Abstract
The Cancer and Leukemia Group B (CALGB) study 9222 tested the hypothesis that treatment intensification of acute myeloid leukemia (AML) in first remission with multiple chemotherapy agents is superior to high-dose cytarabine (HiDAC) alone. We enrolled 474 patients younger than 60 years old with untreated de novo AML. Daunorubicin and cytarabine resulted in complete remission (CR) in 342 patients (72%), and 309 of these patients were randomized to receive one of 2 different intensification regimens. The first regimen consisted of 3 courses of HiDAC. The second regimen consisted of one course of HiDAC, a second course with etoposide and cyclophosphamide, and a third course with diaziquone and mitoxantrone. After a median follow-up time of 8.3 years, the median survival for all randomized patients was 2.8 years (95% CI, 1.9-6.8 years). There was no difference in disease-free survival (DFS) between the 2 regimens (P = .66). The median DFS was 1.1 years (95% CI, 0.9-1.7 years) for patients receiving HiDAC and 1.0 year (95% CI, 0.9-1.3 years) for those receiving multiagent chemotherapy. Cytogenetics was the only pretreatment characteristic prognostic for DFS, but there was no evidence of a differential treatment effect within cytogenetic risk groups. Toxicity was greater with multiagent chemotherapy. These 2 postremission regimens produced similar outcomes.
Introduction
The treatment of acute myeloid leukemia (AML) remains unsatisfactory. Although the rate of complete remission (CR) after induction chemotherapy has steadily increased over the past 2 decades and postremission chemotherapy is routinely used, most patients experience a relapse of AML, and eventually die from their disease. Barriers to a higher cure rate for AML include drug-resistant disease and treatment toxicity.
Treatment with 7 days of cytarabine (Ara-C) and 3 days of daunorubicin has been a standard remission induction regimen used by the Cancer and Leukemia Group B (CALGB) and others for patients with AML. This regimen produced CR in 71% and 77% of patients younger than 60 years of age enrolled on 2 previous CALGB AML studies.1,2 The challenge of translating such remissions into cure for patients with this disease has been the recent focus of clinical research. It was shown in CALGB study 8525 that Ara-C administered as a single agent at a dose of 3000 mg/m2 (HiDAC) for 4 courses during intensification was feasible for young and middle-aged adults with AML in first CR, but cerebellar toxicity was increased among patients older than 60 years, limiting its utility.1 Four courses of HiDAC provided significantly longer disease-free survival (DFS) than 4 courses of cytarabine given for 5 days at intermediate doses (400 mg/m2) or conventional doses (100 mg/m2). For patients younger than 60 years old, the likelihood of remaining alive and free of disease after 4 years was 44% after HiDAC consolidation, only 29% after intermediate-dose Ara-C, and 24% after conventional doses of Ara-C (P = .002).
There has been considerable interest in developing chemotherapy programs that might be non-cross-resistant with cytarabine-based treatment. Agents such as diaziquone (AZQ), mitoxantrone, cyclophosphamide, and etoposide (VP-16) have been tested in phase 1 and 2 studies in relapsed and newly diagnosed AML.3-7 Brown et al3 demonstrated that a combination of cyclophosphamide and etoposide in high doses could induce CR both in untreated AML patients as well as in those who had a relapse after previous remission or had proven refractory to HiDAC therapy. Furthermore, previous CALGB studies established the effectiveness of a combination of mitoxantrone and AZQ in producing a second CR in patients with relapsed AML.4-7
The feasibility of administering sequential courses of potentially non-cross-resistant multiagent chemotherapy to AML patients 16 to 59 years of age in first CR was explored in a pilot study conducted by the CALGB.2 After remission induction, patients who were enrolled on CALGB study 9022 received one course of HiDAC consolidation followed by one course of cyclophosphamide and etoposide followed by one course of mitoxantrone and AZQ.2 The initial patients treated with mitoxantrone and AZQ experienced prolonged bone marrow suppression. However, when the hematopoietic growth factor filgrastim (granulocyte colony-stimulating factor [G-CSF]) was given following completion of mitoxantrone/AZQ therapy, the duration of severe granulocytopenia decreased significantly.2
The current study (CALGB 9222) was designed to test the hypothesis that intensification of AML in first CR with 3 courses of multiagent chemotherapy (MCT) is superior to an equal number of courses of HiDAC alone with respect to the duration of DFS. CALGB 9222 was a direct randomized comparison of a modification of the best consolidation regimen from the CALGB 8525 study (HiDAC alone) and the sequential multiagent “non-cross-resistant” consolidation program developed in CALGB study 9022.1,2 The 2 treatment regimens were also evaluated for differences in toxicity and for effectiveness within biologic subsets of AML.
Patients, materials, and methods
Eligibility
Eligible patients were between 15 and 59 years of age with newly diagnosed AML, morphologically defined as French-American-British (FAB) M0 to M7 by the French-American-British classification of acute leukemias.8 At least 30% replacement of nonerythroid marrow elements by myeloid blasts was required. Initially, patients with FAB M3 (acute promyelocytic leukemia) were treated on another CALGB AML study using tretinoin, but they became eligible for the current study when that study closed. Megakaryocytic leukemia was diagnosed by ultrastructural peroxidase or appropriate monoclonal antibodies. FAB M0 was defined by morphologically undifferentiated leukemia blasts that were reactive with antibodies against at least one myeloid antigen (CD33, CD13, CD11b, or CD14) and no lymphoid antigens.
Patients were excluded for significant cardiac, hepatic, or pulmonary dysfunction not directly attributable to leukemia. Patients with leukemia known to involve the central nervous system were not eligible. Patients were not eligible if they had received any previous chemotherapy or radiation therapy or had any previous hematologic malignancy, myeloproliferative disorder, or myelodysplastic syndrome.
Treatment plan
Remission induction chemotherapy consisted of cytarabine 200 mg/m2 daily for 7 days by continuous intravenous infusion and daunorubicin 45 mg/m2 daily for 3 days by rapid intravenous infusion. A bone marrow aspirate was performed on day 14 to determine the degree of marrow hypoplasia and weekly thereafter until remission status could be determined. If more than 5% blasts remained and if the cellularity were at least 15%, a second induction course was given consisting of 5 days of cytarabine and 2 days of daunorubicin in the same doses described.
Patients achieving CR with adequate laboratory values were randomized to one of 2 postremission treatment regimens. The first regimen consisted of HiDAC given as 3000 mg/m2 intravenously over 3 hours every 12 hours on days 1, 3, and 5 (a total of 6 doses over 5 days). Three courses were prescribed with each course to begin within 2 weeks after hematopoietic recovery from the preceding course. The second regimen consisted of sequential courses of HiDAC (as defined in the first regimen), cyclophosphamide/etoposide, and mitoxantrone/AZQ. The second intensification course in this regimen was begun within 2 weeks after recovery of normal blood counts following a single course of HiDAC. Etoposide was given at 1800 mg/m2 continuous intravenous infusion over 25 to 26 hours followed by cyclophosphamide at 50 mg/kg given as a 2-hour infusion on days 2 and 3 for a total dose of 100 mg/kg. Following recovery from this course, patients received AZQ at 28 mg/m2 in 1 L normal saline by continuous intravenous infusion each day for 3 days together with mitoxantrone 12 mg/m2 by slow intravenous infusion each day for 3 days. Twenty-four hours after completion of the mitoxantrone/AZQ therapy, patients began G-CSF at 5 μg/kg daily by subcutaneous injection and continued this treatment until the neutrophil count exceeded 1500/μL for 2 days. Use of G-CSF was otherwise not permitted on this study. No further therapy was given after the final intensification course.
Quality control, quality assurance, and monitoring
Institutional Review Board approval was obtained at each participating institution, and each patient gave written informed consent. All data forms were sent to the CALGB Statistical Center, where standard CALGB quality control procedures were followed and data were entered into the official CALGB database. The study chair reviewed the eligibility of each patient as well as all data forms to verify the institutional assessments of toxicity and response.
Members of the Data Audit Committee of the CALGB visit all participating institutions at least once every 3 years to verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, response, and follow-up.9 The medical records of 25 of the 249 patients (10%) treated in this study, drawn from all 25 participating institutions (see “Appendix” for the full list) and their affiliates, were randomly selected and reviewed. The CALGB Data and Safety Monitoring Board (DSMB) confidentially reviewed the data in each of the treatment regimens for safety and efficacy during the conduct of the study. As part of this process, the Statistical Center carried out formal interim analyses of DFS twice a year as described in “Experimental design and statistical methods.”
Enrollment and randomization procedures
Patients were enrolled prior to treatment by means of a telephone call to the CALGB Statistical Center. Direct registrations were allowed only from CALGB main member institutions; registrations from affiliates of the main members were made through the appropriate main member. Randomization to one of the 2 consolidation treatment arms was done only after CR was confirmed, and the Statistical Center provided the treatment assignment. The randomization was an unstratified permuted-block design with a preassigned block size of 8.10 As usual in studies with complex treatment regimens, the treatment assignments were not masked to the patients or treating physicians.
Concurrent laboratory studies
All CALGB acute leukemia studies require confirmatory cytologic and cytochemical studies to be performed on pretreatment blood smears and bone marrow specimens to support the diagnosis of AML. In addition, it was recommended that specimens be submitted for cytogenetic analysis as part of CALGB study 8461 and for immunophenotyping studies, using multiparameter flow cytometry, as part of CALGB study 8361. However, participation in these ancillary studies was not mandatory.
Risk stratification based on cytogenetic abnormalities
Patients randomized to postremission therapy were classified into risk groups according to their pretreatment karyotype, based on CALGB criteria described by Byrd et al.11 Each patient was assigned to a unique group with respect to risk for cumulative incidence of relapse (CIR). All patients with core-binding factor (CBF) leukemia, that is, t(8;21)(q22;q22), inv(16)(p13q22), or t(16;16)(p13;q22), were classified as favorable risk. Patients were classified as intermediate risk if they had a normal karyotype, -Y, del(9q), +8, +11, or +13. Patients were classified as unfavorable risk if they had complex cytogenetics (karyotypes with 3 or more abnormalities), -7, or +21. Patients with all other recurring and miscellaneous abnormalities were not classified because outcomes for these abnormalities have not been determined for CIR.11 Except for patients with CBF AML, patients with 2 abnormalities of different prognostic significance were assigned to the less favorable group.
Evaluation and response criteria
All patients were evaluated with detailed medical history and physical examination at the initiation of therapy, throughout the induction period, and during each intensification course. CR was defined by National Cancer Institute criteria and required bone marrow cellularity more than 20% with less than 5% blasts and normal myeloid maturation.12 The response had to be maintained for at least 30 days unless consolidation therapy was begun sooner. The definition of CR also required an absolute granulocyte count more than 1500/μL, platelet count more than 100 000/μL, and the absence of any leukemia cells in the peripheral blood or extramedullary disease. These same criteria also had to be met before each course of intensification therapy. Patients were considered to have failed to respond to induction therapy if CRs were not achieved after 2 courses of Ara-C/daunorubicin therapy. Relapse was defined as either marrow infiltration with more than 25% leukemia cells in a patient with a previous CR, the detection of leukemia cells in the central nervous system, or any other evidence of leukemic relapse.12
Experimental design and statistical methods
The study was designed primarily to compare the distributions of DFS in the 2 randomized treatment groups. DFS was defined to be the time from date of randomization after CR to relapse or death from any cause. This end point was analyzed twice each year using the principles of stochastic curtailment and the calculation of conditional power.13,14 Results of these interim analyses were reported confidentially by the statisticians on this study to the DSMB. Based on accrual to previous CALGB AML studies, it was estimated that 150 patients would be entered each year, of whom 90 would be randomized to postremission intensification. In the design, it was assumed that a proportional hazards model would be approximately correct and that a hazard ratio of 1.6 or larger would be the minimal effect size to be detected (2-sided type I error of 0.05, log-rank test). Based on these assumptions, an accrual period of 3 years and a follow-up period of 1.5 years would yield a power of approximately 0.80. Hence, the total sample size was established at 450.
Survival was another end point in this study, defined to be the time from study entry to death from any cause when the interest is on overall survival of all patients, and the time from randomization to death from any cause when the interest is in comparing the survival distributions of the 2 randomized regimens. Median follow-up time is defined as the median survival time of all surviving patients. Survival and DFS curves were based on Kaplan-Meier estimates, and differences between groups were tested using the log-rank statistic.15,16 Testing for treatment-covariate interactions was carried out using proportional hazards regression models. Ninety-five percent confidence intervals (CIs) for median survival times were calculated by the method of Brookmeyer and Crowley.17 Ninety-five percent CIs for survival and DFS probabilities were calculated by the method of Simon and Lee.18 All reported P values are based on 2-sided significance tests. All comparisons of the randomized regimens used the intent-to-treat principle, including all randomized patients in the analysis, regardless of treatment received or other events after randomization.
Results
CALGB study 9222 was activated in July 1992 and closed in December 1995 with a total accrual of 474 patients from 25 participating main member institutions and their affiliated hospitals. The study did not reach any statistical stopping boundary at any interim analysis during the study and accrual was completed on schedule. One patient received no protocol-specified treatment and was not included in the analysis. Thus, 473 patients were analyzed for this report. The pretreatment characteristics of these patients are given in Table 1. The median age was 43 years with a range of 17 to 59 years, 52% were men; 77% were white, and 76% had a performance status of 0 or 1. The cytologic evaluation, assessed by central review (by F.R.D.) when available (n = 439) or otherwise by local institutional assessment, included 47% with a FAB classification of M1 or M2 (Table 2). In 9 cases, central morphology review changed the cytologic diagnosis from AML to myelodysplasia (MDS) due to less than 30% blast cells counted in the marrow specimen that was submitted. Following the principle of intention-to-treat, these patients are included in all analyses.
Immunophenotyping studies, though not required, were recommended as part of this protocol and were performed on samples of bone marrow or peripheral blood in a central CALGB laboratory. Of the 473 patients, 345 (73%) had samples submitted for immunophenotyping by multiparameter flow cytometry. Antigen expression was evaluable in 301 (87%) of these samples. Cytogenetic analysis was performed in CALGB institutional laboratories on pretreatment samples from 393 (83%) patients. Of these, 323 (82%) were considered adequate after central review (Table 3). Clonal cytogenetic abnormalities were observed in 56% of all cases. Sixty-one patients (19%) had a favorable cytogenetic abnormality involving rearrangement of a CBF gene, either t(8;21), inv (16), or t(16;16).
Response to induction therapy and postremission randomization
CR was achieved by 342 (72%) of the 473 patients. In 264 patients (77% of the responders), CR was achieved following one course of induction therapy. Ninety patients (19%) failed to respond, discontinued the study regimen, and were followed for survival only, and another 41 patients (9%) died during induction.
Of the 342 patients who achieved CR, 309 (90%) were randomized to one of the 2 intensification regimens: 153 to HiDAC and 156 to sequential MCT. The reasons for not being randomized included early relapse (8), residual toxicity from induction therapy (6), bone marrow transplantation (6), other nonprotocol treatment (5), withdrawal by physician (3), death (2), refusal (1), and unknown (2). Table 4 shows the distribution of pretreatment risk factors in the 2 treatment regimens. As would be expected, the randomized treatment groups had similar distributions of these risk factors.
Tolerance and toxicity of postremission therapy
The number of postremission courses completed on each of the treatment regimens is shown in Table 5. There were 2 fatal toxicities with the HiDAC regimen (one from respiratory failure and one from infection), and 3 (all due to infection) with the MCT regimen. All patients experienced grade 3 or 4 myelosuppression following each course of intensification therapy. Table 6 lists the nonhematologic grade 3 to 5 toxicities that occurred in at least 10% of the patients in one of the regimens, based on 294 evaluable cases. The toxicity of the postremission intensification was generally more pronounced in the MCT regimen.
Survival and DFS
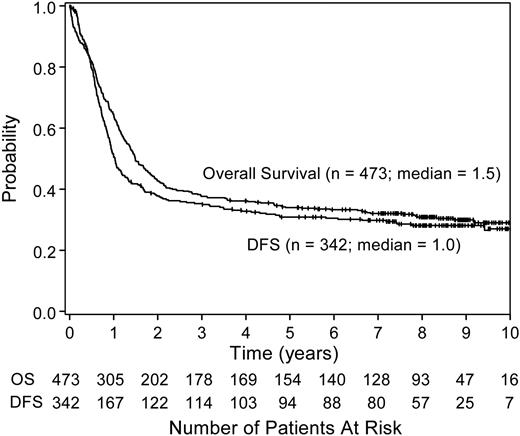
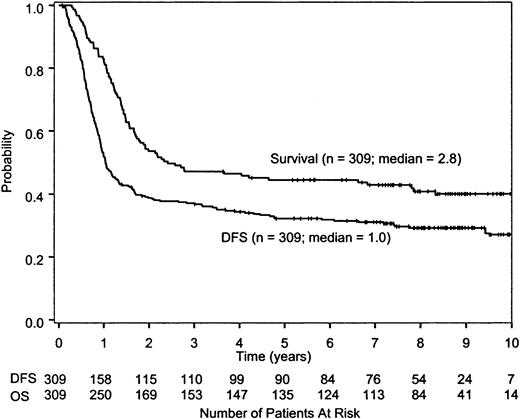
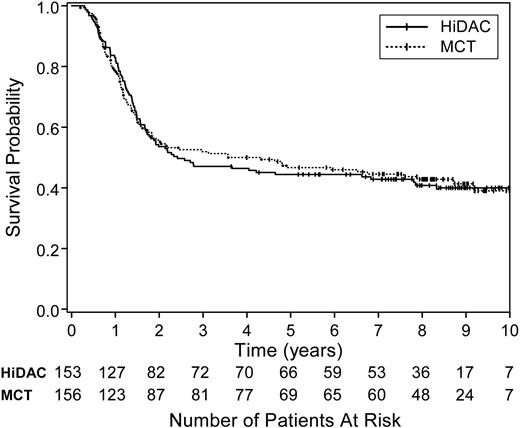
The overall survival and DFS for all treated patients are shown in Figure 1. The 5-year survival rate for all 473 patients was 34% (95% CI, 30%-38%). After a median follow-up time of 8.3 years (minimum, 3.6 years for surviving patients; maximum, 11.4 years), the median survival time for all randomized patients on study 9222 is 2.8 years (95% CI, 1.9-6.8 years), whereas the median DFS is 1.0 year (95% CI, 0.9-1.3 years; Figure 2). There was no difference in DFS between the 2 randomized treatment regimens (P = .66 by log-rank test; Figure 3). The median DFS from date of randomization for patients receiving HiDAC was 1.1 years (95% CI, 0.9-1.7 years) and 1.0 year (95% CI, 0.9-1.3 years) for those receiving MCT. The percent of patients alive and disease free 5 years after achieving CR was 35% (95% CI, 27%-42%) for patients who received HiDAC intensification and 30% (95% CI, 23%-37%) for those who received MCT intensification. The results were similar if the 9 patients with acute promyelocytic leukemia (FAB M3) who were randomized were removed from the analysis. Survival by treatment regimen is shown in Figure 4.
Overall survival and DFS of all treated patients. The median survival time for the 473 treated patients enrolled on CALGB study 9222 was 1.5 years (95% CI, 1.3-1.8 years). The median DFS for the 342 patients who achieved a CR was 1 year (95% CI, 0.9-1.3 years). OS indicates overall survival.
Overall survival and DFS of all treated patients. The median survival time for the 473 treated patients enrolled on CALGB study 9222 was 1.5 years (95% CI, 1.3-1.8 years). The median DFS for the 342 patients who achieved a CR was 1 year (95% CI, 0.9-1.3 years). OS indicates overall survival.
Survival and DFS of all randomized patients. The median survival time from randomization was 2.8 years (95% CI, 1.9-6.8 years). Approximately 46% survived for at least 5 years after randomization (95% CI, 42%-52%). The median DFS was 1.0 year (95% CI, 0.9-1.3 years). Approximately 32% survived free of disease for at least 5 years after randomization (95% CI, 27%-38%).
Survival and DFS of all randomized patients. The median survival time from randomization was 2.8 years (95% CI, 1.9-6.8 years). Approximately 46% survived for at least 5 years after randomization (95% CI, 42%-52%). The median DFS was 1.0 year (95% CI, 0.9-1.3 years). Approximately 32% survived free of disease for at least 5 years after randomization (95% CI, 27%-38%).
DFS by treatment regimen. The DFS for the 309 randomized patients did not differ by postremission treatment arm (log-rank test, P = .66). The medians were 1.1 years (95% CI, 0.9-1.7 years) for those assigned to receive 3 courses of HiDAC (solid line) and 1.0 year (95% CI, 0.9-1.3 years) for those assigned to receive sequential MCT (dotted line). Approximately 35% (95% CI, 27%-42%) survived disease free for at least 5 years after randomization to HiDAC and 30% (95% CI, 23%-37%) after randomization to MCT.
DFS by treatment regimen. The DFS for the 309 randomized patients did not differ by postremission treatment arm (log-rank test, P = .66). The medians were 1.1 years (95% CI, 0.9-1.7 years) for those assigned to receive 3 courses of HiDAC (solid line) and 1.0 year (95% CI, 0.9-1.3 years) for those assigned to receive sequential MCT (dotted line). Approximately 35% (95% CI, 27%-42%) survived disease free for at least 5 years after randomization to HiDAC and 30% (95% CI, 23%-37%) after randomization to MCT.
Survival by treatment regimen. The survival for the 309 randomized patients did not differ by postremission treatment arm (log-rank test, P = .89). The 5-year survival proportions were 44% (95% CI, 36%-52%) for those assigned to receive 3 courses of HiDAC (solid line) and 46% (95% CI, 38%-54%) for those assigned to receive sequential MCT (dotted line).
Survival by treatment regimen. The survival for the 309 randomized patients did not differ by postremission treatment arm (log-rank test, P = .89). The 5-year survival proportions were 44% (95% CI, 36%-52%) for those assigned to receive 3 courses of HiDAC (solid line) and 46% (95% CI, 38%-54%) for those assigned to receive sequential MCT (dotted line).
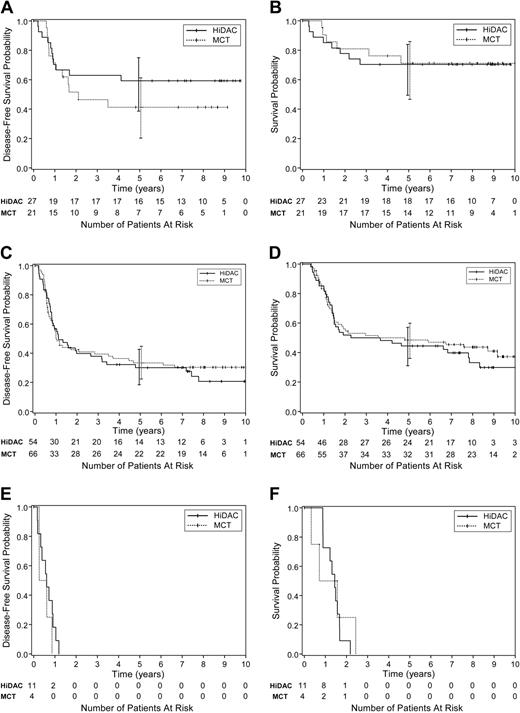
Although there was no evidence of any overall treatment effect, we conducted an exploratory analysis of potential treatment and covariate interactions using standard proportional hazards regression models. The intent was to see whether there was any evidence of differential treatment effects in prognostic subgroups. Pretreatment characteristics such as age, sex, race, performance status, white blood cell (WBC) count, hemoglobin, platelet count, CD56 expression, and FAB subtype were evaluated, and no significant differences were found. Neither DFS nor overall survival were significantly different between the HiDAC and MCT postremission regimens when we analyzed all patients with adequate cytogenetic results or when the favorable, intermediate, and unfavorable cytogenetic risk subgroups were analyzed separately (Figure 5A-F). These analyses were limited by the small sample sizes in most of these subsets. Thus, these subset analyses lack the power to completely exclude a benefit of one treatment arm over the other. Details are not presented, but the only pretreatment variable prognostic for DFS in this study overall was the cytogenetic risk group. Postremission treatment regimen was a nonsignificant factor as expected from the log-rank analysis. Thus, there is no evidence that the treatment effect varies by cytogenetic risk group even though the overall results vary by risk group.
The DFS and overall survival by treatment arm for favorable, intermediate, and unfavorable cytogenetic risk groups. Results are shown according to the CALGB classification for cumulative incidence of relapse.11 There were only small numbers of patients within several subsets, and the 95% CIs at 5 years (vertical bars) overlap for each comparison. (A) DFS by treatment regimen among patients with favorable risk cytogenetics. (B) Survival by treatment regimen among patients with favorable risk cytogenetics. (C) DFS by treatment regimen among patients with intermediate risk cytogenetics. (D) Survival by treatment regimen among patients with intermediate risk cytogenetics. (E) DFS by treatment regimen among patients with unfavorable risk cytogenetics. (F) Survival by treatment regimen among patients with unfavorable risk cytogenetics.
The DFS and overall survival by treatment arm for favorable, intermediate, and unfavorable cytogenetic risk groups. Results are shown according to the CALGB classification for cumulative incidence of relapse.11 There were only small numbers of patients within several subsets, and the 95% CIs at 5 years (vertical bars) overlap for each comparison. (A) DFS by treatment regimen among patients with favorable risk cytogenetics. (B) Survival by treatment regimen among patients with favorable risk cytogenetics. (C) DFS by treatment regimen among patients with intermediate risk cytogenetics. (D) Survival by treatment regimen among patients with intermediate risk cytogenetics. (E) DFS by treatment regimen among patients with unfavorable risk cytogenetics. (F) Survival by treatment regimen among patients with unfavorable risk cytogenetics.
Discussion
AML continues to be a therapeutic challenge. Although most patients under 60 years of age achieve an initial CR after 1 or 2 courses of combination chemotherapy, the majority of these individuals suffer a relapse of their disease within 2 years. Only about one third of those who achieve a CR survive free of disease more than 5 years. The failure to cure more patients with AML is due in large part to the inherent level of resistance to cytotoxic chemotherapy agents within the leukemia stem cells. Various strategies have been developed and tested, ranging from prolonged maintenance treatment with nonmyelosuppressive doses of common antileukemic agents, to postremission intensification using doses that often exceed those successfully used for inducing remissions, to allogeneic stem cell transplantation, exploiting myeloablative chemotherapy and the antileukemic activity of the donor immune system.19-23 Barriers to a higher cure rate for AML thus include drug-resistant leukemia, prolonged and repeated pancytopenia, and treatment-related morbidity and mortality.
In a previous CALGB study (8525), 44% of patients younger than 60 years old who achieved a CR after conventional doses of cytarabine and daunorubicin survived free of disease for 4 years after receiving up to 4 courses of HiDAC as postremission consolidation therapy followed by 4 less intensive courses of maintenance therapy.1 In the present randomized trial, we evaluated the outcomes when a novel regimen that substituted different antileukemia drugs for cytarabine was compared to HiDAC alone for postremission therapy. The trial had sufficient power to detect a hazard ratio of 1.6 between the arms. However, we observed no significant differences in any outcome measure, either DFS or survival, for the 2 treatments, nor was there any evidence of a differential treatment effect in the only important prognostic subgroups as defined by cytogenetics. The latter analyses were limited by small numbers of patients within each subset.
Why did we think that the MCT arm might improve on the outcome after HiDAC alone? The doses and drugs used in the 2 novel consolidation combinations in this study were determined to demonstrate activity in patients with AML in relapse through a series of phase 1/2 trials previously reported by the CALGB or its member institutions.3-7 The sequence of HiDAC followed by cyclophosphamide/etoposide and by mitoxantrone/diaziquone was itself tested in CALGB 9022, a large group-wide phase 2 study.2 After a median follow-up time of 63 months for surviving patients, the median survival time for all 243 eligible patients in that study was 21 months, whereas the median DFS for the 186 (77%) who achieved a CR was 12 months. These results are consistent with those observed on the MCT arm of the current study.
In a similar way, we compared the results of the HiDAC arm of this study with the HiDAC arm of the CALGB 8525 trial.1 In the latter study, 467 patients younger than 60 years old achieved a CR after 1 or 2 courses of conventional cytarabine and daunorubicin. One hundred fifty-six were assigned to receive HiDAC for consolidation. However, only 62% received all 4 of the planned courses. Following the HiDAC, 4 monthly maintenance courses with one dose of daunorubicin (45 mg/m2) and 5 days of cytarabine (100 mg/m2/12 h subcutaneously) were also given. After a median follow-up time from randomization of 64 months, the estimated probability of remaining alive and disease free after 5 years was 42%, and the probability of survival after 5 years was 49%. The comparable estimates for the HiDAC arm on the present study were 35% and 44%, slightly, but not significantly, lower than the CALGB 8525 results. Although not a randomized comparison, this suggests that one additional course of HiDAC plus 4 subsequent courses of daunorubicin/cytarabine maintenance do not add appreciably to the results achieved with 3 courses of HiDAC.
Cytogenetics are important independent determinants of response in AML. When large numbers of patients receive uniform treatment, the outcomes of cytogenetically determined subsets can be compared. Previously, we have reported that HiDAC is more effective postremission therapy for CBF AML than is intermediate (400 mg/m2) or conventional doses (100 mg/m2) of cytarabine.24 Pooled data from several CALGB trials including the one reported here suggest that multiple exposures to HiDAC may also improve the cure rate for certain subsets such as AML patients with t(8;21) or with inv(16) or t(16;16).25,26 HiDAC is not very effective for unfavorable cytogenetic subgroups, yielding a DFS of only about 20%.24 Better antileukemia agents are needed for more effective postremission therapy.
The interactions between a number of complex survival and resistance mechanisms determine whether AML cells are eradicated by treatment. The CALGB is currently testing different strategies designed to overcome multiple mechanisms of drug resistance, using inhibitors of p-glycoprotein (MDR1)-mediated drug efflux pathways and myeloablative chemotherapy with autologous stem cell transplantation.27,28 It remains to be determined whether these approaches can improve the survival of patients with AML.
Appendix
The following CALGB main member institutions, with principal investigator (grant support from the National Cancer Institute), participated in this study: CALGB Statistical Center, Durham, NC, Stephen George (CA33601); Dana Farber Cancer Institute, Boston, MA, George P. Canellos (CA32291); Dartmouth Medical School, Norris Cotton Cancer Center, Lebanon, NH, Marc S. Ernstoff (CA04326); Duke University Medical Center, Durham, NC, Jeffrey Crawford (CA47577); Finsen Institute, Copenhagen, Denmark, Philip Preben; Massachusetts General Hospital, Boston, MA, Michael L. Grossbard (CA12449); Mount Sinai School of Medicine, New York, NY, Lewis R. Silverman (CA04457); North Shore-Long Island Jewish Medical Center, Manhasset, NY, Daniel R. Budman (CA35279); Rhode Island Hospital, Providence, RI, William Sikov (CA08025); SUNY Upstate Medical University, Syracuse, NY, Stephen L. Graziano (CA21060); University of Alabama Birmingham, Birmingham, AL, Robert Diasio (CA47545); University of California at San Diego, San Diego, CA, Stephen L. Seagren (CA11789); University of Chicago Medical Center, Chicago, IL, Gini Fleming (CA41287); University of Iowa, Iowa City, IA, Gerald Clamon (CA47642); University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman (CA31983); University of Massachusetts Medical Center, Worcester, MA, Pankaj Bhargava (CA37135); University of Minnesota, Minneapolis, MN, Bruce A. Peterson (CA16450); University of Missouri/Ellis Fischel Cancer Center, Columbia, MO, Michael C. Perry (CA12046); University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea (CA47559); University of Tennessee Memphis, Memphis, TN, Harvey B. Niell (CA47555); Vermont Cancer Center, Burlington, VT, Hyman B. Muss (CA77406); Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd (CA03927); Walter Reed Army Medical Center, Washington, DC, Joseph J. Drabeck (CA26806); Washington University School of Medicine, St Louis, MO, Nancy Bartlett (CA77440); and Weill Medical College of Cornell University, New York, NY, Scott Wadler (CA0796).
Prepublished online as Blood First Edition Paper, November 30, 2004; DOI 10.1182/blood-2004-08-2977.
This study was conducted by the Cancer and Leukemia Group B and was supported in part by Public Health Service grants 16058, 31946, 33601, and 101140 from the National Cancer Institute (Bethesda, MD) and the Coleman Leukemia Research Fund. The following grants from the National Cancer Institute provided support: CA47577 (J.O.M., S.L.G., R.K.D.), CA33601 (S.L.G., R.K.D.), CA12449 (P.C.A.), CA03927 (B.L.P.), CA35279 (J.E.K.), CA02599 (M.R.B.), CA21060 (F.R.D.), CA77658 (C.D.B.), CA41287 (R.A.L.), and CA31946 (the CALGB). A complete list of the members of the CALGB appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are indebted to Richard K. Dodge, who is recently deceased, for his major contributions to the design, conduct, and analysis of this study. We thank Michael Kelly for editorial assistance; Robert C. Barrier Jr for statistical assistance; and Erin Trikha, Ajiri Smith, and Eva Hoke for central data management on this study.