Abstract
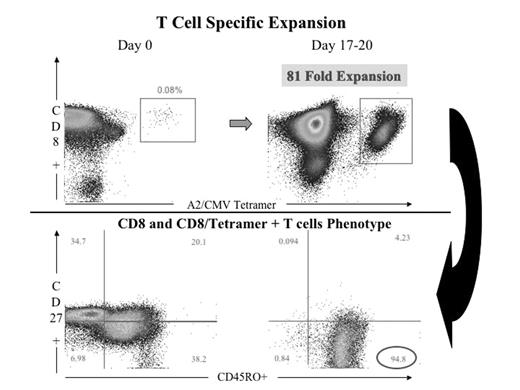
One of the main problems of T cell mediated immunotherapy in delivering significant clinical impact and benefit to patients with malignant diseases and life threatening viral infections is the expansion of adequate numbers of functional antigen specific cytotoxic T cells. The current approaches for expanding T cells possess significant drawbacks in terms of timing, reproducibility and reliability. Many if not all these approaches rely on ex-vivo cell manipulation, which often leads to short T cell survival in-vivo after infusion. In-vivo artificial systems should be the ideal. There is no artificial APC system capable of both ex-vivo and, more importantly, in-vivo antigen specific T cell expansion. In order to address this we have developed a novel artificial nanotechnology system capable of priming and expanding antigen specific T cells in-vivo. As defined by the NIH, nanotechnology uses nanoscale injectable, targeted and traceable devices capable of important immunological/clinical functions. This nano-system was constructed using the latest generation of nanoscale immuno liposomes (100nm; 50 times smaller than average cells and same size as most human viruses), approved for in-vivo human use since they are non-toxic, biodegradable, avoid fast recognition by the reticulo-endothelial system, are safe in terms of size, have good stability and favourable pharmacokinetic behaviour for safe in-vivo trafficking. We have coated these liposomes with an optimised number of MHC Class I / peptide complexes and a specific and selected range of ligands for adhesion (ICAM-1), early activation (CD28, CD27), late activation (4-1BB) and survival receptors (CD40L). We have made these immuno liposomes traceable, either via a fluorescent lipid or iron oxide nano particles (13nm each), which make them traceable in vivo using Magnetic Resonance Imaging. Production of this system in a ready to use form is achievable in less than 48 hrs. We are currently working on an HLA-A2 transgenic mouse model to validate in-vivo behaviour of the system. After ex-vivo stimulation with this artificial system (using CMV pp65 as model antigen), we have measured successful expansions of high antigen specific T cell numbers (55 to 80 fold) in CMV positive individuals, which are superior when compared with other systems such as DCs (30 fold), beads (non antigen specific) and soluble tetramers and antibodies (30 fold). Expanded T cells are functional; they produce INF-γ and are predominantly of effector-memory and memory phenotype. We have demonstrated by double fluorescent staining that these liposomes are recognised directly on CD8+ T cells in an antigen specific fashion and also indirectly by being incorporated on the surface of the natural APCs as exosomes do. When tested in naive individuals, this system is also capable of priming naive T cells without additional adjuvants, as other APC systems use. In conclusion we have established the optimal conditions for an efficient artificial APC system, which embodies a powerful, controllable and superior approach with enormous potential for T cell immunotherapy in vivo.
Author notes
Corresponding author