SDF-1 levels were analyzed using enzyme-linked immunosorbent assay (ELISA) as previously described.22 The ELISA was performed by well coating with mouse monoclonal SDF-1 antibodies (MAB350, Clone: 79018.11 and MAB310, Clone: 79 014.111, each at 5 μg/mL; R&D Systems, Minneapolis, MN). Human recombinant SDF-1 standard (11-24 300 pg/mL; R&D Systems) or test sera (1:10 and higher dilution in phosphate-buffered saline [PBS] containing 0.05% Tween-20) were added.22 Biotinylated goat antihuman SDF-1 antibody (BAF310, 200 ng/mL; R&D Systems) was used as a secondary antibody. The assay is linear between 11 and 2700 pg/mL and has a lower limit of sensitivity of approximately 33 pg/mL.22 No difference in assay sensitivity and linearity was observed when the standard SDF-1 preparation was diluted in 10% human serum. The assay is specific because it does not detect human interferon-inducible protein-10 (IP-10), human Exodous-2, human granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), or interleukin-6 (IL-6) at concentrations ranging between 100 pg and 10 ng/mL. The assay is reproducible as determined by repetitive testing of SDF-1 levels in a panel of 5 normal human sera tested 10 times over a period of approximately 10 months. In addition, levels of SDF-1 detected in 39 normal sera ranged between 14.4 and 16.4 ng/mL.
Immunohistochemistry of SDF-1 in the bone marrow was performed as previously described.23 Tissue sections were incubated with mouse monoclonal SDF-1 antibody (clone 79 018.111, dilution 1:50; R&D Systems). Bound antibody was detected with a biotin-conjugated secondary antibody formulation for recognition of rabbit and mouse immunoglobulins (Ventana Medical System, Tucson, AZ).
Statistical methods
Statistical analyses were performed using Statview (Cary, NC) version 5 statistical analysis program by F.H. Spearman nonparametric correlations involving changes (Δ) in B cells over the recovery period were performed in patients with mantle cell lymphoma. To control for differences in the biologic milieu associated with B-cell recovery, patients were separated into one group with rapid recovery of 40 or more B cells/mm3 (n = 16), approximating the normal lower limit of 50 B cells/mm3 in our institution, and into a second group with recovery of 25 or fewer B cells/mm3 (n = 7). One patient with inadequate B-cell data was excluded. Correlations of patients with rapid B-cell recovery are reported. The Wilcoxon signed rank test was performed to compare SDF-1 levels from paired samples obtained at pretreatment and posttreatment time points. The chi-squared and 2-sided Fisher exact t test were used to compare patient characteristics and the frequency of LON.
Results
Incidence and natural history
We retrospectively evaluated 130 patients for the occurrence of LON. Of these patients, 54 received 334 cycles of DA-EPOCH alone and 76 received 462 cycles of DA-EPOCH with rituximab (Table 1). The 2 groups had similar characteristics. With at least 12 months (range, 12-132 months) of follow-up, 6 (8%) of 76 patients who received DA-EPOCH with rituximab developed LON compared with 0 of 54 patients who received DA-EPOCH alone (p2 = .04; Table 1). The incidence of LON was similar in patients with mantle cell lymphoma and diffuse large B-cell lymphoma (5%-8%), but higher in patients with AIDS-related lymphoma (25%; Table 1). To control for the imbalance of mantle cell patients between the 2 groups, we also analyzed the incidence of LON without the mantle cell patients and identified LON in 4 (8%) of 52 patients who received DA-EPOCH with rituximab compared with 0 of 54 patients who received DA-EPOCH alone (p2 = .05).
The median time to onset of LON was 175 days (range, 77-204 days), with a median neutrophil nadir of 0.2 × 109/L (range, 0.023-0.298 × 109/L; Table 2). The duration of LON from time of detection was available for patients 3, 4, 5, and 6, and lasted for 16, 11, 12, and 16 days, respectively. In all cases, the slopes of the neutrophil recovery curves were steep and complete (Figure 1). The duration of LON was unavailable for patient 1, in whom the neutropenia was retrospectively noted, and for patient 2, in whom filgrastim was administered for fever and buccal cellulitis. Following one dose of filgrastim and intravenous antibiotics in patient 2, the neutrophils increased from 0.023 to 2.050 × 109/L and the buccal cellulitis rapidly resolved.
Bone marrow biopsies performed in patients 2 and 6 during LON showed normal and mildly decreased cellularity, respectively. Both biopsies revealed mildly decreased granulocytes, a left shift, and normal megakaryocytes consistent with prior chemotherapy. Antineutrophil antibodies were negative in both patients.
B-cell recovery and granulocyte dynamics
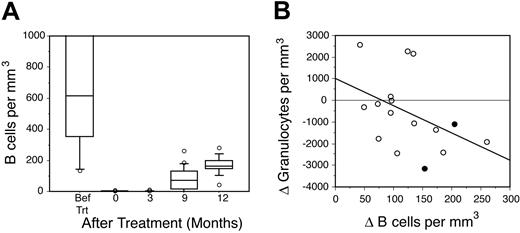
Based on the observation that the median times to LON and B-cell recovery following rituximab were similar, we hypothesized that the events were biologically related. To investigate this premise, the kinetics of B-cell recovery following DA-EPOCH-R treatment in 24 patients with mantle cell lymphoma was analyzed. Prior to treatment, patients had a median of 615 B cells/mm3 (range, 133-70 948 B cells/mm3), which included both normal and leukemic B cells. DA-EPOCH-R produced severe and protracted B-cell depletion with a median of 0 cells/mm3, both immediately following treatment and at 3 months (range, 0-12 months; Figure 2A). By 9 months, 67% of patients had achieved significant B-cell recovery of more than 40 cells/mm3, with all but one patient recovering by 18 months. In all cases, this represented recovery of normal B cells, as there was no evidence of circulating leukemic cells detected by flow cytometry.
Kinetics of late-onset neutropenia. The kinetics of the absolute neutrophil counts over the observation period is shown for the 6 cases of LON observed in this study. The duration of LON was not evaluable in patient (Pt) 1 because LON was retrospectively observed and in patient 2 because filgrastim was administered for fever.
Kinetics of late-onset neutropenia. The kinetics of the absolute neutrophil counts over the observation period is shown for the 6 cases of LON observed in this study. The duration of LON was not evaluable in patient (Pt) 1 because LON was retrospectively observed and in patient 2 because filgrastim was administered for fever.
To assess whether LON is biologically related to B-cell recovery, we investigated if there was a decline in circulating granulocyte levels concurrent with the period of rapid B-cell recovery (Figure 2B). For this analysis, we compared the changes (Δ) in B-cell and granulocyte counts between the 3- and 9-month posttreatment time periods in which LON occurred. Among patients with a high Δ B-cell count, there was an inverse correlation with the Δ granulocyte count (R = –0.53; P = .04), which is consistent with this hypothesis (Figure 2B). Overall, 9 (56%) of the 16 patients who recovered their B cells during this time period had a drop of at least 0.5 × 109 granulocytes/L.
Stromal derived factor-1 and B-cell recovery
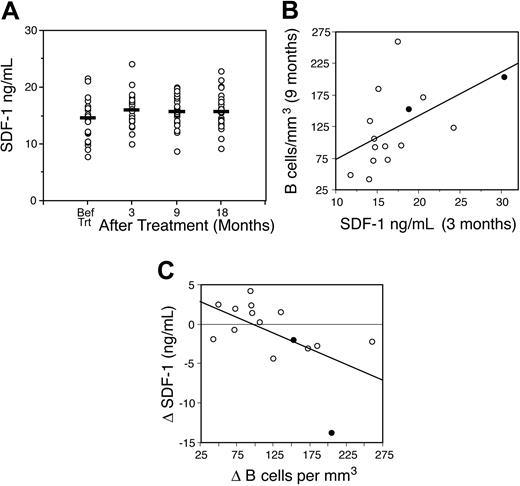
The graded decrement in granulocytes during rapid B-cell recovery suggested that LON may be due to perturbations of neutrophil homeostasis. We focused on the SDF-1 chemokine because of its central role in both the regulation of neutrophil egress from the bone marrow and early B-cell lymphopoiesis.18,24,25 Because these processes are tightly regulated by SDF-1 within the bone marrow microenvironment, SDF-1 is not directly accessible to quantitative analysis.24,25 However, biologically meaningful changes of SDF-1 within the bone marrow could be reflected by small changes in circulating levels. Therefore, we analyzed circulating SDF-1 levels in 19 patients with mantle cell lymphoma. Two patients with pretreatment SDF-1 levels over twice the maximum normal value were excluded because the levels were abnormally high.22 Prior to treatment, the median SDF-1 level was 14.5 ng/mL (range, 7.8-21.6 ng/mL), which is similar to that found in healthy volunteers (Figure 3A).22 SDF-1 levels significantly increased after treatment, reaching a median level of 16.1 ng/mL (range, 10-30.3 ng/mL) at 3 months (P = .006).
B-cell recovery and granulocyte dynamics. Flow cytometric quantitation of circulating B cells and granulocytes was performed in 24 patients with mantle cell lymphoma. (A) The kinetics of B-cell recovery following DA-EPOCH-R is shown. Before treatment, both normal and neoplastic B cells were present. After treatment, only normal circulating B cells were present. B-cell recovery of more than 40 cells/mm3 was achieved in 14 of 21 evaluable patients at 9 months and in 23 of 24 patients at 12 months. Horizontal lines in bars represent the 50th percentile (median). Error bars represent the 90th percentile (above) and 10th percentile (below). Open circles indicate outliers greater than the 90th and less than the 10th percentiles. (B) Between 3 and 9 months after treatment, the change (Δ) in B cells inversely correlated with the change in granulocytes, indicating that rapid B-cell recovery is associated with a fall in circulating granulocytes (R = –0.53; P = .04). • designates the 2 patients with LON.
B-cell recovery and granulocyte dynamics. Flow cytometric quantitation of circulating B cells and granulocytes was performed in 24 patients with mantle cell lymphoma. (A) The kinetics of B-cell recovery following DA-EPOCH-R is shown. Before treatment, both normal and neoplastic B cells were present. After treatment, only normal circulating B cells were present. B-cell recovery of more than 40 cells/mm3 was achieved in 14 of 21 evaluable patients at 9 months and in 23 of 24 patients at 12 months. Horizontal lines in bars represent the 50th percentile (median). Error bars represent the 90th percentile (above) and 10th percentile (below). Open circles indicate outliers greater than the 90th and less than the 10th percentiles. (B) Between 3 and 9 months after treatment, the change (Δ) in B cells inversely correlated with the change in granulocytes, indicating that rapid B-cell recovery is associated with a fall in circulating granulocytes (R = –0.53; P = .04). • designates the 2 patients with LON.
We first examined if early SDF-1 levels correlated with later B-cell expansion, because SDF-1 acts on early B cells. There was a direct correlation between the SDF-1 level at 3 months and the number of circulating B cells at 9 months among the group with rapid B-cell expansion (R = 0.65; P = .015; Figure 3B). We next explored if the rapid expansion of B cells produced a concurrent drop in SDF-1 levels, which might reflect SDF-1 utilization or decreased production within the bone marrow once B cells recovered. In this analysis, a decrease in SDF-1 levels between 3 and 9 months inversely correlated with an increase in B-cell numbers at 9 months among the group with rapid B-cell expansion (R = –0.67; P = .013; Figure 3C).
If the perturbations in SDF-1 levels observed in the mantle cell patients are due to SDF-1–dependent B-cell expansion, we would not expect to observe significant changes in SDF-1 levels in the absence of prolonged and severe B-cell depletion. To assess this hypothesis, we measured serial SDF-1 levels in 17 patients with diffuse large B-cell lymphoma who received DA-EPOCH alone; one patient with pretreatment SDF-1 levels over twice the maximum normal value was excluded.22 Although we were unable to measure the concomitant B cells in these patients due to an absence of samples, our previous results indicate that B-cell recovery occurs within 1 to 3 months following non–rituximab-based chemothearpy.26 Specifically, in 20 patients who received a high-dose EPOCH variant regimen for relapsed lymphoma, B-cell recovery occurred as early as one month after treatment and near normal levels were attained by 3 months after treatment in all patients (F.H. and W.H.W., manuscript in preparation; data not shown). Consistent with our hypothesis, we found no significant change in SDF-1 levels in the patients who received DA-EPOCH alone. When compared with the pretreatment median SDF-1 level of 9.7 ng/mL (range, 4.6-16.3 ng/mL), there was no significant change following treatment, with median levels of 10.2 ng/mL (range, 3.5-18.3 ng/mL; P = .8) at 3 months, 11.3 ng/mL (range, 5.2-14.3 ng/mL; P = .1) at 9 months, and 9.7 ng/mL (range, 6.1-17 ng/mL; P = .9) at 18 months. Furthermore, unlike that observed in the mantle cell patients, there was no decrement in SDF-1 levels between 3 and 9 months, which we hypothesized was due to SDF-1 consumption during B-cell expansion.
Stromal derived factor-1 and granulocyte dynamics
Our findings of a correlation between B-cell recovery and plasma SDF-1 supported the notion that LON may be related to SDF-1. Experimental studies, however, suggest that the mechanisms of SDF-1 regulation of bone marrow neutrophil egress are quite complex compared with early B-cell lymphopoiesis where SDF-1 serves a growth factor function.24,25 In the former case, regulation is achieved through SDF-1 gradients within the bone marrow microenvironment, and both high and low concentrations of SDF-1 can inhibit neutrophil release into the blood stream. Hence, plasma SDF-1 concentrations would not accurately or directly reflect quantitative or temporal changes in SDF-1 bone marrow gradients. Furthermore, biologically meaningful disruption of the SDF-1 gradients could occur during the early rises or late declines in SDF-1 levels, further obscuring a relationship between plasma SDF-1 levels and granulocyte decrements. Nevertheless, we compared changes (Δ) in plasma SDF-1 concentrations between the 3- and 9-month time periods, during which time the SDF-1 levels often fell, and changes in the granulocyte counts over the same time period, and found no correlation (R = 0.19; P = .47). We also compared the plasma SDF-1 concentrations at 3 months, which correlated with later B-cell recovery, with the change (Δ) in granulocyte counts between 3 and 9 months, and again found no correlation (R = –0.20; P = .46).
Statistical model of late-onset neutropenia
It is likely that the true incidence of LON in the present series is higher than the observed 7.9% because LON was usually asymptomatic and blood counts were obtained only every 3 months following treatment. Furthermore, our results suggest that granulocyte decrements during rapid B-cell recovery are a predictable biologic phenomenon that, in some cases, led to severe neutropenia. Based on these observations, we were interested in developing a statistical model to estimate the true incidence of LON.
SDF-1 kinetics and B-cell recovery. (A) The kinetics of circulating SDF-1 following DA-EPOCH-R in 19 mantle cell patients is shown. Compared with before treatment, SDF-1 levels are significantly higher at 3 (P = .006), 9 (P = .02), and 18 (P = .02) months after treatment. Horizontal bars represent median values. (B) Circulating SDF-1 levels at 3 months directly correlated with the recovery of B cells at 9 months after treatment (R = 0.65; P = .015). (C) Between 3 and 9 months after treatment, the change (Δ) in B cells inversely correlated with a reduction in circulating SDF-1 levels, indicating that SDF-1 levels decline as B cells recover (R = –0.67; P = .013). • designates the 2 patients with LON.
SDF-1 kinetics and B-cell recovery. (A) The kinetics of circulating SDF-1 following DA-EPOCH-R in 19 mantle cell patients is shown. Compared with before treatment, SDF-1 levels are significantly higher at 3 (P = .006), 9 (P = .02), and 18 (P = .02) months after treatment. Horizontal bars represent median values. (B) Circulating SDF-1 levels at 3 months directly correlated with the recovery of B cells at 9 months after treatment (R = 0.65; P = .015). (C) Between 3 and 9 months after treatment, the change (Δ) in B cells inversely correlated with a reduction in circulating SDF-1 levels, indicating that SDF-1 levels decline as B cells recover (R = –0.67; P = .013). • designates the 2 patients with LON.
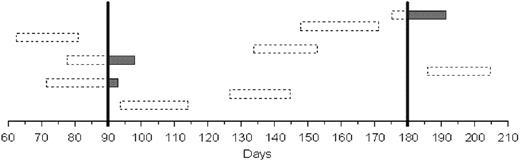
To estimate the incidence of LON, we assumed that the duration of neutropenia was normally distributed but recognized that it had an unknown mean and an unknown standard deviation of more than 1 day. It was also assumed that the date of neutropenia onset was independent of the blood count dates. In order to provide an actual estimate of the mean and variance of the duration of neutropenia, data from the 2 published studies of LON in previously treated patients from Chaiwatanatorn et al10 and Voog et al11 were combined with data from the current study, taking into account that the date of LON onset may not be equal to the date of detection. Since treatment with filgrastim could act to reduce the duration of the neutropenia, patients treated with filgrastim were excluded from this portion of the analysis. Based on the estimates of the duration, and assuming a 90-day interval between blood counts, we estimated the proportion of LON that went undetected in our study to be 79%. Using this estimate along with the observed neutropenia rate of 7.9% led to an estimate of 35.5% for the true rate of LON. Bootstrapping was used to form a confidence distribution of the proportion of undetected LON and yielded a 95% confidence interval of 76% to 86% for the proportion of undetected cases.27 This distribution was convolved with the binomial distribution to form a 95% confidence interval of 16% to 74% for our LON rate estimate.
A simulation based on our model of the occurrence of LON in 9 patients up to an observation period of 210 days is shown in Figure 4. The boxes represent individual episodes of LON, with the dashed areas showing undetected and the solid areas showing detected periods of LON during the routine CBCs obtained at 90 and 180 days. Note that 6 of the 9 cases of LON went entirely undetected, and even in cases in which LON was detected, the true duration of neutropenia was often significantly longer than the observed duration.
Discussion
This study demonstrates that the addition of rituximab to doxorubicin-based chemotherapy produces LON. It is characterized by a transient clinical course, absence of infection, presence of granulocytes in the bone marrow, and a rapid response to filgrastim.10,11,13 Although 3 publications have reported “late”-onset neutropenia in hematologic malignancies following rituximab-based treatment, the studies used variable treatments and lacked appropriate control groups.10,11,13 In contrast, the present results are independent of chemotherapy-related bone marrow damage confirmed by the absence of LON among the control group that received only DA-EPOCH.
Simulation of cases of late-onset neutropenia. Simulation of the occurrence of LON in 9 patients up to an observation period of 210 days. Boxes represent individual episodes of LON, with the dashed areas showing undetected periods and shaded areas showing detected periods of LON. The vertical bars show routine blood counts obtained at 90 and 180 days. Note that 6 of the 9 cases of LON went entirely undetected, and even in cases in which LON was detected, the true duration of neutropenia was often significantly longer than the observed duration.
Simulation of cases of late-onset neutropenia. Simulation of the occurrence of LON in 9 patients up to an observation period of 210 days. Boxes represent individual episodes of LON, with the dashed areas showing undetected periods and shaded areas showing detected periods of LON. The vertical bars show routine blood counts obtained at 90 and 180 days. Note that 6 of the 9 cases of LON went entirely undetected, and even in cases in which LON was detected, the true duration of neutropenia was often significantly longer than the observed duration.
To control for potential effects from the imbalance in mantle cell patients and idiotype vaccination among the 2 groups, we performed several analyses. First, we showed that the incidence of LON and its significance was unaffected by the exclusion of mantle cell patients from the analysis. Secondly, we analyzed 46 patients with untreated follicular lymphoma who received PACE (prednisone, doxorubicin, cyclophosphamide, and etoposide) chemotherapy, a non–rituximab-containing regimen, followed by the same idiotype vaccine formulation and schedule as administered to the mantle cell patients to assess if idiotype vaccination is associated with LON. In this retrospective analysis, no episodes of LON were identified, suggesting that idiotype vaccination is not associated with LON (L.K. and Barry Gause, unpublished observations; data not shown). Finally, all biologic end points in the mantle cell patients were obtained at least 4 weeks after idiotype vaccination, and, in most cases, they were obtained either before any vaccination or 8 weeks after the last idiotype vaccination. These results suggest that the imbalance in mantle cell patients between the 2 groups does not confound the interpretation of our results.
While recognizing the pitfalls of comparing results among studies, it is instructive to evaluate our findings in the context of 3 published reports (Table 3). The median time to LON in our series was somewhat longer (174 days versus 128 days) and the ANC nadir higher (0.2 × 109/L compared with 0.138 × 109/L) compared with the combined results of the published series, although the median duration of neutropenia was similar at 14 versus 11 days, respectively. In the published series, many patients had received considerable myelotoxic therapy including stem cell transplantation. Among 16 bone marrow biopsies obtained in these patients, 5 (31%) were reported to show significant hypocellularity suggesting bone marrow damage. In contrast, the other 11 bone marrow biopsies were reported to show variable degrees of maturation arrest, which is similar to our findings. Most patients in our study were asymptomatic, although 11 (50%) of the patients in the published series had a fever and/or infection and 4 (19%) patients had prolonged neutropenia of 20 to 148 days. These results suggest that the LON in some patients from the published series may not have been primarily related to rituximab.
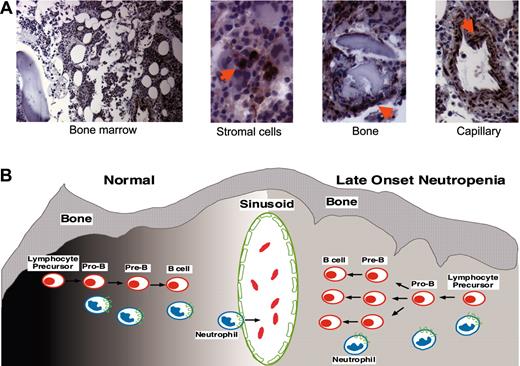
The median time to LON led us and others to hypothesize that it is related to B-cell recovery.10,11 In the present study, we show that rapid B-cell recovery is associated with a decline in circulating granulocytes, suggesting that LON is biologically intertwined with B-cell recovery. The transient nature of LON and the effects of B-cell recovery on granulocyte dynamics suggest that perturbations of neutrophil homeostasis rather than autoimmune dyscrasias are a more likely etiology. There is compelling evidence that granulocyte egress from the bone marrow is regulated through interactions between the SDF-1 chemokine produced by stromal cells and its unique receptor, CXCR4, expressed on hematopoietic cells (Figure 5A).16,17,19,24 SDF-1 is also essential for B-cell lymphopoiesis where it triggers cell division and migration of early lineage B cells.15,25 These observations raised the possibility that SDF-1 concentrations may be disrupted during rapid B-cell expansion resulting in reduced neutrophil egress from the bone marrow. Accurate measurement of SDF-1 levels in the bone marrow microenvironment and its change over time is currently not possible. However, we found that circulating SDF-1 levels obtained at 3 months following rituximab therapy correlated with later B-cell recovery at 9 months, likely reflecting an increase in bone marrow SDF-1 early in the recovery period.22 In addition, we found a correlation between a decrease in circulating SDF-1 levels after recovery and B-cell recovery at 9 months, consistent with a subsequent decline in bone marrow SDF-1 following B-cell expansion. In the absence of severe B-cell depletion, however, one would not expect to observe B-cell–dependent changes in SDF-1 levels. As a test of this hypothesis, we analyzed SDF-1 levels in patients who received DA-EPOCH alone and found no significant change following treatment. Importantly, based on our previous work, we anticipate that these patients would achieve B-cell recovery by 3 months after treatment, suggesting minimal B-cell loss.
Bone marrow SDF-1 immunohistochemistry and model of late-onset neutropenia. (A) SDF-1 detection in the bone marrow of a patient with LON. SDF-1 is expressed by bone marrow stromal cells, which are dispersed throughout the bone marrow; osteoblasts that are juxtaposed to the bone trabeculae; and endothelial cells that line the blood capillaries. Sections immunostained for SDF-1 with specific antibodies. Images were collected using a Nikon Eclipse 6600 microscope (10×/0.45 DICL and 40×/0.95 DICM lenses; Nikon, Tokyo, Japan) with a Retiga 1300 digital camera (QImaging, Burnaby, BC, Canada) and IP Lab acquisition software (Scanalytics, Fairfax, VA). Images were imported into Adobe Photoshop software (Adobe Systems, San Jose, CA). (B) A model of late-onset neutropenia is shown. Early B-cell lymphopoiesis and neutrophil egress into the bone marrow sinusoid is regulated by SDF-1 gradients (dark to light shading, left) within the bone marrow microenvironment, as shown on the left. In a patient with LON, we hypothesize that the SDF-1 gradients are transiently disrupted (even gray shading, right) within the bone marrow microenvironment due to SDF-1 consumption by rapidly expanding B cells, resulting in the temporary inhibition of neutrophil egress across the sinusoid, as shown on the right. SDF-1–positive cells are indicated by arrows.
Bone marrow SDF-1 immunohistochemistry and model of late-onset neutropenia. (A) SDF-1 detection in the bone marrow of a patient with LON. SDF-1 is expressed by bone marrow stromal cells, which are dispersed throughout the bone marrow; osteoblasts that are juxtaposed to the bone trabeculae; and endothelial cells that line the blood capillaries. Sections immunostained for SDF-1 with specific antibodies. Images were collected using a Nikon Eclipse 6600 microscope (10×/0.45 DICL and 40×/0.95 DICM lenses; Nikon, Tokyo, Japan) with a Retiga 1300 digital camera (QImaging, Burnaby, BC, Canada) and IP Lab acquisition software (Scanalytics, Fairfax, VA). Images were imported into Adobe Photoshop software (Adobe Systems, San Jose, CA). (B) A model of late-onset neutropenia is shown. Early B-cell lymphopoiesis and neutrophil egress into the bone marrow sinusoid is regulated by SDF-1 gradients (dark to light shading, left) within the bone marrow microenvironment, as shown on the left. In a patient with LON, we hypothesize that the SDF-1 gradients are transiently disrupted (even gray shading, right) within the bone marrow microenvironment due to SDF-1 consumption by rapidly expanding B cells, resulting in the temporary inhibition of neutrophil egress across the sinusoid, as shown on the right. SDF-1–positive cells are indicated by arrows.
Although the regulation of SDF-1 levels in the bone marrow microenvironment is incompletely understood, consumption by rapidly expanding B cells could result in disruption of bone marrow SDF-1 gradients, which regulate neutrophil egress from the bone marrow (Figure 5B).17,19,28 Several additional observations support this model. Immunohistochemical staining for SDF-1 in bone marrow biopsies obtained before treatment and at the time of LON in 2 patients showed similar patterns, suggesting that SDF-1 production by stromal cells does not substantially change during the neutropenia (comparative data not shown; Figure 5A). The bone marrows of patients with LON showed evidence of granulocyte maturation. Furthermore, filgrastim usually produced rapid granulocyte mobilization, suggesting that neutrophil egress rather than myeloid cell maturation is affected.12,17 Recently, enzymatic cleavage of SDF-1 and CXCR4 has been proposed to mediate filgrastim-induced neutrophil and stem cell mobilization.19,29,30
Unfortunately, because it is not possible to measure bone marrow SDF-1 gradients in clinical samples, we cannot provide direct causative evidence for LON. While recognizing that plasma SDF-1 levels could reflect overall changes in bone marrow SDF-1 production, such as those associated with early B-cell lymphopoiesis, we believe it unlikely they could accurately reflect the complex and dynamic gradients that control neutrophil egress. Indeed, plasma SDF-1 levels are a composite of SDF-1 production, consumption, and clearance of the inactive molecule.22 Hence, we would not have expected to find a correlation between plasma SDF-1 levels and granulocyte changes. If, as we hypothesized, rapidly expanding B cells in the bone marrow transiently disrupts the SDF-1 gradients, one might expect to find a direct correlation with granulocyte decrements, which was observed.
A potentially important observation is the possibly higher incidence of LON in AIDS-related lymphomas. Interestingly, while the levels of circulating SDF-1 are generally normal in AIDS patients, AIDS-related lymphomas are associated with increased levels of circulating SDF-1.22,31 The presence of abnormally high SDF-1 levels and the potential dysregulation of CXCR4, which serves as both the SDF-1 receptor and a T-cell tropic HIV-1 coreceptor, may predispose patients with AIDS-related lymphoma to LON.31,32 This is clinically suggested by our results as well as by a recent randomized study in AIDS-related lymphoma where patients receiving rituximab had a significantly higher incidence of infectious complications and a higher, albeit not quite significant, incidence of neutropenia.33
Our results raise the intriguing notion that B-cell recovery following rituximab involves early stages of B-cell lymphopoiesis. Furthermore, they provide evidence for the dual role of SDF-1 in B-cell regulation and granulocyte homeostasis whereby perturbations of SDF-1 during B-cell recovery can affect neutrophil egress from the bone marrow. With the entry of rituximab into the standard treatment of aggressive lymphomas and its widening indications, it is likely that LON will become a commonly observed phenomenon. These results, however, suggest that LON is self-limited and unlikely to cause significant clinical sequelae when it occurs in patients receiving primary treatment.
Prepublished online as Blood First Edition Paper, February 17, 2005; DOI 10.1182/blood-2004-08-3198.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Mr Harry Schafer for technical support and Drs Robert Yarchoan and Richard Little for collaborations on AIDS-related lymphoma.