Abstract
The International Prognostic Scoring System (IPSS) requires cytogenetic data to evaluate risk in MDS. Cytogenetic data were not available for all patients from the CALGB trial 9221 (
Silverman et al, JCO 2002; 20:2429
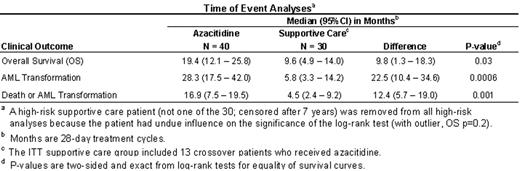
). Therefore, we developed an alternative prognostic model that identified a homogeneous subgroup of high-risk MDS patients based on the following risk factors: percent marrow blasts, number of cytopenias, age, gender, FAB classification, and time since diagnosis (IPSS used data at time of diagnosis; CALGB 9221 used data at time of randomization). The model was validated using 2,318 patients from the MDS Registry, Düsseldorf. Using these baseline prognostic factors, we predicted survival for each of the 191 CALGB patients, identified a high-risk subgroup with a survival prognosis of ≤ 1.2 years (the IPSS INT-2 survival median), and compared azacitidine to supportive care. Patients were analyzed as randomized (azacitidine or supportive care) according to the intention-to-treat (ITT) principle. The two groups had similar demographic and disease characteristics. All 70 high-risk patients were followed until death. There was a statistically significant difference in overall survival curves between the azacitidine and supportive care groups (p=0.03). The one-year survival rate was 63% (95% CI: 47 to 78%) in the azacitidine group and 37% (95% CI: 19 to 54%) in the supportive care group (p=0.03; 26% difference with a 95% CI of 3 to 49%). The two-year survival rate was 35% (95% CI: 20 to 50%) in the azacitidine group and 13% (95% CI: 1 to 25%) in the supportive care group (p=0.03; 22% difference with a 95% CI of 3 to 41%). Similarly, statistically significant differences in time to AML transformation, and death or AML transformation, were observed. Transfusion independence was defined as free from transfusions for at least 2 months. Among patients who were RBC transfusion dependent at baseline, a significantly greater number of patients from the azacitidine group (11/25, 44%) compared with patients from the supportive care group (1/14, 7%) achieved transfusion independence (p=0.03; 37% difference with a 95% CI of 7 to 59%). Additionally, patients in the azacitidine group with baseline transfusion independence experienced significantly prolonged duration of RBC (p<0.0001) and platelet (p=0.0002) transfusion independence compared with those in the supportive care group. Use of this prognostic model has identified a subgroup of high-risk MDS patients who significantly benefited from treatment with azacitidine.Author notes
Corresponding author
2005, The American Society of Hematology
2005