Abstract
There is currently no standard salvage chemotherapy regimen in patients with relapsed/ refractory DLBCL. We have previously shown that GEM-P is an effective salvage regimen in patients with relapsed/refractory lymphoma; 10 patients in this study had DLBCL (
Br J Cancer 2005;92:1352–7
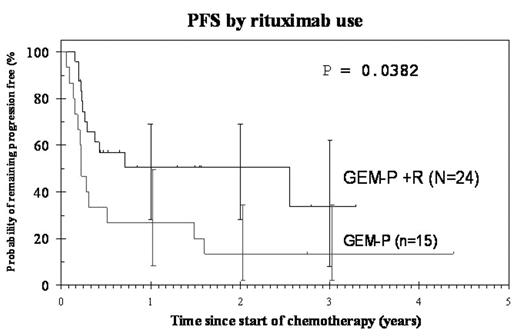
). The aim of this study was to evaluate the combination of GEM-P in a larger subset of DLBCL patients. This has not been reported before. Between 1/01 and 3/05, 39 (10 patients had transformed lymphoma) heavily pre-treated relapsed and refractory DLBCL patients received GEM-P; 24 (61%) of these patients also received Rituximab (R). The median age was 49 y (range, 18–68); 28 males; 64% patients had Stage III/IV disease; 69% had IPI ≥2. The median time from diagnosis to start of GEM-P ± R was 17 mo (range, 2mo-7 yrs). All patients had previously received an anthracycline-based regimen. The disease status at start of GEM-P ± R was: 24 patients were in 1st relapse, 7 had primary refractory disease, 5 were in 2nd relapse and 3 in 3rd relapse. Median number of cycles of GEM-P ± R received were 2 (range, 1–4). The overall response rate (RR) was 59% (95% CI 42.1–74.4). 11 (28%) patients attained complete response, 12 partial response and 15 patients did not respond; 1 patient died of progressive disease. The median response duration to GEM-P ± R in 23 responding patients was 332 days (range, 36–1530) compared to 170 days (range, 26–2264) in 32 responding patients to 1st-line chemotherapy. Of the 24 patients who received R (not randomised; given when available via NHS funding network) with GEM-P, the overall RR was 67% (95% CI 45–84) compared to 47% (95% CI 21–73) in those who did not receive R (P=0.2). Eight patients were consolidated with high-dose therapy which was an autologous stem cell transplant (ASCT) in 7 (all are alive and well except one who relapsed 4 mo later and died of progressive disease) and a reduced intensity conditioning matched unrelated donor transplant in 1 patient who died of transplant-related complications. The remaining patients did not undergo an ASCT due to treatment failure (n=16), cardiac insufficiency (n=2) and subclavian vein thrombosis (n=1). ASCT was not planned in the other 12 patients because of indolent histology (n=2), inadequate stem cell collection (n=1), lost-to follow-up (n=1), previous high-dose therapy (n=3) and stage 1 disease (n=1), physicians decision (n=1), patients choice (n=1) multiply relapsed disease (n=2). Progression-free survival (PFS) and overall surveival (OS) are shown in the Table. 23 patients are alive at a median follow-up of 543 days (range, 76–1598). In conclusion, GEM-P is an effective salvage regimen with long response duration in patients with relapsed or refrcatory DLBCL. The additional benefit with Rituximab on outcome warrants further studies in new and relapsed patients with DLBCL in a prospective, randomised trial.Survival
| . | OS . | PFS . |
|---|---|---|
| GEM-P (n=15) | 3-y 40%(95%CI 16–63) | 1-y 27%(95%CI 8–49) |
| GEM-P +R (n=24) | 3-y 65%(95%CI 40–82) | 1-y 51% (95%CI 28–69) |
| GEM-P ±R (n=39) | 3-y 53.4% (95% CI 35–69) | Median 157d (95% CI 42–271) |
| . | OS . | PFS . |
|---|---|---|
| GEM-P (n=15) | 3-y 40%(95%CI 16–63) | 1-y 27%(95%CI 8–49) |
| GEM-P +R (n=24) | 3-y 65%(95%CI 40–82) | 1-y 51% (95%CI 28–69) |
| GEM-P ±R (n=39) | 3-y 53.4% (95% CI 35–69) | Median 157d (95% CI 42–271) |
PFS by rituximab use
Author notes
Corresponding author
2005, The American Society of Hematology
2005