In vitro studies suggest that bone marrow endothelial cells contribute to multilineage hematopoiesis, but this function has not been studied in vivo. We used a Cre/loxP-mediated recombination to produce mice that lacked the cytokine receptor subunit gp130 in hematopoietic and endothelial cells. Although normal at birth, the mice developed bone marrow dysfunction that was accompanied by splenomegaly caused by extramedullary hematopoiesis. The hypocellular marrow contained myeloerythroid progenitors and functional repopulating stem cells. However, long-term bone marrow cultures produced few hematopoietic cells despite continued expression of gp130 in most stromal cells. Transplanting gp130-deficient bone marrow into irradiated wild-type mice conferred normal hematopoiesis, whereas transplanting wild-type bone marrow into irradiated gp130-deficient mice did not cure the hematopoietic defects. These data provide evidence that gp130 expression in the bone marrow microenvironment, most likely in endothelial cells, makes an important contribution to hematopoiesis.
Introduction
The interleukin-6 (IL-6) family of cytokines includes IL-6, IL-11, oncostatin M, leukemia inhibitory factor, ciliary neurotrophic factor, and cardiotropin-1. All these cytokines bind to receptors that share the signaling subunit gp130.1,2 Because many cells express these receptors, gp130-dependent signaling is thought to have numerous important functions. Indeed, mice lacking gp130 in all cells die of diverse morphologic abnormalities during development,3 and postnatally induced deletion of gp130 in all cells causes defects in many systems.4 Because gp130 is so widely expressed, clarifying gp130-dependent functions requires targeted deletion of gp130 in specific cell types. For example, myocyte-restricted deletion of gp130 in mice causes apoptosis that results in heart failure.5
Global deletion of gp130 decreases hematopoietic progenitors in fetal liver,3 and postnatally induced deletion of gp130 causes hematopoietic defects.4 It is unknown whether these abnormalities result from loss of gp130 signaling in hematopoietic cells, other cells in the microenvironment, or both. Cultured hematopoietic cells respond directly to IL-6–related cytokines.6-10 However, endothelial cells also express gp130,11 and there is a close developmental relationship between hematopoietic and endothelial cells that may arise from hemangioblasts or specialized hemogenic endothelial cells in early fetal life.12-16 Cultured endothelial cells derived from adult bone marrow or fetal yolk sac support multilineage hematopoiesis,12,13,17,18 but this broad function has not been demonstrated in vivo. Stimulation of megakaryocyte progenitors with some chemokines, but not with IL-6 or IL-11, causes them to adhere to bone marrow endothelial cells, which enhances thrombopoiesis in thrombopoietin-deficient mice.19 This study provides evidence that endothelial cells contribute to hematopoiesis in one lineage, but it does not address whether IL-6 family cytokines participate in cross-talk between hematopoietic and endothelial cells during hematopoiesis.
Here, we used the Cre/loxP-mediated gene recombination to selectively delete gp130 in hematopoietic and endothelial cells of mice. Although hematopoiesis was normal throughout the fetal and neonatal periods, bone marrow dysfunction developed in adult mice. Intrinsic defects in hematopoietic stem and progenitor cells were not apparent even when anemia was severe. Transplantation experiments revealed that hematopoiesis required the expression of gp130 in the bone marrow microenvironment. These data provide evidence that gp130-dependent signaling in bone marrow endothelial cells contributes significantly to hematopoiesis.
Materials and methods
Mice
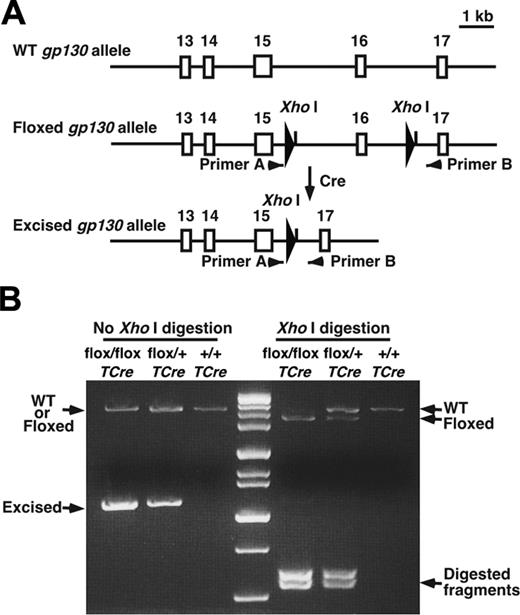
All animal experiments were conducted after the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation approved the experimental protocols. TCre transgenic mice were provided by Dr Masashi Yanagisawa (University of Texas Southwestern Medical Center, Dallas, TX),20 and gp130flox/flox mice were provided by Dr Werner Müller (University of Cologne, Germany).4 TCre mice were bred with gp130flox/flox mice. From the progeny, heterozygous gp130flox/+/TCre mice were bred with gp130flox/+ mice to obtain gp130flox/flox/TCre mice. The wild-type (WT), floxed, and deleted floxed gp130 alleles were detected by polymerase chain reaction (PCR) of genomic DNA, digestion of the amplified DNA with XhoI, and electrophoresis on agarose gels (Figure 1A-B). Mice backcrossed at least 6 generations into the C57/BL6J background were used in most experiments, and littermate controls were used to ensure genetic comparability. To obtain ROSA26R/TCre mice, TCre mice were bred with ROSA26 reporter mice obtained from The Jackson Laboratory (Bar Harbor, ME).21
Antibodies
Monoclonal antibodies to signal transducer and activator of transcription-3 (Stat3) and platelet endothelial cell adhesion molecule-1 (PECAM-1) and rabbit polyclonal antibodies to Tie2 and gp130 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti–rabbit immunoglobulin G (IgG) conjugated to Alexa 568 and donkey anti–rat IgG conjugated to Alexa 488 were from Molecular Probes (Eugene, OR). Fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies and all other unlabeled monoclonal antibodies were from BD Biosciences (San Diego, CA).
Confocal microscopy
Frozen tissue sections were fixed in acetone for 10 minutes at -20°C. The sections were incubated with rabbit anti-gp130 antibodies and rat anti–PECAM-1 monoclonal antibody, followed by goat anti–rabbit IgG conjugated to Alexa 568 and donkey anti–rat IgG conjugated to Alexa 488. Images were visualized on a confocal microscope (TCS NT; Leica Microsystems, Heidelberg, Germany).
Hematologic analysis and histology
Blood was collected by retro-orbital puncture into heparinized tubes. Complete blood counts were determined using a cell counter (Serono 9000; Serono-Parker Diagnostics, Allentown, PA). Blood smears were stained with May-Grünwald/Giemsa. Formalin-fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin-eosin. Tissues from ROSA26R and ROSA26R/TCre mice were fixed and stained with X-Gal for 16 hours at 37°C.22 For immunocytochemistry, methanol-fixed stromal cells from long-term bone marrow cultures were incubated with rabbit anti–gp130 IgG or control rabbit IgG or with rat anti–PECAM-1 monoclonal antibody or control rat IgG. Bound antibody was detected by sequential incubations with biotinylated swine anti–rabbit IgG or swine anti–rat IgG, streptavidin/horseradish peroxidase, and diaminobenzidine (DakoCytomation, Carpinteria, CA). Murine femurs were fixed in 4% paraformaldehyde and then decalcified in 0.5 M EDTA (ethylenediaminetetraacetic acid) for 7 days at 4°C. The bones were embedded in optimum temperature cutting compound (OCT) and frozen. Frozen sections were incubated with rabbit anti–von Willebrand factor (anti-VWF) polyclonal antibody (DakoCytomation) or rat anti-gp130 monoclonal antibody (R&D Systems, Minneapolis, MN), followed by biotinylated goat anti–rabbit IgG or rabbit anti–rat IgG (Vector Laboratories, Burlingame, CA). The sections were incubated sequentially with Vectastain ABC Reagent and peroxidase substrate according to the instructions of the manufacturer (Vector Laboratories). Images were obtained with a Zeiss AxioCam MRC 12-bit color digital camera (Carl Zeiss Microimaging, Thornwood, NY) with a Zeiss Plan Neofluar 0.75 NA objective and Zeiss Axiovision 4.1 software.
Generation of gp130flox/flox/TCre mice. (A) Schematic diagram of portions of the WT gp130 allele, the gp130flox/flox allele, and the gp130flox/flox allele after Cre-mediated excision of the floxed exon.4 Exon numbers are shown. The loxP sites are depicted as large arrowheads adjacent to the introduced XhoI restriction sites. Small arrowheads indicate the sites of PCR primers that were used to amplify each allele. (B) Genotype analysis. gp130flox/+/TCre mice were bred with gp130flox/+ mice. PCR products of genomic DNA from the offspring were resolved on agarose gels before or after digestion with XhoI. Arrows indicate the products corresponding to the WT allele, the floxed allele, and the floxed allele after Cre-mediated excision of the floxed exon.
Generation of gp130flox/flox/TCre mice. (A) Schematic diagram of portions of the WT gp130 allele, the gp130flox/flox allele, and the gp130flox/flox allele after Cre-mediated excision of the floxed exon.4 Exon numbers are shown. The loxP sites are depicted as large arrowheads adjacent to the introduced XhoI restriction sites. Small arrowheads indicate the sites of PCR primers that were used to amplify each allele. (B) Genotype analysis. gp130flox/+/TCre mice were bred with gp130flox/+ mice. PCR products of genomic DNA from the offspring were resolved on agarose gels before or after digestion with XhoI. Arrows indicate the products corresponding to the WT allele, the floxed allele, and the floxed allele after Cre-mediated excision of the floxed exon.
Immunoblots
Murine tissues were lysed with buffer (Tissue-PE LB; Geno Technology, St Louis, MO). Splenic T cells and cultured endothelial cells or stromal cells were lysed in 50 mM cold NaCl, 50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL aprotinin, 5 μg/mL leupeptin, 1 mM Na3VO4, and 5 mM EDTA. Proteins (30 μg) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon P (Millipore, Bedford, MA) membranes, and probed with antibodies using a chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ).
Cell sorting and flow cytometry
Bone marrow cell suspensions were enriched for lineage-negative cells by incubation with monoclonal antibodies to CD45RA, CD19, Mac-1, Gr-1, and TER-119, followed by negative selection on a magnetic cell sorting (MACS) separation system (Miltenyi Biotec, Auburn, CA). The enriched cells were stained with FITC-conjugated monoclonal antibodies to the lineage markers B220, Mac-1, Gr-1, CD2, CD3, and CD8, with PE-conjugated monoclonal antibody to Sca-1 and with allophycocyanin (APC)–conjugated monoclonal antibody to c-kit. Cells were sorted into Lin-c-kitHiSca-1+, Lin-c-kitHiSca-1-, and Lin-c-kitLoSca-1-/Lo fractions on a MoFlo cell sorter (Cytomation, Fort Collins, CO). To identify donor-derived cells after transplantation, these cell fractions were stained with biotinylated monoclonal antibody to CD45.2 followed by streptavidin-PE-Texas Red (Caltag, Burlingame, CA). Alternatively, bone marrow or spleen cells obtained after transplantation were stained with biotinylated anti-CD45.2 and FITC–anti-Mac-1/Gr-1, PE–anti-CD19, or APC–anti-CD4/CD8. Expression of β-galactosidase in cell suspensions of thymus, spleen, and bone marrow from ROSA26R and ROSA26R/TCre mice was measured by loading the cells, after incubation with 150 mM NH4Cl, 10 mM NaHCO3, and 10 mM EDTA to lyse erythrocytes, with fluorescein di-D-galactopyranoside (Molecular Probes). The cells were then stained with PE-conjugated monoclonal antibodies to Thy1.2, B220, Mac-1, or Ter-119 and were analyzed on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences).
Colony-forming assays
Clonal growth of myeloerythroid progenitors was measured as described.23 Briefly, 2 × 104 bone marrow cells, 1 × 105 spleen cells, or 200 sorted Lin-c-kitHiSca-1+ cells were cultured in individual dishes in Iscove modified Dulbecco-based methylcellulose medium (Methocult M3231; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 50 ng/mL recombinant murine stem-cell factor, 10 ng/mL recombinant murine IL-3, and 3 U/mL recombinant human erythropoietin (R&D Systems, Minneapolis, MN). After 8 to 10 days, colonies visualized on an inverted microscope were counted and classified as granulocyte macrophage/megakaryocyte colony-forming unit (CFU-GM/M), erythroid blast-forming unit (BFU-E), or granulocyte macrophage–erythroid [megakaryocyte] colony-forming unit (CFU-GEM[M]).
Endothelial-cell and long-term bone marrow cultures
Endothelial cells isolated from murine lung were cultured as described.24 Purity of the cells was confirmed by staining the cells with monoclonal antibodies to the endothelial-cell markers intercellular adhesion molecule-2 (ICAM-2) and PECAM-1. Production of myeloid cells in long-term bone marrow cultures was performed as described.25 Briefly, bone marrow cells (1.2 × 107 cells per 25-cm2 flask) were cultured at 33°C in α-modified Eagle medium (α-MEM) supplemented with 100 nM hydrocortisone (Stem Cell Technologies) and 20% horse serum (Hyclone, Logan, UT). Cultures were maintained by replacing half the medium weekly. Production of hematopoietic stem and progenitor cells in long-term bone marrow cultures was measured with the cobblestone area–forming cell (CAFC) assay.26,27 Bone marrow cells were cultured at 33°C for 2 weeks as described. After nonadherent cells were removed, the confluent stromal-cell monolayer was trypsinized, irradiated (15 Gy [1500 rad]), and subcultured in 96-well plates at a density of 3 × 105 cells/well. In some experiments, murine OP9 stromal cells28 were cultured in 96-well plates under identical conditions. Bone marrow cells pooled from 2 mice of each genotype were seeded at 2-fold dilutions (3 × 104-7.5 × 103 cells/well) into 12 wells for each dilution. Cultures were incubated at 33°C and were maintained by replacing half the medium weekly. After 4 weeks, the frequency of CAFC was scored using L-CALC software (Stem Cell Technologies).
Transplantation
The following congenic mice were used as donors and recipients: WT C57BL/6J mice expressing CD45.2, WT C57BL/6SJL mice expressing CD45.1, and gp130flox/flox/TCre backcrossed 8 generations to C57BL/6J mice expressing CD45.2. All donor and recipient mice were 10 weeks of age at the time of transplantation. Donor bone marrow cells were injected intravenously into lethally irradiated (9.5 Gy [950 rad]) recipient mice (1 × 106 cells/recipient). After 6 weeks, reconstitution of hematopoiesis was examined in peripheral blood, bone marrow, spleen, and thymus. For serial transplantation, bone marrow cells recovered from CD45.1+ WT mice that underwent transplantation with CD45.2+ WT or gp130flox/flox/TCre cells were transplanted into irradiated CD45.1+ WT secondary recipients (1 × 106 cells/recipient). After 16 weeks, reconstitution of hematopoiesis was examined.
Results
Tie2-Cre transgenic mice efficiently excise a loxP-flanked reporter gene in hematopoietic and endothelial cells
The Tie2 receptor is expressed by hematopoietic stem cells and endothelial cells and by their common precursors that appear early during development.14 Transgenic mice expressing Cre recombinase under the control of a Tie2 promoter/enhancer quantitatively excise loxP-flanked (floxed) genes in endothelial cells.20,29,30 However, excision efficiency in hematopoietic cells varies, depending on the site of integration of the Tie2-Cre transgene.30,31 To determine the tissue specificity and efficiency of recombination, we crossed our Tie2-Cre (TCre) mice 20 to ROSA26 reporter mice that express β-galactosidase after Cre-mediated excision of a floxed stuffer fragment.21 Histochemical analysis of tissues from the offspring revealed widespread β-galactosidase expression in endothelial cells of heart, lung, liver, and brain and in hematopoietic cells of spleen (Figure S1, available at the Blood website; see the Supplemental Figures link at the top of the online article; also, L.Y. and R.P.M., unpublished data, January 2000). Furthermore, flow cytometry of permeabilized cells from bone marrow, thymus, and spleen demonstrated β-galactosidase expression in more than 90% of hematopoietic cells of B lymphoid, T lymphoid, myeloid, and erythroid lineage (Supplemental Figure S2 and unpublished data). Hematopoietic-cell expression of β-galactosidase was stable in mice until they were at least 10 months of age. These results indicate that Tie2-Cre–mediated recombination is efficient and specific for endothelial cells and hematopoietic cells. Furthermore, they are consistent with a developmental relationship between these cell types.
Mice lacking gp130 in hematopoietic and endothelial cells develop normally in the fetal and neonatal periods
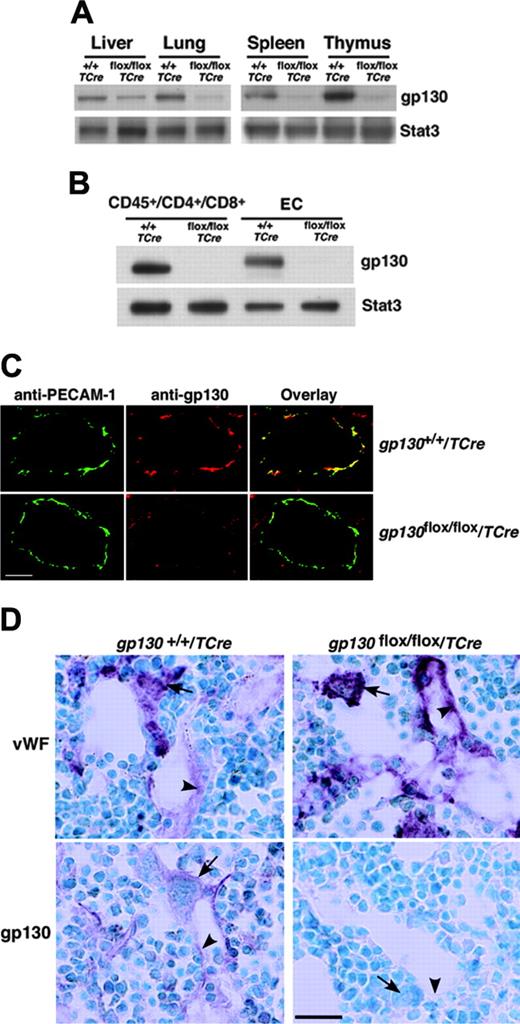
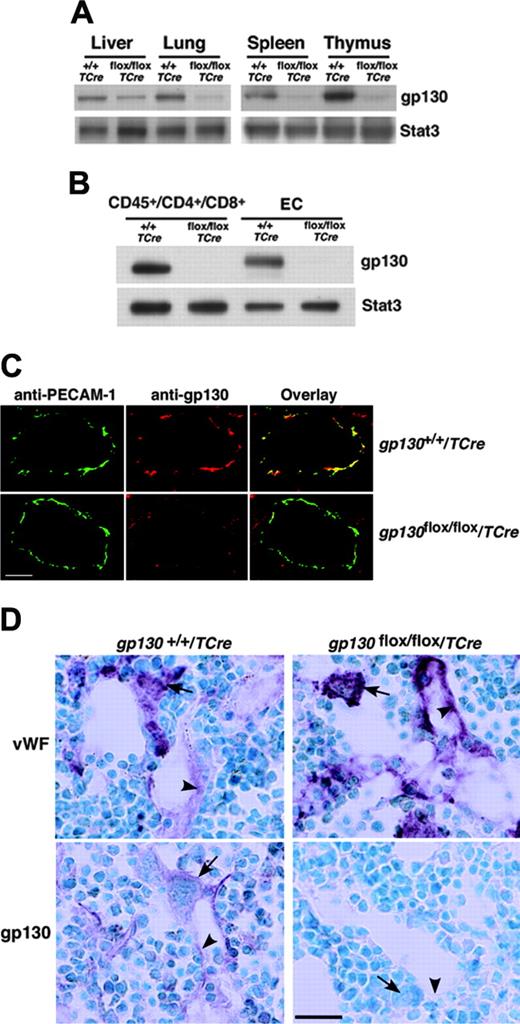
We next crossed TCre transgenic mice with mice bearing a gp130 gene with loxP sites flanking the exon encoding the transmembrane domain on each allele (gp130flox/flox) (Figure 1A). Cre-mediated excision of this exon causes loss of gp130 from the plasma membrane and eliminates gp130-dependent signaling.4 From the progeny, male heterozygous (gp130flox/+/TCre) mice were mated to female gp130flox/+ mice. PCR analysis of DNA demonstrated that approximately 50% of these offspring expressed the TCre transgene with genotypes of the expected frequencies. These included mice lacking the gp130 exon in both alleles (gp130flox/flox/TCre), mice lacking the gp130 exon on 1 allele (gp130flox/+/TCre), and mice expressing 2 normal alleles (gp130+/+/TCre) (Figure 1B). We also bred male gp130flox/+/TCre mice with female gp130flox/flox mice, yielding mice lacking gp130 on either or both alleles in the expected frequency (L.Y. and R.P.M., unpublished data, September 2000). Mice of all genotypes appeared normal and were indistinguishable during the neonatal period. Immunoblots of extracts of tissues enriched in endothelial or hematopoietic cells demonstrated marked decreases in gp130 protein in adult gp130flox/flox/TCre mice, suggesting efficient deletion of the floxed gp130 exon (Figure 2A). Immunoblots of T cells from spleen and of endothelial cells from lung revealed essentially complete elimination of gp130 in gp130flox/flox/TCre mice (Figure 2B). PCR analysis of DNA also demonstrated nearly total excision of the floxed gp130 allele in lineage marker-negative (Lin-) c-kitHiSca-1+ cells, which are enriched in hematopoietic stem and progenitor cells (unpublished data). Confocal immunofluorescence microscopy of multiple organs revealed the expected colocalization of gp130 with the endothelial-cell protein PECAM-1 in blood vessels of gp130+/+/TCre mice but not in vessels of gp130flox/flox/TCre mice (Figure 2C). As assessed by immunoperoxidase staining, bone marrows of both gp130+/+/TCre and gp130flox/flox/TCre mice contained megakaryocytes and sinusoidal endothelial cells that expressed VWF (Figure 2D). In bone marrows of gp130+/+/TCre mice, endothelial cells, megakaryocytes, and a subset of other hematopoietic cells expressed gp130. In sharp contrast, neither bone marrow endothelial cells nor hematopoietic cells expressed gp130 in gp130flox/flox/TCre mice (Figure 2D). These data demonstrate that gp130flox/flox/TCre mice develop normally through the neonatal period, despite the virtually complete absence of gp130 in hematopoietic cells and endothelial cells in bone marrow and other tissues.
Bone marrow dysfunction develops in mice lacking gp130 in hematopoietic and endothelial cells
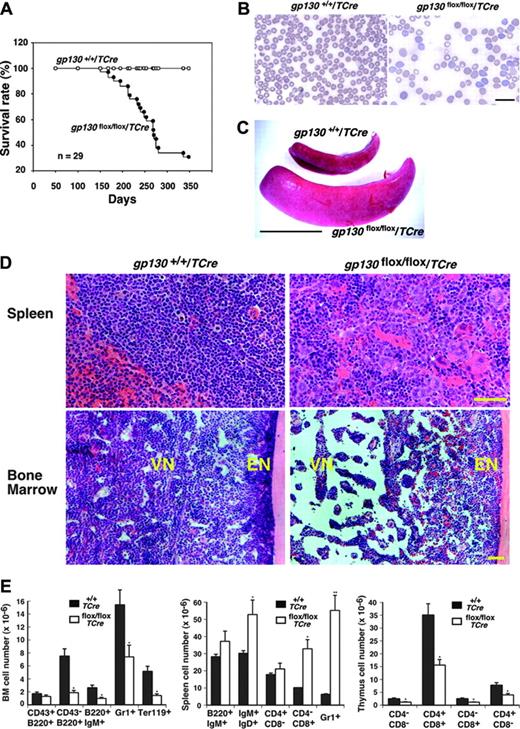
Although the gp130flox/flox/TCre mice appeared normal at birth, most of them died by 12 months of age (Figure 3A). Reduced body weight often preceded death. Histologic examination of older survivors or of the mice that died revealed no obvious morphologic abnormalities in the heart or in other nonhematopoietic organs. At 1 week of age, the mice had normal peripheral-blood counts (L.Y. and R.P.M., unpublished data, April 2002), but they had anemia and myeloid leukocytosis at 4 weeks of age that became more pronounced at 12 weeks of age (Table 1) but that did not change further at 7 to 10 months of age (L.Y. and R.P.M., unpublished data, April 2003). The premature deaths of the gp130flox/flox/TCre mice could not be directly linked to the hematologic abnormalities. None of the mice developed leukemia. Platelet counts remained in the normal range. Peripheral-blood smears of mice revealed increased reticulocytes and anisocytosis/poikilocytosis of erythrocytes (Figure 3B). After 4 weeks of age, the mice gradually developed splenomegaly (Figure 3C) with a mean 2.5-fold increase in spleen weight. Large numbers of immature myeloerythroid cells and megakaryocytes replaced the normal splenic architecture (Figure 3D). In sharp contrast, the vascular niche of the bone marrow became hypocellular and developed expanded sinusoidal spaces (Figure 3D). The sole exception to the decreased cellularity of the vascular niche was the retention of normal numbers of megakaryocytes. As in normal bone marrow,19 the megakaryocytes were closely associated with sinusoidal endothelial cells (Figure 2D). Silver staining revealed no evidence of bone marrow fibrosis (L.Y. and R.P.M., unpublished data, October 2003). Based on morphologic criteria, maturation of the residual myeloid and erythroid cells appeared normal. Unlike the decreased cellularity in the vascular niche, the endosteal niche adjacent to bone retained normal numbers of cells (Figure 3D). Consistent with the histologic appearance, the number of cells expressing myeloid-, erythroid-, or B-cell markers decreased in bone marrow and increased in spleen (Figure 3E). The gp130flox/flox/TCre mice also developed cortical atrophy of the thymus (L.Y. and R.P.M., unpublished data, August 2001) with reduced numbers of thymic T cells at all stages of development (Figure 3E).
Specific deletion of gp130 in hematopoietic and endothelial cells of gp130flox/flox/TCre mice. (A) Immunoblots of tissue lysates probed with antibodies to gp130 or to the control protein Stat3. (B) Immunoblots of sorted CD45+ splenic T cells expressing CD4 or CD8 and of cultured lung endothelial cells (ECs) probed with antibodies to gp130 or Stat3. Cells were pooled from 3 mice from each genotype. (C) Confocal immunofluorescence microscopy of lung sections stained with antibodies to the endothelial-cell marker PECAM-1 (green) and to gp130 (red). Yellow staining indicates colocalization of PECAM-1 and gp130 in the endothelial cells of gp130+/+/TCre mice. Only green staining for PECAM-1 is present in the endothelial cells of gp130flox/flox/TCre mice. Both sections exhibit red staining for gp130 in nonendothelial cells. Scale bar represents 20 μm. Objective, × 40/0.75 NA. (D) Immunoperoxidase staining of bone marrow sections with antibodies to VWF or to gp130. Red staining for VWF is present in megakaryocytes (arrows) and sinusoidal endothelial cells (arrowheads) of both gp130+/+/TCre mice and gp130flox/flox/TCre mice. Staining for gp130 is present in megakaryocytes, a subset of other hematopoietic cells, and endothelial cells of gp130+/+/TCre mice. By contrast, there is no staining for gp130 in hematopoietic or endothelial cells of gp130flox/flox/TCre mice. Scale bar represents 50 μm. Data are representative of 3 to 4 independent experiments with 12-week-old mice. Objective, × 40/0.75 NA.
Specific deletion of gp130 in hematopoietic and endothelial cells of gp130flox/flox/TCre mice. (A) Immunoblots of tissue lysates probed with antibodies to gp130 or to the control protein Stat3. (B) Immunoblots of sorted CD45+ splenic T cells expressing CD4 or CD8 and of cultured lung endothelial cells (ECs) probed with antibodies to gp130 or Stat3. Cells were pooled from 3 mice from each genotype. (C) Confocal immunofluorescence microscopy of lung sections stained with antibodies to the endothelial-cell marker PECAM-1 (green) and to gp130 (red). Yellow staining indicates colocalization of PECAM-1 and gp130 in the endothelial cells of gp130+/+/TCre mice. Only green staining for PECAM-1 is present in the endothelial cells of gp130flox/flox/TCre mice. Both sections exhibit red staining for gp130 in nonendothelial cells. Scale bar represents 20 μm. Objective, × 40/0.75 NA. (D) Immunoperoxidase staining of bone marrow sections with antibodies to VWF or to gp130. Red staining for VWF is present in megakaryocytes (arrows) and sinusoidal endothelial cells (arrowheads) of both gp130+/+/TCre mice and gp130flox/flox/TCre mice. Staining for gp130 is present in megakaryocytes, a subset of other hematopoietic cells, and endothelial cells of gp130+/+/TCre mice. By contrast, there is no staining for gp130 in hematopoietic or endothelial cells of gp130flox/flox/TCre mice. Scale bar represents 50 μm. Data are representative of 3 to 4 independent experiments with 12-week-old mice. Objective, × 40/0.75 NA.
Bone marrow from gp130flox/flox/TCre mice retains progenitor cells but does not produce hematopoietic cells in long-term cultures
Marrow dysfunction in gp130flox/flox/TCre mice could have resulted from intrinsic abnormalities in hematopoietic cells or in components of the microenvironment. Although the bone marrow was hypocellular, it retained normal numbers of clonable myeloid and erythroid progenitor cells responsive to IL-3, stem-cell factor, and erythropoietin, whereas the spleen had large increases in progenitor cells (Figure 4A). Lin-c-kitHiSca-1+ cells are enriched in multipotent stem/progenitor cells, whereas Lin-c-kitHiSca-1- and Lin-c-kitLoSca-1-/Lo cells are, respectively, enriched in myeloid and lymphoid progenitors.32-34 Flow cytometric analysis revealed that gp130flox/flox/TCre bone marrow preserved the Lin-c-kitHiSca-1+ fraction (Figure 4B). Furthermore, sorted Lin-c-kitHiSca-1+ cells from gp130flox/flox/TCre bone marrow generated normal numbers of colonies of all lineages (Figure 4C), and the morphology of the cells was normal. These data demonstrate that hematopoietic stem/progenitor cells are not detectably altered in bone marrow of gp130flox/flox/TCre mice.
To examine the potential contributions of the marrow environment to hematopoiesis, we established long-term bone marrow cultures that required adherent stromal cells to support hematopoietic-cell growth. Stromal cells in gp130flox/flox/TCre marrow cultures appeared to develop normally and had normal numbers of adipocytes (Figure 5A). However, the cultures produced virtually no hematopoietic cells (Figure 5B). Mixed cultures established with equal numbers of gp130+/+/TCre and gp130flox/flox/TCre marrow cells generated normal numbers of hematopoietic cells (L.Y., T.Y., and R.P.M., unpublished data, April 2003), suggesting that the hematopoietic defect did not result from the secretion of soluble gp130 or another protein that might act as an inhibitor.4 To directly examine the effects of stromal cells on the development of hematopoietic stem and progenitor cells, we measured the number of “cobblestones” that formed under irradiated stromal-cell monolayers. The number of CAFCs correlates linearly with the in vivo repopulating potential of hematopoietic stem and progenitor cells.26,27 Bone marrow cells from gp130+/+/TCre and gp130flox/flox/TCre mice established equal numbers of CAFCs when they were plated on gp130+/+/TCre stromal cells (Figure 5C) or on the murine OP9 stromal cell line (L.Y. and R.P.M., unpublished data, May 2005). In sharp contrast, bone marrow cells of either genotype formed significantly fewer CAFCs when plated on gp130flox/flox/TCre stromal cells (Figure 5C). Immunocytochemical analysis indicated that most gp130flox/flox/TCre stromal cells still expressed gp130 (Figure 5D), and immunoblots revealed no detectable decreases in gp130 protein in stromal-cell lysates (Figure 5E). Endothelial cells, a less common component of the stromal-cell layer,35 were not morphologically identifiable. However, immunoperoxidase analysis revealed that approximately 1% to 2% of the stromal cells in gp130+/+/TCre and gp130flox/flox/TCre cultures expressed PECAM-1, consistent with the presence of a minor population of endothelial cells (L.Y. and R.P.M., unpublished data, September 2003). These collective data indicate that bone marrow dysfunction in gp130flox/flox/TCre mice results from a microenvironmental defect. This defect probably reflects the loss of gp130 in the rare endothelial cells of the stromal-cell layer.
Shortened survival and defective hematopoiesis in gp130flox/flox/TCre mice. (A) Shortened survival of gp130flox/flox/TCre mice. (B) Peripheral-blood smear of a 16-week-old gp130flox/flox/TCre mouse exhibits polychromasia and anisopoikilocytosis of erythrocytes. Scale bar represents 20 μm. Objective, × 63/1.4 NA. (C) Splenomegaly in a 12-week-old gp130flox/flox/TCre mouse. Scale bar represents 1 cm. (D) Spleen of a 12-week-old gp130flox/flox/TCre mouse exhibits extramedullary hematopoiesis with abundant megakaryocytes. Bone marrow of a 16-week-old gp130flox/flox/TCre mouse exhibits normal cellularity in the endosteal niche (EN) adjacent to bone. In contrast, the vascular niche (VN) is markedly hypocellular, except for retention of normal numbers of megakaryocytes. Scale bar represents 50 μm. Objective, × 20/0.5 NA. (E) Quantification of blood cells of B-cell, T-cell, myeloid, and erythroid lineage expressing the indicated markers in bone marrow (BM), spleen, and thymus of gp130+/+/TCre and gp130flox/flox/TCre mice. Data represent the mean ± SD of 5 mice (age 12 weeks) in each genotype. *P < .05 and **P < .01 compared with gp130+/ +/TCre.
Shortened survival and defective hematopoiesis in gp130flox/flox/TCre mice. (A) Shortened survival of gp130flox/flox/TCre mice. (B) Peripheral-blood smear of a 16-week-old gp130flox/flox/TCre mouse exhibits polychromasia and anisopoikilocytosis of erythrocytes. Scale bar represents 20 μm. Objective, × 63/1.4 NA. (C) Splenomegaly in a 12-week-old gp130flox/flox/TCre mouse. Scale bar represents 1 cm. (D) Spleen of a 12-week-old gp130flox/flox/TCre mouse exhibits extramedullary hematopoiesis with abundant megakaryocytes. Bone marrow of a 16-week-old gp130flox/flox/TCre mouse exhibits normal cellularity in the endosteal niche (EN) adjacent to bone. In contrast, the vascular niche (VN) is markedly hypocellular, except for retention of normal numbers of megakaryocytes. Scale bar represents 50 μm. Objective, × 20/0.5 NA. (E) Quantification of blood cells of B-cell, T-cell, myeloid, and erythroid lineage expressing the indicated markers in bone marrow (BM), spleen, and thymus of gp130+/+/TCre and gp130flox/flox/TCre mice. Data represent the mean ± SD of 5 mice (age 12 weeks) in each genotype. *P < .05 and **P < .01 compared with gp130+/ +/TCre.
gp130flox/flox/TCre bone marrow retains progenitor cells. (A) Quantification of the indicated myeloerythroid colonies that developed from bone marrow or spleen cells suspended in methylcellulose. Data represent the mean ± SD of triplicate determinations and are representative of 3 independent experiments. *P < .001 compared with gp130+/+/TCre. (B) To determine the relative proportions of hematopoietic stem/progenitor cells, Lin- bone marrow cells were stained with PE–anti-Sca-1 and APC–anti-c-kit and then were analyzed by flow cytometry. The c-kitHiSca-1+, c-kitHiSca-1-, and c-kitLoSca-1-/Lo populations are gated with open boxes. The percentage of cells in each population is shown. (C) Quantification of the indicated myeloerythroid colonies that developed from 200 sorted Lin-c-kitHiSca-1+ cells suspended in methylcellulose. Data represent the mean ± SD of triplicate determinations from cells pooled from 5 mice per genotype and are representative of 2 independent experiments.
gp130flox/flox/TCre bone marrow retains progenitor cells. (A) Quantification of the indicated myeloerythroid colonies that developed from bone marrow or spleen cells suspended in methylcellulose. Data represent the mean ± SD of triplicate determinations and are representative of 3 independent experiments. *P < .001 compared with gp130+/+/TCre. (B) To determine the relative proportions of hematopoietic stem/progenitor cells, Lin- bone marrow cells were stained with PE–anti-Sca-1 and APC–anti-c-kit and then were analyzed by flow cytometry. The c-kitHiSca-1+, c-kitHiSca-1-, and c-kitLoSca-1-/Lo populations are gated with open boxes. The percentage of cells in each population is shown. (C) Quantification of the indicated myeloerythroid colonies that developed from 200 sorted Lin-c-kitHiSca-1+ cells suspended in methylcellulose. Data represent the mean ± SD of triplicate determinations from cells pooled from 5 mice per genotype and are representative of 2 independent experiments.
Bone marrow transplantation does not transfer or cure the hematopoietic defects in gp130flox/flox/TCre mice
The failure of gp130flox/flox/TCre marrow to initiate long-term cultures suggests that hematopoiesis requires gp130 expression in some type of marrow cell. To determine whether this cell type is transplantable, we first injected WT or gp130flox/flox/TCre marrow bearing the CD45.2 marker into lethally irradiated WT recipient mice bearing the CD45.1 marker. After 6 weeks, more than 95% of the circulating mononuclear cells expressed the CD45.2 marker, confirming a major donor contribution to hematopoiesis. Remarkably, gp130flox/flox/TCre bone marrow cells completely reconstituted hematopoiesis in irradiated WT mice (Table 2). We found no abnormalities in cells of peripheral blood, bone marrow, spleen, or thymus 6 weeks after transplantation. To further study the long-term reconstituting and self-renewal potential of gp130-deficient hematopoietic stem cells, we performed serial transplantation. Bone marrow cells recovered from CD45.1+ WT mice that received transplanted CD45.2+ WT or gp130flox/flox/TCre marrow were injected into a second group of irradiated CD45.1+ WT recipient mice. Like WT marrow, gp130flox/flox/TCre marrow successfully reconstituted myeloid, B-cell, and T-cell lineages in bone marrow and spleens of the secondary recipients 16 weeks after transplantation (Figure 6A). None of the mice that underwent transplantation developed hematologic disease. To confirm that gp130-deficient hematopoietic stem cells were responsible for reconstitution, we sorted Lin-c-kitHiSca-1+CD45.2+ multipotent progenitor cells, Lin-c-kitHiSca-1-CD45.2+ myeloid progenitor cells, and Lin-c-kitLoSca-1-/LoCD45.2+ lymphoid progenitor cells from the marrow of secondary recipients. PCR analysis of DNA demonstrated complete excision of the gp130 allele in cells from secondary recipients of gp130flox/flox/TCre marrow (Figure 6B).
We next injected WT marrow bearing the CD45.1 marker into irradiated WT or gp130flox/flox/TCre recipient mice bearing the CD45.2 marker. After 6 weeks, more than 95% of bone marrow, spleen, and peripheral-blood mononuclear cells expressed the CD45.1 marker, confirming successful reconstitution. Nevertheless, transplantation of WT marrow did not cure the hematopoietic abnormalities in gp130flox/flox/TCre recipients (Table 2) and did not reverse the development of bone marrow hypocellularity, splenomegaly, and thymic atrophy. Taken together, these data demonstrate that bone marrow dysfunction in gp130flox/flox/TCre mice is not attributable to the loss of gp130 in hematopoietic stem cells. Moreover, the gp130-responsive marrow cells that might rescue the hematopoietic defects are not easily engrafted in irradiated recipient mice. These cells are most likely endothelial cells of the bone marrow microenvironment.
gp130flox/flox/TCre bone marrow does not produce hematopoietic cells in long-term cultures. (A) Photomicrographs of long-term bone marrow cultures. The stromal-cell monolayer appears normal in the gp130flox/flox/Cre culture, but there are markedly reduced numbers of nonadherent hematopoietic cells. Magnification, × 100. (B) Quantification of myeloid cells produced in long-term bone marrow cultures. Data represent the mean ± SD of triplicate determinations and are representative of at least 3 independent experiments. *P < .05 and **P < .01 compared with gp130+/+/TCre. (C) Quantification of CAFC frequency in long-term bone marrow cultures plated on irradiated stromal cells of the indicated genotype. *P < .05 compared with bone marrow cells plated on gp130+/+/TCre stromal cells. (D) Immunocytochemistry of stromal cells in gp130flox/flox/TCre cultures probed with control or anti-gp130 antibodies. Scale bar represents 10 μm. (E) Immunoblots of lysates of stromal cells from long-term bone marrow cultures probed with antibodies to gp130 or Tie2. Data are representative of 3 independent experiments.
gp130flox/flox/TCre bone marrow does not produce hematopoietic cells in long-term cultures. (A) Photomicrographs of long-term bone marrow cultures. The stromal-cell monolayer appears normal in the gp130flox/flox/Cre culture, but there are markedly reduced numbers of nonadherent hematopoietic cells. Magnification, × 100. (B) Quantification of myeloid cells produced in long-term bone marrow cultures. Data represent the mean ± SD of triplicate determinations and are representative of at least 3 independent experiments. *P < .05 and **P < .01 compared with gp130+/+/TCre. (C) Quantification of CAFC frequency in long-term bone marrow cultures plated on irradiated stromal cells of the indicated genotype. *P < .05 compared with bone marrow cells plated on gp130+/+/TCre stromal cells. (D) Immunocytochemistry of stromal cells in gp130flox/flox/TCre cultures probed with control or anti-gp130 antibodies. Scale bar represents 10 μm. (E) Immunoblots of lysates of stromal cells from long-term bone marrow cultures probed with antibodies to gp130 or Tie2. Data are representative of 3 independent experiments.
Discussion
We have demonstrated that mice lacking gp130 in hematopoietic and endothelial cells developed bone marrow dysfunction with anemia that persisted despite compensatory extramedullary hematopoiesis. Long-term bone marrow cultures and transplantation experiments revealed that the hematopoietic defect resulted from gp130 deficiency in the bone marrow microenvironment rather than in the hematopoietic cells themselves. The microenvironmental defect appeared to result from gp130 deficiency in endothelial cells because expression of the TCre transgene was highly specific and, other than in hematopoietic cells, was restricted to endothelial cells. These data suggest that bone marrow endothelial cells support hematopoiesis in vivo by responding to signals from IL-6 family cytokines.
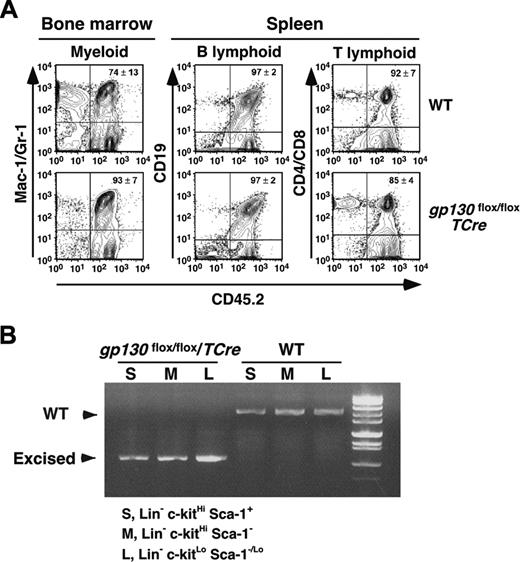
Serial transplantation of gp130-deficient bone marrow reconstitutes hematopoiesis in irradiated mice. (A) Bone marrow cells recovered from CD45.1+ WT mice that underwent transplantation with CD45.2+ WT or gp130flox/flox/TCre cells were transplanted into irradiated CD45.1+ WT secondary recipients. After 16 weeks, flow cytometry was used to determine the level of CD45.2 donor-cell contribution to the myeloid, B-lymphoid, and T-lymphoid populations in the reconstituted bone marrow. (B) The c-kitHiSca-1+, c-kitHiSca-1-, and c-kitLoSca-1-/Lo populations were sorted from the reconstituted bone marrow. Genomic DNA from each population was subjected to PCR analysis (Figure 1A-B) to identify the WT gp130 allele or the excised floxed gp130 allele. DNA was pooled from 4 mice for each genotype. Data are representative of 2 independent experiments.
Serial transplantation of gp130-deficient bone marrow reconstitutes hematopoiesis in irradiated mice. (A) Bone marrow cells recovered from CD45.1+ WT mice that underwent transplantation with CD45.2+ WT or gp130flox/flox/TCre cells were transplanted into irradiated CD45.1+ WT secondary recipients. After 16 weeks, flow cytometry was used to determine the level of CD45.2 donor-cell contribution to the myeloid, B-lymphoid, and T-lymphoid populations in the reconstituted bone marrow. (B) The c-kitHiSca-1+, c-kitHiSca-1-, and c-kitLoSca-1-/Lo populations were sorted from the reconstituted bone marrow. Genomic DNA from each population was subjected to PCR analysis (Figure 1A-B) to identify the WT gp130 allele or the excised floxed gp130 allele. DNA was pooled from 4 mice for each genotype. Data are representative of 2 independent experiments.
Our TCre transgenic mice quantitatively excised a floxed lacZ reporter gene in all endothelial cells, confirming previous reports.20,29,30 Moreover, the mice efficiently deleted the reporter gene in all lineages of hematopoietic cells; this excision remained uniform and complete in mice that reached at least 10 months of age. During development, primitive hemangioblasts are thought to generate blood cells and endothelial cells, and the first hematopoietic stem cells bud from specialized patches of hemogenic endothelium.12-16 Tie2, a receptor for angiopoietin and a known endothelial-cell marker, is transiently expressed in the earliest stem/progenitor cells required for hematopoiesis and is indeed required for definitive hematopoiesis.14 It has been suggested that some stem cells produce blood cells during early life and are then replaced by later cohorts of stem cells.36 However, our findings demonstrate that the Tie2 locus is active in stem/progenitor cells that direct the formation of all types of blood cells throughout adult life.
Consistent with the reporter gene experiments, the crossing of Tie2-Cre mice with gp130flox/flox mice resulted in nearly complete excision of the floxed gp130 allele in endothelial cells and hematopoietic cells. Importantly, gp130 was not expressed in either endothelial cells or hematopoietic cells of bone marrow. Unlike the embryonic lethality observed in mice lacking gp130 in all cells,3 mice lacking gp130 only in hematopoietic and endothelial cells developed normally and thrived for the first 4 weeks after birth. Thereafter, the mice developed dysfunctional bone marrow and experienced a general loss of progenitors of all cell lineages except megakaryocytes. Compensatory extramedullary hematopoiesis in the spleen caused splenomegaly and elevated leukocyte counts in the peripheral blood but did not prevent the anemia. The inability of long-term bone marrow cultures to produce blood cells confirmed an intrinsic defect in the bone marrow. Because clonable stem/progenitor cells in bone marrow were not diminished, this defect appeared to reside in the adherent stromal cells rather than in the hematopoietic stem cells. The defect was probably in the minor endothelial-cell population because gp130 expression was preserved in the more common stromal cells, which are fibroblasts, adipocytes, or smooth muscle cells.37
Although considerable evidence indicates the contribution of other mesenchymal stromal cells to hematopoiesis,37 the role of bone marrow endothelial cells has been less well studied. In vivo, the adhesion of hematopoietic stem cells to osteoblasts in the bone marrow endosteal niche supports their quiescence and survival.38-40 Matrix metalloproteinase 9–mediated release of soluble kit-ligand shifts these cells to the vascular niche, which permits differentiation and proliferation into multiple lineages.41 In vitro, endothelial cells derived from early fetal tissue or from adult bone marrow or other organs support long-term hematopoiesis.12,13,17,18 In vivo, chemokine-mediated adhesion of megakaryocytes to bone marrow endothelial cells in the vascular niche contributes to thrombopoiesis.19 Our data provide the first evidence that bone marrow endothelial cells contribute to multilineage hematopoiesis in vivo. Thus, endothelial cells of the vascular niche appear to use a gp130-dependent mechanism to foster differentiation and proliferation of erythroid, myeloid, and lymphoid cells in the bone marrow. In contrast, the persistent association of megakaryocytes with bone marrow sinusoids of gp130flox/flox/TCre mice indicates that endothelial cells do not require gp130 to support thrombopoiesis. Inactivation of the gp130 gene in adult mice has been achieved by crossing gp130flox/flox mice with transgenic mice expressing Cre under the control of an interferon-responsive promoter.4 Despite efficient deletion of gp130 in hematopoietic cells and many other tissues, those mice developed a milder hematopoietic defect than we observed. This milder phenotype probably resulted from incomplete excision of the gp130 allele in endothelial cells of mice after interferon administration, as indicated by persistence of the WT allele in Southern blots of lung DNA.4
Our bone marrow transplantation experiments demonstrated that mice lacking gp130 only in hematopoietic cells produced normal numbers of blood cells for sustained periods. These data extend previous observations that transplanting gp130-deficient fetal liver hematopoietic cells into irradiated WT mice restores hematopoiesis for at least 9 weeks.42 The transplantation results contrast with the ability of IL-6 to regulate the proliferation and differentiation of hematopoietic cells in some in vitro assays.6-10 Mice lacking IL-6 or a related cytokine, leukemia inhibitory factor, exhibit abnormal hematopoiesis.43,44 Although this observation is consistent with a requirement for IL-6 stimulation of hematopoietic stem/progenitor cells in vivo, it does not address whether IL-6 acts directly on hematopoietic cells or indirectly through other bone marrow cell types. It remains possible that blood-cell formation requires gp130-dependent signaling in hematopoietic stem/progenitor cells under conditions of stress, which we did not evaluate. Competitive repopulation experiments might help address this possibility. Positive and negative signals may be delivered through gp130 because mice expressing gp130 that cannot activate Stat proteins have increased stem/progenitor cells in bone marrow and spleen.45 Recent studies indicate that IL-6 augments the ability of stromal cells to support hematopoiesis in long-term bone marrow cultures.46 Our findings suggest that IL-6 acts in part by gp130-dependent signaling in stromal endothelial cells. Mice lacking the oncostatin M receptor β subunit, which pairs with gp130, have mild anemia and thrombocytopenia, in part because of loss of the receptor in the marrow environment.47 Our results suggest that the environmental defect might have developed in the endothelial cells of these mice. It remains to be determined how gp130-dependent signaling enables bone marrow endothelial cells to support hematopoiesis.
Although we observed normal hematopoiesis in irradiated WT mice that underwent reconstitution with gp130-deficient bone marrow, we could not rescue the hematologic abnormalities in irradiated gp130flox/flox/TCre mice reconstituted with WT bone marrow. Thus the gp130-responsive environmental cells, most likely endothelial cells, detected in long-term bone marrow cultures are not readily transplantable in vivo. This observation may be relevant to hematologic disorders that are refractory to bone marrow transplantation.48,49 Adult bone marrow has attracted considerable attention as a potential source of multipotential stem cells for the regeneration of diverse tissues.50,51 Although hemangioblasts were originally thought to be restricted to early embryos, recent studies suggest that they are also present in adult bone marrow.52-56 If so, the hemangioblasts in WT marrow could not readily replace the gp130-deficient endothelial cells that arose from Tie2-expressing progenitors in recipient mice. It will be important to determine whether other sources of stem cells can restore marrow function in these mice.
Our findings provide evidence that interactions of IL-6 family cytokines with endothelial cells contribute to hematopoiesis. Thus, signals in the bone marrow environment enable endothelial cells to support the differentiation and proliferation of hematopoietic cells, further supporting the intimate relationship between these cell types.
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-02-0671.
Supported by National Institutes of Health grants HL 54502 (R.P.M.) and AI 20069 and AI 45864 (P.W.K.).
L.Y. and T.Y. contributed equally to this study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Werner Müller for providing gp130flox/flox mice and Masashi Yanagisawa for providing Tie2-Cre mice. We also thank Zoltan Laszik, Jonathan Miner, Yang Song, Sophia Gregory, and Patricia Ann Clark for advice on experimental protocols. Tissue processing and microscopy were performed in the imaging core facility of the Oklahoma Medical Research Foundation.