Abstract
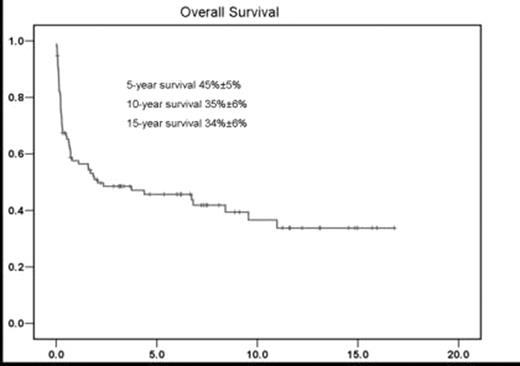
We report here the long-term outcome of 95 patients with myelodysplastic syndromes (MDS) who underwent allogeneic stem cell transplantation (SCT) following busulfan-containing regimens. The median age was 43 (range:3–62) and 55 patients were male. Eighty-four patients had primary MDS, 11 had secondary MDS. Twenty patients received induction therapy for leukemic transformation prior to SCT. Pre-transplantation cytogenetic studies were available in 82 patients, and were categorized according to the international prognostic scoring system (IPSS) karyotype groups. The bone marrow biopsies/aspirates were reviewed and classified according to WHO classification (Table 1). Sixty one patients received related donor transplant (54 patients were 6/6 HLA-matched), and 34 patients received unrelated donor transplant (26 patients were 6/6 HLA-matched). Ninty patients received consecutive administration of busulfan 4 mg/kg/day orally for 4 days, cytosine arabinoside 2 g/m2 IV every 12 hours for 4 doses, and cyclophosphamide 60 mg/kg IV daily for 2 days (BAC); and 5 received other busulfan-containing regimens. GHVD prophylaxes were either cyclosporine (75 patients) or tacrolimus (12 patients) regimens, and 8 patients received other regimens. The median duration of transplant hospitalization was 34 days (range:13–121 days). Grade I–IV regimen-related toxicities (RRT) included GI (diarrhea, 32; stomatitis, 38), liver (28), cardiac (19), kidney (15), lung (25), neurological (7) and skin (5). Acute and chronic GHVD occurred in 48 (50%) and 21 (22%) patients, respectively. With a median follow up of survivors of 6.7 years, the Kaplan-Meier estimates for overall survival were 45%±5% (±SE), 35%±6% and 34%±6% at five, ten and fifteen years, respectively (Figure 1). Fifty-four patients died. The causes of death were GHVD (14 patients), relapse (12), infection (11), pulmonary toxicity (3), multiorgan failure (3), hepatic (2), renal (1), cardiac (1) and other causes (7). Non-relapse mortality at 100 days and three years was 22% and 40% respectively. The long-term follow up of patients receiving high-dose busulfan-based regimens for allogeneic SCT in this study indicated durable overall survival with acceptable toxicity.
Patient Characteristics
| BAC: Busulfan/Ara-C/Cyclophosphamide, BU: Busulfan, CY: Cyclophosphamide, TLI: Total lymphoid irradiation, FLU: Fludarabine, ATG: Antithymocyte globulin, CS: Cyclosporin | |
| # of Patients | 95 |
| Median Age (range) | 43 (3–62) |
| Male/Female | 55/40 |
| Cytogenetic risk (IPSS) | |
| Good/Intermediate/Poor | 38/14/30 |
| Not available | 13 |
| Disease Duration Median (range) | 182 (40–1944) |
| Etiology | |
| Denovo/Secondary | 84/11 |
| Donor | |
| HLA-identical sibling | 54 |
| HLA-nonidentical sibling | 7 |
| Unrelated HLA-identical | 26 |
| Unrelated HLA-nonidentical | 6 |
| WHO Classification | |
| RA | 5 |
| RARS | 2 |
| RAEB-1 | 4 |
| RAEB-2 | 16 |
| RCMD | 14 |
| CMML | 4 |
| AML | 20 |
| MDS-U | 6 |
| Not available | 24 |
| Source of stem cells | |
| BM/PBSC | 69/26 |
| GVHD prophylaxis | |
| CSA/Methylprednisolone | 32 |
| CSA/Methotrexate | 43 |
| Tacrolimus/Mycelophenolate | 7 |
| Tacrolimus/Methotrexate | 5 |
| Busulfan regimen | |
| BAC | 90 |
| BU/ATG/CS/TLI | 2 |
| BU/CY | 1 |
| BU/FLU/TLI | 2 |
| BAC: Busulfan/Ara-C/Cyclophosphamide, BU: Busulfan, CY: Cyclophosphamide, TLI: Total lymphoid irradiation, FLU: Fludarabine, ATG: Antithymocyte globulin, CS: Cyclosporin | |
| # of Patients | 95 |
| Median Age (range) | 43 (3–62) |
| Male/Female | 55/40 |
| Cytogenetic risk (IPSS) | |
| Good/Intermediate/Poor | 38/14/30 |
| Not available | 13 |
| Disease Duration Median (range) | 182 (40–1944) |
| Etiology | |
| Denovo/Secondary | 84/11 |
| Donor | |
| HLA-identical sibling | 54 |
| HLA-nonidentical sibling | 7 |
| Unrelated HLA-identical | 26 |
| Unrelated HLA-nonidentical | 6 |
| WHO Classification | |
| RA | 5 |
| RARS | 2 |
| RAEB-1 | 4 |
| RAEB-2 | 16 |
| RCMD | 14 |
| CMML | 4 |
| AML | 20 |
| MDS-U | 6 |
| Not available | 24 |
| Source of stem cells | |
| BM/PBSC | 69/26 |
| GVHD prophylaxis | |
| CSA/Methylprednisolone | 32 |
| CSA/Methotrexate | 43 |
| Tacrolimus/Mycelophenolate | 7 |
| Tacrolimus/Methotrexate | 5 |
| Busulfan regimen | |
| BAC | 90 |
| BU/ATG/CS/TLI | 2 |
| BU/CY | 1 |
| BU/FLU/TLI | 2 |
Figure
Author notes
Corresponding author