Abstract
Mutation of p53 is a rare event in multiple myeloma, but it is unknown if p53 signaling is functional in myeloma cells, and if targeted nongenotoxic activation of the p53 pathway is sufficient to kill tumor cells. Here, we demonstrate that treatment of primary tumor samples with a small-molecule inhibitor of the p53–murine double minute 2 (MDM2) interaction increases the level of p53 and induces p53 targets and apoptotic cell death. Significantly, given the importance of the bone marrow microenvironment for the support and drug resistance of myeloma cells, tumor cells undergo effective apoptosis also in the presence of stromal cells, which themselves appear to tolerate exposure to nutlin-3. The in vitro toxicity of nutlin-3 was similar to that of the genotoxic drug melphalan. Because nutlin-mediated p53 activation is not dependent on DNA damage, MDM2 antagonists may help to avoid or reduce the severe genotoxic side effects of chemotherapeutic agents currently used to treat multiple myeloma. Therefore, MDM2 antagonists may offer a new treatment option for this disease.
Introduction
Multiple myeloma (MM) is an incurable plasma cell tumor that accounts for about 1% of human cancers.1 Neoplastic transformation is assumed to involve certain primary genetic lesions, such as translocations between immunoglobulin enhancers and oncogenes,2 which are subsequently complemented by secondary events that lead to activation of growth and survival pathways and to disruption of apoptotic signaling.2,3 The principal site of tumor growth and propagation is the bone marrow (BM) compartment, where cells from the microenvironment are thought to supply growth and survival factors and to provide contact-mediated drug resistance.4-6
The tumor suppressor p53, known as “the gatekeeper” of cellular integrity, orchestrates the induction of cell cycle arrest or apoptosis (or both) in response to diverse stress factors.7,8 p53 is a transcription factor that can activate multiple genes involved in cell cycle regulation, DNA repair, and programmed cell death.8 In response to cellular stress, such as DNA damage induced by genotoxic drugs, the p53 level is elevated by a posttranslational mechanism that increases the half-life of the protein.9 In the absence of stress, p53 is tightly controlled by its negative regulator murine double minute 2 (MDM2). Association of the 2 proteins impairs p53 activity due to inhibition of its transactivation domain by MDM2 as well as MDM2-mediated ubiquitin-dependent degradation in the proteasome.10 Because the MDM2 gene is itself a transcriptional target of p53, both proteins form an autoregulatory feedback loop that keeps their levels low in unstressed cells.
The central role of p53 in tumor suppression is underscored by the fact that it is the most frequently mutated protein in human cancer. The prevalence of p53 mutations, however, differs considerably between tumor types and stages of cancer, and approximately 50% of all human tumors retain wild-type p53.11 Activation of p53 in these tumors may lead to cell cycle arrest or apoptosis or both and may thus offer a therapeutic benefit.12,13 In MM, mutation14 or deletion15-19 of the gene for p53 is rarely detected at diagnosis, although both become more frequent in advanced disease.20,21 Therapeutic activation of p53 might therefore be particularly suitable for the treatment of MM. However, it is currently unknown if p53 signaling is functional in primary myeloma cells and if activation of p53 could lead to their demise. Here, we use nutlin-3, a recently developed small-molecule activator of p53, that efficiently disrupts the p53-MDM2 interaction,22 to probe the functionality of the p53 pathway in a range of primary myeloma samples and in MM cell lines. Because the BM microenvironment has been shown to be critical for the malignant growth, survival, and drug resistance of MM cells, we also analyzed the effect of p53 activation on nonmalignant cells of the BM and investigated the influence of BM stromal cells (BMSCs) on nutlin-induced apoptosis of cocultured tumor cells. Lastly, because many cytotoxic drugs exert at least some of their apoptotic effect through DNA damage-mediated activation of p53, we compared the effectiveness of currently used cytotoxic drugs with that of nutlin-induced nongenotoxic activation of p53. Our results support the utility of MDM2 antagonists for the treatment of MM.
Patients, materials, and methods
Cell lines
Human MM cell lines ANBL-6, MM.1s, OPM-2, and RPMI-8226 were maintained in RPMI 1640, supplemented with 10% fetal calf serum (FCS; both from Biochrom, Berlin, Germany), 100 U/mL penicillin, 100 μg/mL streptomycin (both from PAN Biotech, Aidenbach, Germany), 2 mM glutamine, and 1 mM sodium pyruvate (both from Gibco, Karlsruhe, Germany). MM cell lines INA-6 and NCI-H929 were kept with 20% and 15% FCS, respectively; cultures of the latter contained 0.0004% 2-mercaptoethanol. ANBL-6 and INA-6 cells were grown in the presence of 2 ng/mL recombinant interleukin 6 (IL-6). All cells were grown at 37°C and 5% CO2.
Preparation of primary myeloma cells and BMSCs
Primary myeloma cells and BMSCs were obtained from BM aspirates after Ficoll density gradient centrifugation followed by separation of myeloma cells with CD138 microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany). Cytospins of purified samples stained according to the Pappenheim method routinely confirmed plasma cell morphology for more than 95% of cells. Purified myeloma cells were seeded onto BMSCs or kept in RPMI medium (20% FCS) supplemented with 10 ng/mL IL-6. BMSCs were derived from the column run-through after culture in Dulbecco modified Eagle medium (DMEM) supplemented with 20% FCS. BMSCs for coculture studies were obtained from 9 patients with MM and maintained in culture for up to 5 months. BMSCs used to assess the effects of nutlin-3 were derived from 3 different patients with MM and had not been kept in culture for more than 2 months. All primary cells were obtained from routine diagnostic samples after informed consent was provided by the patients and permission given by the Charité Ethics Committee, University Medicine Berlin Institutional Review Board. Prior to the study, informed consent was provided in accordance with the Declaration of Helsinki.
Isolation of primary CD34+ cells and colony formation assay
CD34+ cells were collected by progenitor cell apheresis and immunomagnetic selection according to standard operating procedures. All patients gave their written and informed consent. The immunomagnetic selection of CD34+ cells was performed in a semiautomated fashion (Isoflex 300i system software version 1.1.3; Baxter, Munich, Germany) as described previously.23 Cryopreserved CD34+ cells that were used for the analysis of drug effects were thawed and cultured overnight in RPMI medium in the presence of BMSCs. Following density gradient centrifugation with Opti-Prep (Axis-Shield, Oslo, Norway) and reclamation of the live cell fraction, 1 × 104 cells were seeded onto BMSCs (1.5 × 103 cells/well; 96-well plates) and drugs added as described in “Coculture of cells with BMSCs and application of drugs.”
Hematopoietic colony formation was analyzed using standard methyl-cellulose granulocyte-macrophage colony-forming unit (CFU-GM) assays (StemCell Technologies, Meylan, France). Enriched CD34+ cells were seeded at 1.5 × 103 cells/mL and incubated for 21 days in the presence of 8 μM nutlin-3 or an adequate amount of the solvent dimethyl sulfoxide (DMSO), respectively.
In vitro generation of CD38++/CD20- plasmablastic cells
CD19+ B lymphocytes were isolated as described previously.24 The differentiation of CD19+ cells to a plasma cell phenotype was induced by culturing in RPMI (10% FCS, 20 μM 2-mercaptoethanol, 10 mM glutamate, 100 U/mL penicillin, 100 μg/mL streptomycin) with a combination of CpG 2006 (3 μg/mL; TIB MOLBIOL, Berlin, Germany), F(ab)2 fragments of goat anti–human immunoglobulin (2.5 mg/mL; Jackson ImmunoResearch, West Grove, PA), IL-2 (50 U/mL), IL-10 (50 U/mL), IFN-α (200 U/mL), and IFN-β (200 U/mL; AMS Biotechnology, Wiesbaden, Germany). This activation system produced about 35% plasmablastic CD38++/CD20- cells after 7 days in culture. Cells were stained with anti-CD20–fluorescein isothiocyanate (FITC) and anti-CD38–allophycocyanin (APC; BD Biosciences, Heidelberg, Germany), and CD38++/CD20- plasmablastic cells isolated by fluorescence-activated cell sorting (FACS). About 30% of CD38++ cells were also positive for CD138. Cells were subsequently cocultured with BMSCs in RPMI (20% FCS) and drug effects measured after 3 days.
Coculture of cells with BMSCs and application of drugs
Primary BMSCs were taken from cultures with passage numbers no higher than 5, seeded in DMEM at a density of 1.5 × 103 cells/well (96-well plate), and given time to attach for at least 1 day. The medium was exchanged for 50 μL RPMI and 1 × 104 (myeloma, stem, or plasmablastic) cells in 50 μL RPMI were added per well and left to settle overnight. Then, 100 μL of the applicable, doubly concentrated drug solutions was added, and the extent of cell death determined after 3 to 5 days. Control incubations with DMSO (0.1%) were always included, and the relative survival in each assay calculated as percentage of live cells relative to the live cell fraction in the control (= 100%). Experiments with less than 35% viable cells in the control were not evaluated.
MM cell lines NCI-H929 and MM.1s were cultured in their respective media and cell death in response to drugs measured after 3 and 4 days, respectively. To assess the combined effects of melphalan and nutlin-3a, cells were treated as if for sole incubation with melphalan, and after 2 days half of the medium exchanged for the same volume containing twice the desired concentration of nutlin-3, plus the applicable concentration of melphalan. Cell death was measured 5 days after melphalan addition.
Apoptosis assay
Cell death was determined through annexin V–FITC/propidium iodide (PI) staining as detailed in Chatterjee et al.25
INA-6 survival assay
BMSCs were seeded in 96-well plates (1.5 × 103/well) and drugs added after 2 days. After 5, 10, 15, 22, and 27 days, the medium was exchanged for 150 μL fresh RPMI without drugs, and 1 × 104 INA-6 cells, which had been thoroughly washed in phosphate-buffered saline (PBS), added with 50 μL medium. Control incubations of INA-6 without BMSCs and with or without 2 ng/mL IL-6 were included to confirm normal viability and extensive apoptosis after IL-6 starvation, respectively. Viability of INA-6 cells was determined after 3 days in culture.
Western analysis
Procedures followed those detailed in Chatterjee et al.25 Because only small numbers of primary myeloma cells were available for Western analysis, equal numbers of freshly purified cells (between 8 × 104 and 1.5 × 105 cells/assay) were incubated in RPMI medium supplemented with 10 ng/mL IL-6 and treated overnight with 10 μM nutlin-3a, nutlin-3b, or DMSO. Cells were harvested into polymerase chain reaction (PCR) tubes and each complete sample lysed and loaded onto NuPAGE gradient gels (4%-12%; Invitrogen, Karlsruhe, Germany). Blots were cut so that antibody staining could be done in parallel. Staining against β-actin was performed on the same strip that had been used to assess p53 levels and served to confirm that this methodology provided similar amounts of protein within each single patient sample.
TP53 mutation analysis
Mutation analysis for exons 5 to 8 of the gene for p53 in myeloma cell lines was performed as described.26,27 To determine cDNA sequence, 2 overlapping fragments spanning the p53 coding region were amplified by PCR, purified, and sequenced with the same primers that were used in the respective PCR. The primer sequences were: 5-AGACTGCCTTCCGGGTCACT (-23 to -4); 5-TAGGGCACCACCACACTATG (659-640); 5-ATGGCCATCTACAAGCAGTC (477-496); and 5-CAAGAAGTGGAGAATGTCAG (1198-1179). Numbers refer to the human p53 cDNA sequence.
Reagents
Primary antibodies were anti-MDM2 (Santa Cruz Biotechnology, Heidelberg, Germany; SC-965), anti-p53 (CM042A; Biocare Medical, Walnut Creek, CA), anti-p21WAF1 (Santa Cruz Biotechnology; SC-397), anti–β-actin (Sigma, Deisenhofen, Germany; A5316). Secondary antibodies: anti–rabbit horseradish peroxidase (HRP), anti–mouse HRP (Promega, Mannheim, Germany; W4011, W4021). Melphalan was bought from Sigma (M2011) and etoposide from Calbiochem (Schwalbach, Germany; 341205).
Data analysis and statistics
Dose-response curves were calculated from at least 3 independent experiments (MM cell lines), or from a single experiment (primary patient cells), by nonlinear regression (variable slope dose-response curve) analysis using GraphPad Prism 3.0 (GraphPad Software, San Diego, CA). Statistical analysis was done using a 2-tailed unpaired Student t test.
Results
Antitumor activity of nutlin-3 in myeloma cell lines
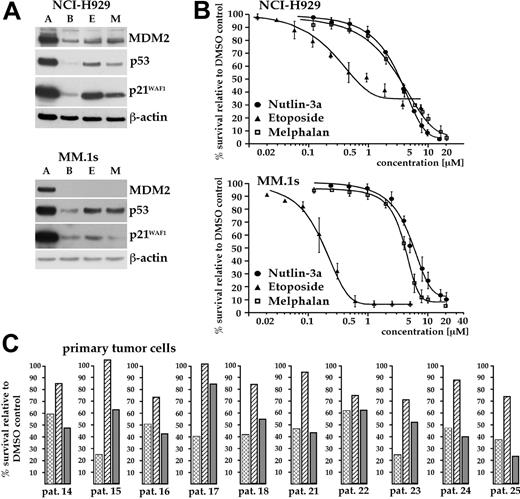
To determine if p53 activation can offer therapeutic utility for the treatment of MM, we initially tested the small-molecule MDM2 antagonist nutlin-3 for its ability to activate the p53 pathway in a panel of MM cell lines. Western analysis revealed strong and specific increases in the levels of p53, MDM2, and p21 in response to the active enantiomer (nutlin-3a) in the MM cell lines MM.1s and NCI-H929 (Figure 1A). The inactive stereoisomer nutlin-3b, which has a 150-fold lower affinity for MDM2,22 did not affect the level of these proteins, confirming selective modulation of the p53 pathway by nutlin-3a. The other MM cell lines tested (ANBL-6, INA-6, OPM-2, RPMI-8226) displayed high levels of p53 protein, normally indicative of p53 mutation. In these 5 cell lines, MDM2 and p21WAF1 were essentially undetectable (Figure 1A).
Sensitivity to p53 activation in MM cell lines. (A) Western analysis for p53, MDM2, and p21WAF1 in MM cell lines after overnight incubation with 10 μM nutlin-3a (A), 10 μM nutlin-3b (B), or DMSO (D). Nutlin-3a selectively augmented the p53 level in MM.1s and NCI-H929, whereas the other lines displayed high and unmodifiable levels of p53. Induction of downstream targets was strictly correlated with induction of p53. These data show that only 2 of the 6 cell lines tested retain functional p53 signaling. (B) Nutlin-3a–induced cell death is restricted to MM cells with wild-type p53. Cells were exposed for 4 days to 10 μM nutlin-3a, nutlin-3b, or DMSO and apoptosis measured. Numbers show the means of 3 independent tests and represent the percentage of live cells relative to the DMSO control. The mutation status of p53 was inferred from sequencing at gDNA and cDNA levels and confirmed that induction of p53 pathway components was strictly correlated with the presence of wild-type p53. (C) Dose-response curves for nutlin-3a–induced cell death in MM.1s and NCI-H929 as assessed by annexin-FITC/PI staining after 4 days (MM.1s) or 3 days (NCI-H929) of incubation with the drug. EC50 values were 5.2 μM for MM.1s and 3.5 μM for NCI-H929. Nutlin-3b was without effect in this concentration range.
Sensitivity to p53 activation in MM cell lines. (A) Western analysis for p53, MDM2, and p21WAF1 in MM cell lines after overnight incubation with 10 μM nutlin-3a (A), 10 μM nutlin-3b (B), or DMSO (D). Nutlin-3a selectively augmented the p53 level in MM.1s and NCI-H929, whereas the other lines displayed high and unmodifiable levels of p53. Induction of downstream targets was strictly correlated with induction of p53. These data show that only 2 of the 6 cell lines tested retain functional p53 signaling. (B) Nutlin-3a–induced cell death is restricted to MM cells with wild-type p53. Cells were exposed for 4 days to 10 μM nutlin-3a, nutlin-3b, or DMSO and apoptosis measured. Numbers show the means of 3 independent tests and represent the percentage of live cells relative to the DMSO control. The mutation status of p53 was inferred from sequencing at gDNA and cDNA levels and confirmed that induction of p53 pathway components was strictly correlated with the presence of wild-type p53. (C) Dose-response curves for nutlin-3a–induced cell death in MM.1s and NCI-H929 as assessed by annexin-FITC/PI staining after 4 days (MM.1s) or 3 days (NCI-H929) of incubation with the drug. EC50 values were 5.2 μM for MM.1s and 3.5 μM for NCI-H929. Nutlin-3b was without effect in this concentration range.
Because lack of responsiveness to nutlin-3a treatment has so far always been correlated with mutation in p53,22 we determined the current genetic status of TP53 in the MM cell lines used in our laboratory. The gDNA and cDNA sequence analyses confirmed the presence of wild-type p53 in MM.1s and NCI-H929 and the presence of inactivating point mutations28 in the other cell lines (Figure 1B). Treatment of MM.1s and NCI-H929 with 10 μM nutlin-3a was sufficient to induce extensive apoptosis, with median effective concentration (EC50) values of 5.2 μM and 3.5 μM, respectively (Figure 1C), whereas p53 mutant MM cell lines were essentially unaffected. Additionally, overnight exposure of NCI-H929 cells to 10 μM nutlin-3a led to complete halt of the cell cycle (T.S., data not shown, June 2005). These experiments provided evidence that disruption of the p53-MDM2 interaction in myeloma cells with wild-type p53 can activate the p53 pathway and can effectively induce apoptotic cell death.
Antitumor activity of nutlin-3 in primary myeloma cells
In contrast to late-stage MM and myeloma-derived cell lines, mutations or deletions in TP53 are relatively rare at diagnosis or in the early stages of the disease. However, it is unknown if the p53 pathway remains functional in this tumor and could thus be exploited for the therapy of MM. We therefore analyzed the activity of nutlin-3 on freshly isolated primary tumor samples collected from 25 patients with myeloma (Figures 2 and 3; Tables 1 and 2).
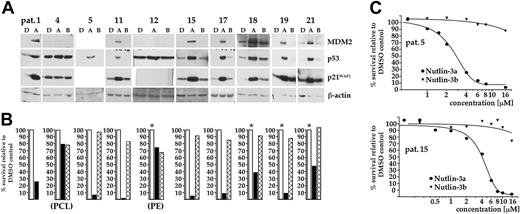
Western analysis confirmed that in the majority of cases (16 of 19) treatment with nutlin-3a led to a selective increase of the p53 level (Figure 2A; also T.S., unpublished data, May 2004-March 2005) that was always mirrored by concomitant increases in the levels of MDM2 and p21. Patient samples that did not conform to this pattern showed high and unaffected levels of p53 and failed to induce either MDM2 or p21 (samples no. 4 and 12, Figure 2A; p21 was constitutively expressed in sample no. 4), or did not display traces of p53 (sample no. 22, not shown). Samples no. 4 and 12 were obtained from myeloma patients with extramedullary manifestations (plasma cell leukemia and pleural effusion) and are thus more representative for advanced-stage disease, where mutation of p53 is frequently encountered. The p53 transcript sequences were analyzed from 4 primary samples (nos. 4, 16, 23, and 25) of which only sample no. 4 (the plasma cell leukemia) was found to contain a mutation (C238R, which is within a p53 mutation hotspot region).28 Western analysis thus clearly showed that in the majority of primary tumor samples p53 appeared to be wild type and active as a transcription factor and that it can be specifically induced by treatment with nutlin-3a.
Next, we determined whether p53 activation was sufficient to induce apoptosis of primary myeloma cells. With the exception of patient samples no. 4 and 12, in which p53 appears mutated, apoptosis was observed in all samples where p53 was induced by treatment with nutlin-3a. Cell viability in these tumor samples decreased by at least 50% and often to below 10% compared to controls (Figure 2B). In total, 22 of 25 primary tumor isolates (88%) displayed sensitivity to treatment with nutlin-3a. The inactive enantiomer nutlin-3b was essentially without effect on cell viability. Dose-response analyses revealed low micromolar EC50 values for nutlin-3a (2.8-5.9 μM; n = 5; Figure 2C), in accordance with the EC50 values determined for nutlin-3–responsive myeloma cell lines.
Analysis of p53 function in primary MM tumor samples. (A) CD138 immunopurified MM cells were incubated overnight with either 10 μM nutlin-3a (A), 10 μM nutlin-3b (B), or DMSO (D). The status of p53, and of MDM2 and p21WAF1, was assessed by Western analysis. The majority of tumor samples showed selective induction of p53 and of both downstream targets in response to nutlin-3a, confirming that these cells most likely contained wild-type p53. High constitutive and unmodified levels of p53 in patient samples no. 4 and 12, which represented cases of plasma cell leukemia (PCL) and pleural effusion (PE), respectively, indicate p53 mutations. (B) Nutlin-3a–induced apoptosis of primary MM cells. Cells from each of the tumor isolates represented in panel A were incubated with either 10 μM nutlin-3a (▪), 10 μM nutlin-3b (( ) or with DMSO (□), and cell death assessed through annexin-FITC/PI staining after 5 days. Height of the bars represents the percentage of live cells relative to the DMSO control. Nutlin-3a proved effective in all samples, except those shown to be defective in p53 signaling. An asterisk indicates that the apoptosis data shown derived from MM cells cocultured with BMSCs. (C) Dose-response curves for nutlin-3a–induced cell death, as assessed through annexin-FITC/PI staining after 5 days of exposure to the drug, in selected primary tumor samples. EC50 values fell within the range identified for nutlin-3a–responsive MM cell lines (sample no. 5, 2.8 μM; sample no. 15, 4.4 μM).
) or with DMSO (□), and cell death assessed through annexin-FITC/PI staining after 5 days. Height of the bars represents the percentage of live cells relative to the DMSO control. Nutlin-3a proved effective in all samples, except those shown to be defective in p53 signaling. An asterisk indicates that the apoptosis data shown derived from MM cells cocultured with BMSCs. (C) Dose-response curves for nutlin-3a–induced cell death, as assessed through annexin-FITC/PI staining after 5 days of exposure to the drug, in selected primary tumor samples. EC50 values fell within the range identified for nutlin-3a–responsive MM cell lines (sample no. 5, 2.8 μM; sample no. 15, 4.4 μM).
Analysis of p53 function in primary MM tumor samples. (A) CD138 immunopurified MM cells were incubated overnight with either 10 μM nutlin-3a (A), 10 μM nutlin-3b (B), or DMSO (D). The status of p53, and of MDM2 and p21WAF1, was assessed by Western analysis. The majority of tumor samples showed selective induction of p53 and of both downstream targets in response to nutlin-3a, confirming that these cells most likely contained wild-type p53. High constitutive and unmodified levels of p53 in patient samples no. 4 and 12, which represented cases of plasma cell leukemia (PCL) and pleural effusion (PE), respectively, indicate p53 mutations. (B) Nutlin-3a–induced apoptosis of primary MM cells. Cells from each of the tumor isolates represented in panel A were incubated with either 10 μM nutlin-3a (▪), 10 μM nutlin-3b (( ) or with DMSO (□), and cell death assessed through annexin-FITC/PI staining after 5 days. Height of the bars represents the percentage of live cells relative to the DMSO control. Nutlin-3a proved effective in all samples, except those shown to be defective in p53 signaling. An asterisk indicates that the apoptosis data shown derived from MM cells cocultured with BMSCs. (C) Dose-response curves for nutlin-3a–induced cell death, as assessed through annexin-FITC/PI staining after 5 days of exposure to the drug, in selected primary tumor samples. EC50 values fell within the range identified for nutlin-3a–responsive MM cell lines (sample no. 5, 2.8 μM; sample no. 15, 4.4 μM).
) or with DMSO (□), and cell death assessed through annexin-FITC/PI staining after 5 days. Height of the bars represents the percentage of live cells relative to the DMSO control. Nutlin-3a proved effective in all samples, except those shown to be defective in p53 signaling. An asterisk indicates that the apoptosis data shown derived from MM cells cocultured with BMSCs. (C) Dose-response curves for nutlin-3a–induced cell death, as assessed through annexin-FITC/PI staining after 5 days of exposure to the drug, in selected primary tumor samples. EC50 values fell within the range identified for nutlin-3a–responsive MM cell lines (sample no. 5, 2.8 μM; sample no. 15, 4.4 μM).
Effect of BMSCs on nutlin-mediated apoptosis of MM cells
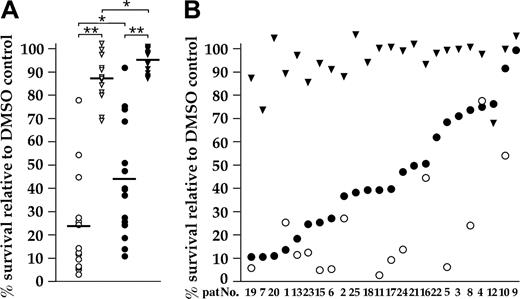
The BM microenvironment plays an important role for the survival and drug resistance of MM cells. We therefore examined the effects of nutlin-3 on myeloma cells cultured either in medium (supplemented with 10 ng/mL IL-6) or in coculture with primary BMSCs. BMSCs did not modify the susceptibility of NCI-H929 cells to nutlin-3a (T.S., unpublished data, July 15, 2004). Fifteen primary MM samples were successfully analyzed in both settings. The median values for survival in medium alone were 23% (nutlin-3a, 10 μM, 5 days) and 87% (nutlin-3b), and, for MM cells cocultured with BMSCs, 43% (nutlin-3a) and 95% (nutlin-3b) as shown in Figure 3A. Statistical differences between nutlin-3a– and nutlin-3b–treated samples were very highly significant. Figure 3B shows the complete set of evaluable patient samples cocultured with BMSCs, arranged according to decrease in viability after nutlin-3a treatment. This arrangement also accentuates the differences in survival between culture conditions for the 15 samples from which the median survival rates were calculated. The MDM2 antagonist specifically induced apoptosis in the large majority of primary MM tumor samples, and in most cases tumor cell death of 50% to 90% was observed even in coculture with BMSCs.
Effect of BMSCs on antitumor activity of nutlin-3a in primary myeloma isolates. (A) Survival of primary MM cells after 5-day incubation with either 10 μM nutlin-3a (•) or 10 μM nutlin-3b (▾) in medium supplemented with 10 ng/ml IL-6 (▿, ○) or in coculture with BMSCs (▾, •). The median values of survival relative to the DMSO control (indicated by bars) were 23% (nutlin-3a) and 87% (nutlin-3b) in medium supplemented with IL-6 and 43% (nutlin-3a) and 95% (nutlin-3b) for coculture with BMSCs (*P < .01, **P < .001). (B) The complete set of primary cells analyzed as detailed (which also includes a number of samples that could exclusively be evaluated after coculture with BMSCs) arranged according to antitumor activity of nutlin-3a in coculture with BMSCs (•). The majority of samples was responsive to nutlin-3a treatment even in the presence of BMSCs. Weak effects sometimes reflected p53 mutation (eg, samples no. 4 and 12), and the functional status of p53 remained untested in other cases (eg, samples no. 3, 9, and 10). Nutlin-mediated cell death of certain tumor isolates was clearly less pronounced in coculture with BMSCs (samples no. 5, 8, and 11). Data for the nutlin-3b control in medium supplemented with IL-6 (essentially similar to the values in coculture; panel A; Table 2), was omitted for clarity.
Effect of BMSCs on antitumor activity of nutlin-3a in primary myeloma isolates. (A) Survival of primary MM cells after 5-day incubation with either 10 μM nutlin-3a (•) or 10 μM nutlin-3b (▾) in medium supplemented with 10 ng/ml IL-6 (▿, ○) or in coculture with BMSCs (▾, •). The median values of survival relative to the DMSO control (indicated by bars) were 23% (nutlin-3a) and 87% (nutlin-3b) in medium supplemented with IL-6 and 43% (nutlin-3a) and 95% (nutlin-3b) for coculture with BMSCs (*P < .01, **P < .001). (B) The complete set of primary cells analyzed as detailed (which also includes a number of samples that could exclusively be evaluated after coculture with BMSCs) arranged according to antitumor activity of nutlin-3a in coculture with BMSCs (•). The majority of samples was responsive to nutlin-3a treatment even in the presence of BMSCs. Weak effects sometimes reflected p53 mutation (eg, samples no. 4 and 12), and the functional status of p53 remained untested in other cases (eg, samples no. 3, 9, and 10). Nutlin-mediated cell death of certain tumor isolates was clearly less pronounced in coculture with BMSCs (samples no. 5, 8, and 11). Data for the nutlin-3b control in medium supplemented with IL-6 (essentially similar to the values in coculture; panel A; Table 2), was omitted for clarity.
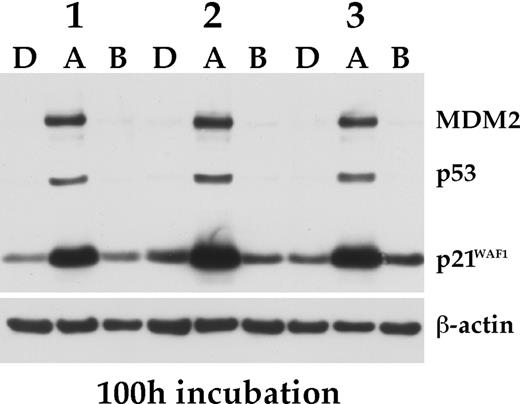
Effect of nutlin-3a on BMSCs. Primary BMSCs from 3 different donors were exposed for 100 hours to nutlin-3a (A), nutlin-3b (B), each at 10 μM, or DMSO (D), and the status of p53, MDM2 and p21WAF1 determined by Western analysis.
Effect of nutlin-3a on BMSCs. Primary BMSCs from 3 different donors were exposed for 100 hours to nutlin-3a (A), nutlin-3b (B), each at 10 μM, or DMSO (D), and the status of p53, MDM2 and p21WAF1 determined by Western analysis.
Two tumor samples that were not affected by nutlin treatment appeared defective in the p53 pathway (see “Antitumor activity of nutlin-3 in primary myeloma cells”), whereas the p53 status of the others remained unknown, because Western analysis was not practical due to insufficient sample size (nos. 3, 9, and 10). Some myeloma samples, however, were very effectively killed by nutlin-3a when kept in medium supplemented with IL-6, but clearly less so in coculture with BMSCs (nos. 5, 8, and 11; Figure 3B).
Effects of nutlins on cells of the BM microenvironment
Because normal cells have wild-type p53 and functional p53 signaling, we examined the effect of nutlin treatment on nonmalignant cells of hematopoietic origin and of the BM microenvironment. Exposure of BMSCs to 10 μM nutlin-3a for 100 hours specifically increased the levels of p53, MDM2, and p21 (Figure 4), confirming that MDM2 antagonists can activate the p53 pathway in normal cells. After treatment with 10 μM nutlin-3a for a week, BMSCs developed a rather thin, spindlelike morphology, but remained attached to the culture dish, and did not display increased numbers of apoptotic nuclei, as assessed by acridine orange staining. This effect was completely reverted once the drug was removed, even after 4 weeks of exposure to nutlin-3a (T.S., unpublished data, January 2005). To test if BMSCs exposed to nutlins would still be able to effectively support myeloma cells, we cocultured INA-6 cells, which without supplementary addition of IL-6 are strictly dependent on the presence of BMSCs for their survival,25 with nutlin-treated BMSCs. The viability of the MM cells was completely unaffected even after coculture for 3 days on BMSCs pretreated with 10 μM nutlin-3a for 30 days (Table 3). Although these data attest to the basic integrity of BMSCs even after prolonged exposure to nutlin-3a, they also strongly suggest that the apoptotic effect of nutlin-3a on primary myeloma cells in coculture with BMSCs is a direct consequence of drug activity on the tumor cells.
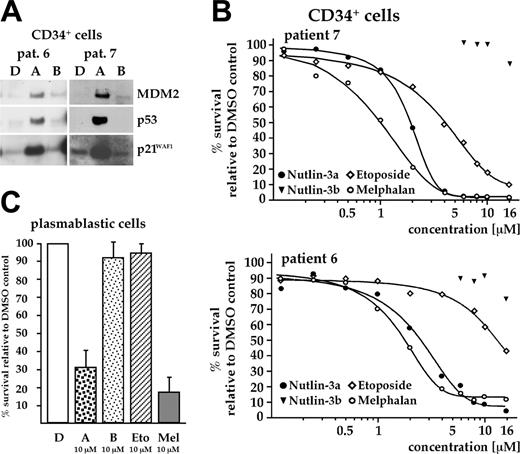
Effect of nutlin-3a on cells of the hematopoietic system. (A) Overnight exposure of CD34+ hematopoietic stem cells to 10 μM nutlin-3a activated p53, as assessed by Western analysis. (B) CD34+ cells were cocultured with BMSCs and cell death assessed after a 3-day exposure to increasing concentrations of nutlins and 2 therapeutically relevant cytostatics. A strong decrease in viability in response to nutlin-3a was recorded (EC50 value about 2.4 μM; range, 1.9-2.9 μM; cells from 3 different patients tested). The genotoxic cytostatic melphalan was equally toxic in this assay (range of EC50 values, 1.1-1.8 μM). (C) Viability of in vitro differentiated plasmablastic cells, cocultured with BMSCs, after a 3-day exposure to either nutlins, melphalan, or etoposide (all at 10 μM). Treatment with nutlin-3a or with melphalan strongly decreased the number of viable cells. Height of the bars represents the median survival relative to DMSO control from experiments with cells from 3 different donors.
Effect of nutlin-3a on cells of the hematopoietic system. (A) Overnight exposure of CD34+ hematopoietic stem cells to 10 μM nutlin-3a activated p53, as assessed by Western analysis. (B) CD34+ cells were cocultured with BMSCs and cell death assessed after a 3-day exposure to increasing concentrations of nutlins and 2 therapeutically relevant cytostatics. A strong decrease in viability in response to nutlin-3a was recorded (EC50 value about 2.4 μM; range, 1.9-2.9 μM; cells from 3 different patients tested). The genotoxic cytostatic melphalan was equally toxic in this assay (range of EC50 values, 1.1-1.8 μM). (C) Viability of in vitro differentiated plasmablastic cells, cocultured with BMSCs, after a 3-day exposure to either nutlins, melphalan, or etoposide (all at 10 μM). Treatment with nutlin-3a or with melphalan strongly decreased the number of viable cells. Height of the bars represents the median survival relative to DMSO control from experiments with cells from 3 different donors.
CD34+ hematopoietic stem cells also responded to overnight incubation with nutlin-3a (10 μM) with activation of the p53 pathway (Figure 5A). However, when the effect of 8 μM nutlin-3 on clonal growth of CD34+ cells was assessed using a colony formation assay, treatment with the MDM2 inhibitor completely blocked colony growth. This effect was probably dependent on p53 because the inactive enantiomer did not show any growth suppressive activity (Table 4). Dose-response analysis showed EC50 values for nutlin-3a–induced cell death ranging from 1.9 to 2.9 μM (median from experiments with cells from 3 different donors, 2.4 μM), which was comparable with EC50 values for the commonly used cytostatic drug, melphalan (1.1-1.8 μM; Figure 5B).
Next, we tested the effect of 10 μM nutlin-3a on the viability of in vitro differentiated plasmablastic cells, cocultured with BMSCs. Again, treatment with nutlin-3a (or with 10 μM melphalan) strongly decreased the number of viable cells, as determined by the annexin V–FITC/PI FACS assay (Figure 5C).
Antitumor activity of nutlin-3a in MM cells is comparable to activity of clinically established cytotoxic agents
Nutlin-3a is a nongenotoxic agent that owes its antitumor activity to activation of the p53 pathway.22 Most currently used chemotherapeutics are genotoxic drugs that may derive some of their activity from p53 activation. However, these drugs may also induce p53-independent apoptotic pathways and could therefore be more effective than p53-selective agents. We compared the antitumor efficacy of nutlin-3a with that of the cytotoxic drugs melphalan and etoposide currently used in the clinic. Western analysis of myeloma cell line NCI-H929 treated with nutlin-3a, melphalan, or etoposide (all at 10 μM) showed activation of the p53 pathway by all 3 drugs (Figure 6A), but the effect was clearly most pronounced in cells treated with the MDM2 inhibitor. Etoposide and melphalan induced a modest increase in p53 levels in MM.1s, but no concomitant rise in the levels of downstream targets was seen. As expected, myeloma cell lines with mutant p53 did not show induction of the p53 pathway (T.S., unpublished data, November 2004). The EC50 values for nutlin-3a and melphalan in NCI-H929 (both 3.5 μM) and MM.1s (5.2 μM and 2.5 μM) were similar, whereas etoposide was more effective (EC50 = 0.32 μM and 0.2 μM in NCI-H929 and MM.1s, respectively). However, a sizeable fraction of NCI-H929 cells remained unaffected by etoposide even at high concentrations (Figure 6B).
Comparison of the antitumor activity of nutlin-3a, etoposide, and melphalan in primary MM cells cocultured with BMSCs revealed that nutlin-3a was most effective in reducing the surviving tumor cell fraction. After exposure for 5 days, nutlin-3a reduced viability to 45% of control (range, 25%-61%), melphalan to 49% (range, 24%-86%), and etoposide to 82% (range, 61%-101%; n = 10; Figure 6C; Table 2). These data indicate that the apoptotic activity of nutlin-3a in myeloma cells is comparable to that of the widely used genotoxic cytostatic melphalan.
Antitumor activities of nutlin-3a and genotoxic drugs in MM cells. (A) Western analysis for p53, MDM2, and p21WAF1 in p53 wild-type MM cells after overnight exposure to either 10 μM nutlin-3a (A), nutlin-3b (B), etoposide (E), or melphalan (M). Etoposide and melphalan activated p53 signaling in NCI-H929, but did so less robustly than the MDM2 inhibitor. A modest increase of p53 levels was seen in MM.1s treated with either genotoxic drug. (B) Dose-response curves revealed EC50 values of 0.32 μM (etoposide) and 3.5 μM (nutlin-3a and melphalan) for NCI-H929 and 0.2 μM (etoposide), 2.5 μM (melphalan), and 5.2 μM (nutlin-3a) for MM.1s, respectively. (C) Effects of either nutlin-3a (( ), etoposide (▨), or melphalan (▦), 10 μM, on the 5-day survival of primary myeloma tumor cells in coculture with BMSCs relative to DMSO controls (= 100%). The apoptotic activity of nutlin-3a was similar to melphalan, whereas etoposide had little effect on primary tumor cells.
), etoposide (▨), or melphalan (▦), 10 μM, on the 5-day survival of primary myeloma tumor cells in coculture with BMSCs relative to DMSO controls (= 100%). The apoptotic activity of nutlin-3a was similar to melphalan, whereas etoposide had little effect on primary tumor cells.
Antitumor activities of nutlin-3a and genotoxic drugs in MM cells. (A) Western analysis for p53, MDM2, and p21WAF1 in p53 wild-type MM cells after overnight exposure to either 10 μM nutlin-3a (A), nutlin-3b (B), etoposide (E), or melphalan (M). Etoposide and melphalan activated p53 signaling in NCI-H929, but did so less robustly than the MDM2 inhibitor. A modest increase of p53 levels was seen in MM.1s treated with either genotoxic drug. (B) Dose-response curves revealed EC50 values of 0.32 μM (etoposide) and 3.5 μM (nutlin-3a and melphalan) for NCI-H929 and 0.2 μM (etoposide), 2.5 μM (melphalan), and 5.2 μM (nutlin-3a) for MM.1s, respectively. (C) Effects of either nutlin-3a (( ), etoposide (▨), or melphalan (▦), 10 μM, on the 5-day survival of primary myeloma tumor cells in coculture with BMSCs relative to DMSO controls (= 100%). The apoptotic activity of nutlin-3a was similar to melphalan, whereas etoposide had little effect on primary tumor cells.
), etoposide (▨), or melphalan (▦), 10 μM, on the 5-day survival of primary myeloma tumor cells in coculture with BMSCs relative to DMSO controls (= 100%). The apoptotic activity of nutlin-3a was similar to melphalan, whereas etoposide had little effect on primary tumor cells.
Increased antitumor activity through combined treatment with nutlin-3a and melphalan
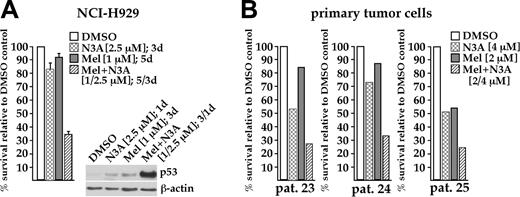
Finally, we tested if melphalan and nutlin-3a could synergize in their antitumor activity against MM cells. Treatment of NCI-H929 cells with a low dose of melphalan (1 μM for 5 days), complemented with a low dose of nutlin-3a (2.5 μM for the last 3 days), reduced cell viability to about 34% of control. The respective single-drug treatments had little effect on the number of viable cells (melphalan, 92%; nutlin-3a, 83%; Figure 7A). Western analysis showed that an initial 2-day incubation with melphalan, followed by additional overnight exposure to the MDM2 antagonist, led to much higher p53 levels than the respective treatments with either drug alone (Figure 7A). Similarly, combined treatment with 2 μM melphalan and 4 μM nutlin-3a for 5 days was more effective than either agent alone in 3 primary myeloma samples (Figure 7B). These observations suggest that a combination of MDM2 antagonist and currently used genotoxic agents might be a useful approach to enhance antitumor efficacy in myeloma cells.
Discussion
Many of the currently used cytotoxic drugs owe their antineoplastic effects to induction of apoptotic pathways. However, their therapeutic activity is a trade-off between efficacy and adverse effects, namely, unspecific genotoxic collateral damage, which may induce genetic instability, selection of drug-resistant tumor subclones, and secondary malignancies. In contrast to the short-term side effects of genotoxic therapy, such as nausea, vomiting, mucositis, myelosuppression, and infection, which are reversible and manageable, these long-term side effects are irreversible and cannot be managed clinically. Nongenotoxic activation of the p53 pathway would therefore be an attractive therapeutic strategy for cancers with intact p53-dependent signaling. Because p53 mutations are relatively rare events in MM, we reasoned that selective pharmacologic activation of p53 might offer an alternative therapeutic strategy for this malignancy. However, it is not known whether p53-dependent apoptotic signaling is functionally intact in primary MM tumor cells and if this might be exploited to instigate cell death. Here, we report on the first molecular and functional analysis of the p53 pathway in MM cell lines, primary MM tumor cells, and in cells of the BM microenvironment, using the recently developed MDM2 antagonist nutlin-322 as a specific p53 inducer.
Enhanced cell death through combination of low doses of melphalan with nutlin-3. (A) Survival of NCI-H929 cells after a 5-day exposure to 1 μM melphalan (▦), 3-day exposure (days 3-5) to 2.5 μM nutlin-3a ( ), and a combination of both drugs (▨), relative to DMSO control (□). Combined treatment significantly reduced viability compared to any single-drug exposure. Western analysis showed that the level of p53 in NCI-H929 cells treated with both drugs was much higher than in cells exposed to either drug alone. (B) A combined low dose of melphalan (2 μM) and nutlin-3a (4 μM) showed increased antitumor activity in primary MM samples cocultured with BMSCs. MM cells were exposed to both drugs for 5 days.
), and a combination of both drugs (▨), relative to DMSO control (□). Combined treatment significantly reduced viability compared to any single-drug exposure. Western analysis showed that the level of p53 in NCI-H929 cells treated with both drugs was much higher than in cells exposed to either drug alone. (B) A combined low dose of melphalan (2 μM) and nutlin-3a (4 μM) showed increased antitumor activity in primary MM samples cocultured with BMSCs. MM cells were exposed to both drugs for 5 days.
Enhanced cell death through combination of low doses of melphalan with nutlin-3. (A) Survival of NCI-H929 cells after a 5-day exposure to 1 μM melphalan (▦), 3-day exposure (days 3-5) to 2.5 μM nutlin-3a ( ), and a combination of both drugs (▨), relative to DMSO control (□). Combined treatment significantly reduced viability compared to any single-drug exposure. Western analysis showed that the level of p53 in NCI-H929 cells treated with both drugs was much higher than in cells exposed to either drug alone. (B) A combined low dose of melphalan (2 μM) and nutlin-3a (4 μM) showed increased antitumor activity in primary MM samples cocultured with BMSCs. MM cells were exposed to both drugs for 5 days.
), and a combination of both drugs (▨), relative to DMSO control (□). Combined treatment significantly reduced viability compared to any single-drug exposure. Western analysis showed that the level of p53 in NCI-H929 cells treated with both drugs was much higher than in cells exposed to either drug alone. (B) A combined low dose of melphalan (2 μM) and nutlin-3a (4 μM) showed increased antitumor activity in primary MM samples cocultured with BMSCs. MM cells were exposed to both drugs for 5 days.
We showed that treatment of MM cells with the active enantiomer nutlin-3a leads to elevated levels of p53 protein and its immediate downstream transcriptional targets, MDM2 and p21WAF1. Nutlin-induced activation of the p53 pathway led to subsequent induction of apoptosis. This was observed in 2 MM cell lines, both of which express wild-type p53, and in the majority of primary MM cell samples analyzed. Wild-type p53 status appears to be predictive of the functional outcome. Without exception, all MM samples that showed nutlin-induced expression of p53 and of p53-inducible downstream targets (n = 16) underwent apoptosis after exposure to the MDM2 antagonist. Two of 3 samples that did not show any up-regulation of the p53 pathway were completely resistant to nutlin-3a and represented extramedullary forms of the disease. These observations confirm the target specificity of nutlin-3a and clearly demonstrate the p53 dependence of the observed biologic effects. Thus, downstream signaling in the p53 pathway appears to be at least partially intact in more than 90% of primary medullary MM cases and might therefore constitute a useful target for specific therapeutic activation. Our functional analysis of the p53 pathway is in agreement with genetic studies that reported TP53 mutations or deletions in 9% to 32% of MM cases.14-20,29 In contrast, 2 of 3 extramedullary samples, as well as the majority of MM cell lines, showed a defective p53 response, most likely due to p53 mutation, and were completely resistant to nutlin-3a. This, too, is in agreement with previous genetic studies that reported an increased frequency of p53 gene mutations in extramedullary myeloma, end-stage myeloma, and MM cell lines.14,20,21
It is important to note that treatment with nutlin-3a resulted in effective cell death (85%-95%) in sensitive cell lines and in some primary MM tumor samples, whereas in other primary samples only a fraction of the cells died. This is likely due to defects in downstream p53-dependent apoptotic signaling and, therefore, combined treatment with drugs that have different mechanisms of action might be required for efficient treatment.
The BM microenvironment has been reported to play an important role in disease progression, malignant growth, and drug resistance in myeloma. We therefore investigated whether the presence of BMSCs would affect the efficacy of nutlin-mediated apoptosis. BMSCs did not influence the activity of nutlin-3a in the MM cell line NCI-H929. In cocultured primary tumor cells, the picture was more complex. In general, cells that were responsive to nutlin treatment in medium supplemented with IL-6 were also sensitive to the drug in coculture with BMSCs. However, there were also some cases in which MM cells were clearly less sensitive to the MDM2 inhibitor when cocultured with BMSCs. Although it is not clear what mechanisms led to reduced apoptosis in this setting, this observation might reflect the biologic heterogeneity of the disease.
Treatment of normal cells of the BM microenvironment with nutlin-3a also showed effective induction of the p53 pathway. However, in contrast to tumor cells, prolonged treatment of BMSCs with 10 μM nutlin-3a did not appear to affect their viability or their growth-supporting effect on MM cells. These results indicate that the proapoptotic activity of nutlin-3a in cocultures with BMSCs is due to a direct effect on MM cells rather than indirect targeting of growth-supporting cells of the BM microenvironment. The reason for the differential effects of p53 activation in different cell types is still unclear and awaits further studies. In contrast to BMSCs, other cell types of the BM microenvironment, such as plasmablastic cells and CD34+ blood stem cells, did not survive exposure to 8 μM nutlin-3a. However, this might reflect the fact that normal hematopoietic cells have an increased sensitivity to apoptotic stimuli when grown in vitro, whereas they appear to be more tolerant to p53 activation in their natural cellular environment in vivo. This notion is supported by the observation that genetic manipulation of MDM2 in mice, leading to 70% reduction of protein expression and corresponding p53 activation, shows mild myelosuppression but is well tolerated and did not lead to a shortened life span.30 Treatment of nude mice bearing human tumor xenografts with nutlin-3a for 3 weeks, at doses that cause significant tumor regression as a result of p53 activation (200 mg/kg, twice a day), also appeared to be well tolerated and the animals showed no weight loss or other signs of toxicity (L.T.V., manuscript in preparation).
Overproduction of MDM2 is a mechanism to inhibit p53 function in human tumor cells with wild-type p53. However, it is unclear if MDM2 overexpression plays such a role in myeloma. One group has reported constitutive expression of MDM2 in MM cell lines and 2 cases of plasma cell leukemia,31 whereas others have found low incidences of MDM2 amplification in cytogenetic studies.18,32 We did not detect elevated MDM2 protein in Western analyses of mutant p53 MM cell lines or in the large majority of primary myeloma tumor samples, although some signal was visible in both MM cell lines with wild-type p53 and in a few primary MM samples. The inconspicuous levels of MDM2 in the bulk of primary myeloma samples might argue against a prominent role for this protein in the protection of MM cells from p53-mediated apoptosis.
Many chemotherapeutic drugs currently used for treatment of MM may exert some of their anticancer effects via genotoxic activation of the p53 pathway, causing irreversible DNA damage and serious side effects. Here, we compared 2 genotoxic drugs and the nongenotoxic MDM2 antagonist nutlin-3a with respect to their abilities to induce apoptosis in MM cells. Although treatment with nutlin-3a and melphalan had similar effects in MM cell lines with wild-type p53 and primary tumor cells, etoposide was slightly more effective in the 2 cell lines, but much less effective in a panel of 10 primary MM samples. All 3 drugs increased p53 levels, but this was much more pronounced with the MDM2 inhibitor. Treatment of NCI-H929 cells with a combined low dose of melphalan and nutlin-3a led to a much higher level of p53 and was considerably more effective to induce apoptosis than treatment with either agent alone. Similar effects were observed in primary myeloma samples. These experiments support the therapeutic utility of MDM2 antagonists as single agents or in combination with genotoxic drugs for the treatment of MM. However, it should also be noted that patients with cytogenetically apparent TP53 defects (17p deletion) have a decidedly poorer prognosis and hardly benefit from conventional chemotherapy or autologous stem cell transplantation.15,16,19 Even if some of these cases represent just monoallelic deletions, it is possible that nutlin treatment would not prove effective. Alternative novel treatments may be required for this therapeutically challenged subgroup of patients.
Taken together, this is the first systematic report on the molecular and functional analysis of p53 downstream signaling in MM. Our data suggest that downstream signaling in the p53 pathway is (at least partially) functional in over 90% of primary medullary MM tumor samples and that specific pharmacologic activation of p53 can be achieved in these cells. Selective p53 activation with the nongenotoxic MDM2 antagonist nutlin-3 induces apoptosis in the vast majority of primary MM tumor samples. Resistant samples were predominantly of extramedullary origin and p53 pathway defective. Coculture with BMSCs was in most cases not sufficient to protect the bulk of primary MM cells from the antitumor effect of nutlin-3. Nutlin-3 proved to be at least as effective as the clinically relevant genotoxic drug melphalan, and, importantly, may support tumor cell death at lower doses of the genotoxic drug. Thus, nongenotoxic activation of the p53 pathway could be a promising future treatment option for patients with MM.
Prepublished online as Blood First Edition Paper, August 4, 2005; DOI 10.1182/blood-2005-04-1489.
Supported in part by grants from the Deutsche Forschungsgemeinschaft (Klinische Forschergruppe KFO 105).
L.T.V. is employed by Hoffman-La Roche, whose potential product was studied in the present work.
T.S. designed, performed, and analyzed research, and wrote the paper; M.H. and P.T.D. designed and performed research; P.H. and L.C. performed research; M.C., K.B., and R.A.M. designed research; L.T.V., H.G., C.G., and S.T. provided essential material; and L.T.V. and R.C.B. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Nutlins were kindly provided by Hoffmann-La Roche (Nutley, NJ). Data on fluorescence in situ hybridization analyses were kindly provided by Dr Peter Liebisch (Ulm, Germany).