Abstract
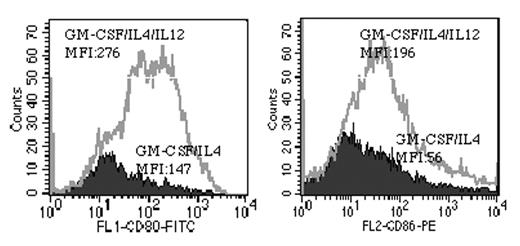
To generate highly AML reactive autologous CTL, the optimal protocol of growth factor combination and sequence of administration were screened and compared in an AML-DC culture system aimed to induce AML cell DC differentiation, activate and expand naïve T cells against AML; and simultaneously activate and expand possible rare existing AML reactive memory T cells. Primary AML peripheral blood or marrow cells containing more than 90% AML blasts were cultured in 96 well plates. Every single well was regarded and treated as an independent culture. Various growth factor combinations of GM-CSF, IL2, IL-3, IL-4, IL7, IL12, CD40L, TNF-α, anti-CTLA-4 mAb were compared for efficiency of AML cell DC differentiation induction and autologous T cell activation in 7 day culture. Significantly enhanced CD80/CD86 expression and total cell number (include both AML-DC and T cells, see attached figure) in the culture was observed when GM-CSF/IL-4 combined with IL-12. The T cells in each well were than expanded with high dose IL-2 (6000 u/ml) and CTL activity against autologous AML were examined by both 51Cr release assay and culture supernatant IFN-γ concentration assay. Multiple wells of AML cell culture from the same patient with the same cell number and culture condition exhibited substantial functional variation demonstrated by MHC-restricted autologous AML cell killing (3 - 70% specific lysis) and IFN-γ secretion (25 - 2994 pg/ml), indicating that the T cells randomly put in the independent wells vary greatly in efficiency of AML antigen recognition and activation. IL-12 alone exerted significant enhanced IFN-γ secretion in 4 of 5 patients. IL-12 combined with GM-CSF/IL-2/IL-4/IL-7 enhanced IFN-γ secretion compared with IL-12 alone in 3 of 5 patients. CTLA-4 blockade further significantly enhanced T cell activity in 5 of 5 patients. Highly active CTL lines were selected from high IFN-γ secretion wells and rapidly expanded using OKT-3/CD28 coated, mitomycin-c treated autologous AML cells. After expansion, the highly active lines still maintained high autologous AML cell killing compared to the low active lines. A therapeutic quantity of AML reactive autologous T cells (20.2±30.1)x109, (n=5) can be obtained after 6 weeks of culture from (6.4±2.9)x107 AML PBMC. This study demonstrated AML cells cultured in IL-12 or IL-12 combined with GM-CSF/IL-2/IL-4/IL-7/anti-CTLA4 mAb activated the potential of the patient immune system and may maximize the possibility of AML reactive CTL generation for adoptive immunotherapy.
Corresponding author