Abstract
We investigated the role of vascular endothelial (VE)–cadherin in blood vessel morphogenesis and established a temporal correlation linking the failure in vessel morphogenesis in VE-cadherin null embryos to a specific step in vasculogenesis. We showed that the sequence in which blood vessels failed followed the order in which they had formed (ie, those forming first—yolk sac, allantoic and endocardial vessels—were the first to display morphologic abnormalities). We next showed that in place of normal reticulated networks of blood vessels, clusters of platelet endothelial cell adhesion molecule–positive (PECAM+) cells formed within cultured allantois explants from VE-cadherin null embryos. Similarly, a function-blocking VE-cadherin antibody, BV13, caused PECAM+ cell clusters to form in cultured allantois explants from normal mice. Finally, we demonstrated that formation of PECAM+ cell clusters in response to BV13 was not due to a disruption in the formation of nascent vessels but was due to the actual disassembly of nascent vessels. Based on these findings, we conclude that the events of de novo blood vessel formation up to the point at which a vascular epithelium forms (ie, nascent vessels with lumens) are not dependent on VE-cadherin and that VE-cadherin, whose expression is up-regulated following vascular epithelialization, is required to prevent the disassembly of nascent blood vessels.
Introduction
De novo blood vessel formation (vasculogenesis) is the process by which endothelial cell progenitors (angioblasts) interact to form vascular epithelia (ie, nascent blood vessels). As with all epithelia, cadherins play an essential role in mediating vascular epithelial adherens junction formation. Despite intense interest in the functional roles of cadherin family members, the specific roles of cadherins in the formation and regulation of vascular epithelium remains to be elucidated. Endothelial cells express both neural (N)–cadherin1,2 and vascular endothelial (VE)–cadherin3 and targeted deletion of these genes in mice leads to early embryonic death with associated severe vascular anomalies.4-6
A mechanistic understanding of the observed vascular defects in N- and VE-cadherin knockouts has not yet been achieved. In N-cadherin–deficient embryos, a number of vascular anomalies were noted, but the anomalies were not attributed to defective vasculogenesis or angiogenesis. In VE-cadherin knockouts, Carmeliet et al5 concluded that because lumenized vessels were evident, the basis of the defects was a failure in the process of angiogenesis. By contrast, Gory-Faure et al,6 noting defects in the yolk sac vessels concomitant with apparent normal intraembryonic vessels (ie, dorsal aortae), concluded that extraembryonic vasculogenesis was dependent on VE-cadherin activity, whereas intraembryonic vasculogenesis was not.
Herein, we investigated the vascular phenotype of VE-cadherin–deficient embryos to reconcile the disparate views as to the whether the protein is required in the process of vasculogenesis. In the first set of studies, we characterized the temporal appearance of vascular anomalies in blood vessel formation occurring in different embryonic tissues of null embryos. In the second series of studies, we used two VE-cadherin monoclonal antibodies, BV13 and BV14, in conjunction with wild-type allantois explant culture to investigate the role of VE-cadherin in the de novo formation of blood vessels. Both BV13, which is directed against the distal extracellular domain of VE-cadherin (EC1), and BV14, which is directed against the more proximal extracellular domain (EC4),7 have been shown to have function-blocking activity as judged by their ability to disrupt the formation of cordlike vascular structures in cultured mouse lung endothelial cells8 and to disrupt tumor neovascularization in vivo.7,9
Our findings establish that VE-cadherin function is not critical to the process by which angioblasts transition to form a nascent vascular epithelium. However, because nascent vessels disassemble in the absence of VE-cadherin activity, we reason that VE-cadherin is critically required to maintain or prevent (or both) the disassembly of nascent embryonic blood vessels.
Materials and methods
Antibodies
Purified or fluorescently conjugated antibodies to platelet endothelial cell adhesion molecule (PECAM/CD31, clone Mec-13.3) and Flk1, fluorescently conjugated isotype control antibodies, and antibodies to VE-cadherin (CD144, clone 11D4.1) were purchased from BD PharMingen (San Diego, CA). Monoclonal antibodies to VE-cadherin, BV13 and BV14, were generated as previously described.7,10 Fluorescently conjugated secondary antibodies were purchased from Jackson ImmunoResearch Labs (West Grove, PA).
Allantois culture
Allantoides were dissected from mouse embryos and cultured as previously described.11,12 Four allantois culture schemes were used. (1) To investigate the role of VE-cadherin in vasculogenesis, prevascularized mesoderm of 7.8-days postcoitum (dpc) murine allantoides was cultured for a total of 18 hours (37°C; 5% CO2) in the presence or absence of experimental reagent. (2) To establish whether VE-cadherin was acting early or late in vasculogenesis, we modified the aforementioned culture scheme and divided the 18-hour culture period into two 9-hour culture periods. Allantois explants were either cultured for 9 hours in the presence of reagent and then cultured an additional 9 hours in serum-containing medium or cultured for 9 hours in serum-containing medium and then exposed to experimental reagent during the last 9 hours of culture. (3) To investigate vascular remodeling associated with vasculogenesis, 8.5-dpc murine allantoides were cultured for a total of 18 hours (37°C; 5% CO2) in the presence or absence of experimental reagent. (4) Finally, to examine the response of established vessels, 8.5-dpc murine allantoides were cultured for 18 hours (37°C; 5% CO2) to allow formation of stable vascular networks and then cultured for an additional 24 hours (37°C; 5% CO2) in the presence or absence of experimental reagent.
Fixation, immunolabeling, and microscopy of mouse embryos and cultured allantoides
Procedures for whole-mount immunolabeling of mouse embryos and for immunolabeling cultured allantoides have been described previously.11,12 Specimens were mounted in an antiphotobleaching mounting medium13 and all images were acquired at approximately 25°C. Conventional fluorescence or differential interference contrast images of allantois cultures were obtained using a Leica DMR research-grade microscope equipped with Leica objectives (5×/0.15 HC PL Fluotar, 10×/0.30 HC PL Fluotar, 20× /0.70 HC Plan Apo, 40×/0.85 HC Plan Apo; Leica, Heidelberg, Germany) and a SPOT-RT camera (Diagnostic Instruments, Sterling Heights, MI). Images were acquired using SPOT-RT 3.5.7 software (Diagnostic Instruments). Laser confocal microscopic images were acquired using Zeiss 5×/0.15 and 10×/0.30 objectives (Zeiss, Thornwood, NY) on a Bio-Rad MRC 1024 laser-scanning confocal microscope (Bio-Rad, Microscopy Division, Cambridge, MA) equipped with Lasersharp 2000 software (Bio-Rad Cell Science Division, Hemel Hempstead, United Kingdom). Confocal z-series were projected using Image J 1.31v (National Institutes of Health, Bethesda, MD). Montages of microscopic images were generated using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Fluorescence-activated cell scanning
Cultured allantoides were dissociated in 1 × trypsin-EDTA (ethylenediaminetetraacetic acid) solution (40 allantoides/0.5 mL) for 7 minutes at 37°C. Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 2% penicillin/streptomycin/L-glutamine (PS-G; 0.5 mL) was added to inhibit the trypsin. The cell suspension was centrifuged at 1100g for 10 minutes using an IEC Centra CL2 benchtop centrifuge (IEC, Needham Heights, MA) and the cell pellet was resuspended in 0.5 mL DMEM containing 10% FBS and 2% PS-G and incubated for 40 minutes at 37°C. The cell suspension was resuspended in 1% bovine serum albumin (BSA), 0.01% NaN3, and 10 ng/mL DNAse (fluorescence-activated cell scanning [FACS] buffer) and pelleted by centrifugation as before. Cells were counted on a hemocytometer, separated into tubes for immunolabeling (final concentration of 1 × 106 cells/mL), and immunolabeled with fluorescently conjugated isotype control antibodies, fluorescein isothiocyanate (FITC)–conjugated PECAM, phycoerythrin (PE)–conjugated Flk-1, or both for 40 minutes on ice. Antibodies were added to the cell suspensions at a final concentration of 100 ng/1 × 106 cells and incubated 40 minutes at 4°C. Cells were pelleted and washed in FACS buffer. The washed cells were resuspended in FACS buffer containing 0.1 mg/mL propidium iodide (Sigma, St Louis, MO). After propidium iodide treatment the cells were washed in FACS buffer, centrifuged, resuspended in 0.2 mL FACS buffer, and subjected to FACS analysis using a FACSCalibur (Becton Dickinson, San Jose, CA).
Results
Temporal failure of vasculogenesis in VE-cadherin–null embryos
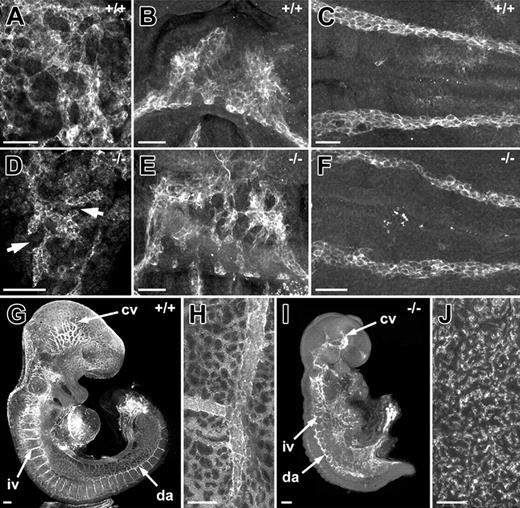
The presence of both normal and abnormal blood vessels in the VE-cadherin nulls, as described by Gory-Faure et al,6 led us to hypothesize that the vascular defects might correlate with the temporal order in which blood vessels are formed de novo in the mouse embryo. This hypothesis is based on our previous findings11 showing that vasculogenesis is initiated regionally at different times during development with extraembryonic vessels being the first to form (yolk sac and allantoic vessels) followed by the endocardium, dorsal aortae/sinus venosus, and the cranial vessels. Our analysis of VE-cadherin null embryos showed that vessels of the allantois and endocardium were abnormal in 8.5-dpc embryos, whereas the dorsal aortae in these embryos appeared normal (Figure 1). Further support for a temporal failure in blood vessels in the VE-cadherin nulls was the finding that the dorsal aortae, which appeared normal at 8.5 dpc, were abnormal in 9.5-dpc null embryos (Figure 1F,I). Based on these observations and the temporal order for the formation of specific embryonic vascular networks (yolk sac, allantois, endocardium, dorsal aortae/sinus venosus, and cranial vasculature), it was evident that the vascular networks that had developed first (ie, yolk sac and allantoic vessels) were the first to fail. Vessels that form later in development, such as the dorsal aortae, failed at a later stage in development.
Temporal failure of vasculogenesis in VE-cadherin null embryos. (A,D) High-magnification images of PECAM-immunolabeled allantoides from normal (A) and VE-cadherin null (D) 8.5-dpc embryos. Note that the extent of vascularization is greatly reduced in the VE-cadherin nulls as compared to controls. Arrows in panel D point to discontinuous PECAM+ vessels not apparent in the vascular network of normal allantois explants. (B,E) PECAM-immunolabeled endocardial cells from normal (B) and VE-cadherin null (E) 8.5-dpc embryos. Note the aggregates of PECAM+ cells in the hearts of the VE-cadherin nulls as compared to the continuous layer of PECAM+ cells that comprise the endocardial tube of the normal heart. (C,F) PECAM-immunolabeled dorsal aortae from normal (C) and VE-cadherin null (F) 8.5-dpc embryos. (G,I) PECAM-immunolabeled 9.5-dpc VE-cadherin wild-type (G) and null (I) embryos. Pronounced abnormalities of the dorsal aortae (da), intersomitic vessels (iv), and cranial vessels (cv) are apparent in the 9.5-dpc VE-cadherin null embryo as compared to the normal 9.5-dpc embryo. (H,J) PECAM-immunolabeled yolk sacs of 9.5-dpc VE-cadherin wild-type (H) and null (J) embryos. The VE-cadherin wild-type yolk sac contains large PECAM+ blood vessels and continuous networks of smaller vessels, whereas the yolk sac of the null contains only clusters of PECAM+ cells. Panels A through J are confocal images. Bars equal 100 μm.
Temporal failure of vasculogenesis in VE-cadherin null embryos. (A,D) High-magnification images of PECAM-immunolabeled allantoides from normal (A) and VE-cadherin null (D) 8.5-dpc embryos. Note that the extent of vascularization is greatly reduced in the VE-cadherin nulls as compared to controls. Arrows in panel D point to discontinuous PECAM+ vessels not apparent in the vascular network of normal allantois explants. (B,E) PECAM-immunolabeled endocardial cells from normal (B) and VE-cadherin null (E) 8.5-dpc embryos. Note the aggregates of PECAM+ cells in the hearts of the VE-cadherin nulls as compared to the continuous layer of PECAM+ cells that comprise the endocardial tube of the normal heart. (C,F) PECAM-immunolabeled dorsal aortae from normal (C) and VE-cadherin null (F) 8.5-dpc embryos. (G,I) PECAM-immunolabeled 9.5-dpc VE-cadherin wild-type (G) and null (I) embryos. Pronounced abnormalities of the dorsal aortae (da), intersomitic vessels (iv), and cranial vessels (cv) are apparent in the 9.5-dpc VE-cadherin null embryo as compared to the normal 9.5-dpc embryo. (H,J) PECAM-immunolabeled yolk sacs of 9.5-dpc VE-cadherin wild-type (H) and null (J) embryos. The VE-cadherin wild-type yolk sac contains large PECAM+ blood vessels and continuous networks of smaller vessels, whereas the yolk sac of the null contains only clusters of PECAM+ cells. Panels A through J are confocal images. Bars equal 100 μm.
VE-cadherin–null allantois explants fail to undergo normal vascular morphogenesis in culture
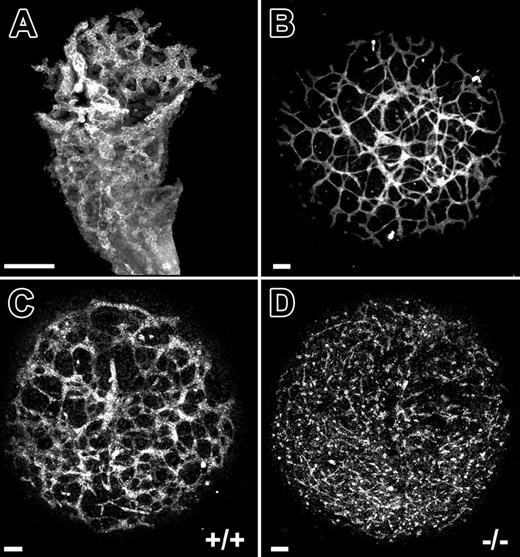
To further assess the role of VE-cadherin in the temporal events of vascular morphogenesis, we turned to the allantois explant culture model.12 At 8.5 dpc, the allantois has an extensive network of blood vessels (Figure 2A). When 8.5-dpc allantois explants are cultured for 18 hours, the explants flatten and the network of PECAM+ capillary-like vessels is evident within the adherent explants (Figure 2B). Following 18 hours of culture, allantois explants from 8.5-dpc VE-cadherin nulls failed to develop interconnected networks of PECAM+ cells as compared to wild-type controls (compare Figure 2C and 2D). Instead, only isolated clusters of PECAM+ cells were present (Figure 2D).
VE-cadherin–null allantois explants fail to undergo normal vascular morphogenesis in culture. (A) PECAM-immunolabeled allantois from a normal 8.5-dpc mouse embryo. (B) PECAM-immunolabeled allantois explant that was derived from an 8.5-dpc normal mouse embryo and cultured for 18 hours. (C-D) PECAM-immunolabeled allantois explants from 8.5-dpc VE-cadherin+/+ (C) and VE-cadherin–/– (D) embryos after 18 hours of culture. The interconnected networks of PECAM+ cells evident in wild-type explants (C) are absent in the VE-cadherin–/– explants (D) and are replaced by isolated clusters of PECAM+ cells. Bars equal 100 μm. All panels are confocal images.
VE-cadherin–null allantois explants fail to undergo normal vascular morphogenesis in culture. (A) PECAM-immunolabeled allantois from a normal 8.5-dpc mouse embryo. (B) PECAM-immunolabeled allantois explant that was derived from an 8.5-dpc normal mouse embryo and cultured for 18 hours. (C-D) PECAM-immunolabeled allantois explants from 8.5-dpc VE-cadherin+/+ (C) and VE-cadherin–/– (D) embryos after 18 hours of culture. The interconnected networks of PECAM+ cells evident in wild-type explants (C) are absent in the VE-cadherin–/– explants (D) and are replaced by isolated clusters of PECAM+ cells. Bars equal 100 μm. All panels are confocal images.
VE-cadherin function-blocking antibody treatment phenocopies vascular anomalies observed in genetic VE-cadherin deficiency
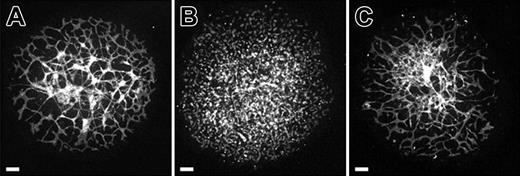
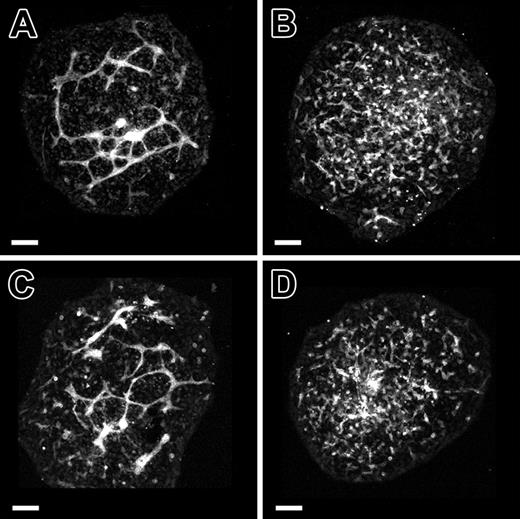
We next evaluated the role of VE-cadherin in vascular morphogenesis using function-blocking VE-cadherin monoclonal antibodies in conjunction with the allantois explant culture model. Treatment of allantois explants from normal 8.5-dpc mouse embryos with BV13 disrupted vascular morphogenesis (Figure 3B). In place of interconnected vessels that are present in control cultures (Figure 3A), only PECAM+ clusters of cells were evident in the treated cultures (Figure 3B). By contrast, BV14 treatment produced little or no effect on vascular morphogenesis occurring in cultured 8.5-dpc allantoides (Figure 3C). The fact that BV13 treatment produced PECAM+ aggregates in 8.5-dpc allantois explants initially possessing extensive vascular networks (Figure 2A) suggested that the antibody was acting to promote blood vessel disassembly.
VE-cadherin function-blocking antibody treatment phenocopies vascular anomalies observed in genetic VE-cadherin deficiency. (A-C) Confocal images of PECAM-immunolabeled allantois explants from normal 8.5-dpc embryos following 18 hours of culture in control medium (A), control medium plus BV13 monoclonal antibody (24 μg IgG/mL; B), or BV14 monoclonal antibody (24 μg IgG/mL; C). The interconnected networks of PECAM+ cells evident in control explants (A) are absent in the BV13-treated explants (B). Instead, only isolated clusters of PECAM+ cells are present. Note that a dose-response series was performed using BV13 IgG and showed a moderate effect at 8 μg IgG/mL and no effect at 3 μg IgG/mL. In contrast to BV13, treatment with BV14 had only a modest effect on allantois explant vascularization (compare panels A and C) when added at concentrations identical to that of BV13 (24 μg IgG/mL) or when added at double the concentration (50 μg IgG/mL) at which BV13 treatment caused the complete dysmorphogenesis of blood vessels. Bars equal 100 μm.
VE-cadherin function-blocking antibody treatment phenocopies vascular anomalies observed in genetic VE-cadherin deficiency. (A-C) Confocal images of PECAM-immunolabeled allantois explants from normal 8.5-dpc embryos following 18 hours of culture in control medium (A), control medium plus BV13 monoclonal antibody (24 μg IgG/mL; B), or BV14 monoclonal antibody (24 μg IgG/mL; C). The interconnected networks of PECAM+ cells evident in control explants (A) are absent in the BV13-treated explants (B). Instead, only isolated clusters of PECAM+ cells are present. Note that a dose-response series was performed using BV13 IgG and showed a moderate effect at 8 μg IgG/mL and no effect at 3 μg IgG/mL. In contrast to BV13, treatment with BV14 had only a modest effect on allantois explant vascularization (compare panels A and C) when added at concentrations identical to that of BV13 (24 μg IgG/mL) or when added at double the concentration (50 μg IgG/mL) at which BV13 treatment caused the complete dysmorphogenesis of blood vessels. Bars equal 100 μm.
Established vessels formed in long-term cultures of normal 8.5-dpc allantoides exhibit incremental disassembly in response to BV13 treatment
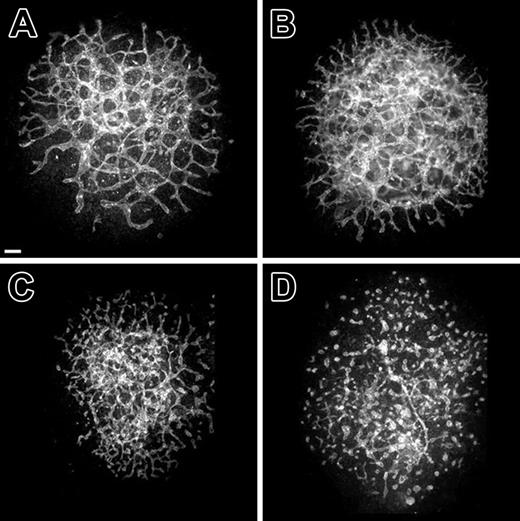
To substantiate the hypothesis that BV13 antibody was promoting the disassembly of blood vessels, we evaluated its effects on established vessels within 8.5-dpc explants cultured for 18 hours prior to administration of the antibody. Within 6 hours after BV13 treatment, allantois vascular networks had discontinuities in the normally interconnected vascular network (Figure 4C). Following 12 hours of BV13 treatment, networks were absent and only clusters of PECAM+ cells were apparent (Figure 4D). Taken together, these findings suggest that VE-cadherin function is required to prevent disassembly of nascent embryonic blood vessels and that in the absence of VE-cadherin activity nascent blood vessels incrementally disassemble over a 12-hour period.
Established vessels formed in long-term cultures of normal 8.5-dpc allantoides exhibit incremental disassembly in response to BV13 treatment. (A-D) Confocal images of PECAM-immunolabeled allantois cultures derived from normal 8.5-dpc embryos. (A) A control explant cultured for 30 hours. (B-D) Explants that were first cultured for 18 hours and then cultured for an additional 2 (B), 6 (C), or 12 hours (D) in the presence of BV13 (24 μg IgG/mL). Alterations in vascular morphogenesis are evident at 6 hours after addition of BV13 (C). By 12 hours after BV13 administration, the now 30-hour-old culture (D), as compared to control (A), shows an almost complete dysmorphogenesis of the blood vessels. Bars equal 100 μm.
Established vessels formed in long-term cultures of normal 8.5-dpc allantoides exhibit incremental disassembly in response to BV13 treatment. (A-D) Confocal images of PECAM-immunolabeled allantois cultures derived from normal 8.5-dpc embryos. (A) A control explant cultured for 30 hours. (B-D) Explants that were first cultured for 18 hours and then cultured for an additional 2 (B), 6 (C), or 12 hours (D) in the presence of BV13 (24 μg IgG/mL). Alterations in vascular morphogenesis are evident at 6 hours after addition of BV13 (C). By 12 hours after BV13 administration, the now 30-hour-old culture (D), as compared to control (A), shows an almost complete dysmorphogenesis of the blood vessels. Bars equal 100 μm.
VE-cadherin antibody-mediated blood vessel disassembly does not involve alteration of mesodermal or endothelial subpopulations
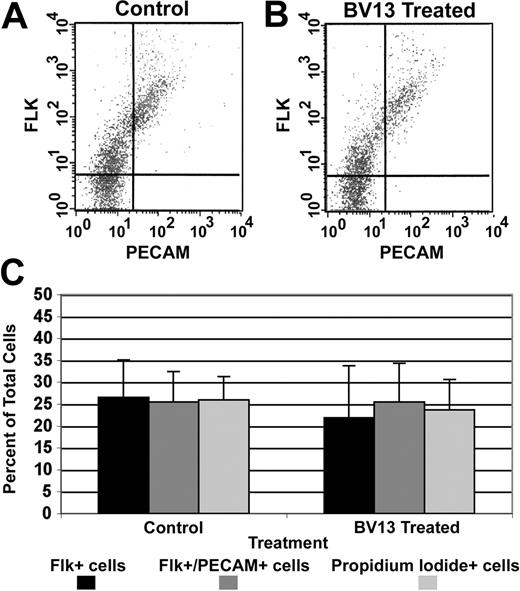
We next used flow cytometry to evaluate the effects of BV13 antibody on subpopulations of cells within the allantois. The analysis revealed that BV13 antibody treatment did not elicit significant changes in either the number of angioblasts (Flk1+/PECAM– population) or endothelial cells (Flk1+/PECAM+ population) present in 8.5-dpc explant cultures (Figure 5). Furthermore, the antibody treatment did not influence the number of dead cells in the explant cultures as measured by propidium iodide exclusion. These findings suggest that blood vessel disassembly induced by BV13 treatment is not the result of significant levels of cell death.
VE-cadherin antibody-mediated blood vessel disassembly does not involve alteration of mesodermal or endothelial subpopulations. (A-B) Representative FACS profiles of cells isolated from control and BV13-treated (24 μg IgG/mL) 8.5-dpc cultured allantoides, respectively. (C) A graphic compilation of the FACS data from 4 experiments comparing control versus BV13-treated cultures. Together these analyses show no significant changes in the Flk1+ (angioblasts; filled bars), Flk1+/PECAM+ (endothelial cells; dark gray bars), or propidium iodide+ (dead cells; light gray bars) cell populations in BV13-treated allantois cultures as compared to control cultures. Error bars indicate the standard deviation of the mean.
VE-cadherin antibody-mediated blood vessel disassembly does not involve alteration of mesodermal or endothelial subpopulations. (A-B) Representative FACS profiles of cells isolated from control and BV13-treated (24 μg IgG/mL) 8.5-dpc cultured allantoides, respectively. (C) A graphic compilation of the FACS data from 4 experiments comparing control versus BV13-treated cultures. Together these analyses show no significant changes in the Flk1+ (angioblasts; filled bars), Flk1+/PECAM+ (endothelial cells; dark gray bars), or propidium iodide+ (dead cells; light gray bars) cell populations in BV13-treated allantois cultures as compared to control cultures. Error bars indicate the standard deviation of the mean.
VE-cadherin function-blocking antibody treatment disrupts vascular morphogenesis in the 7.8-dpc allantois explant culture model
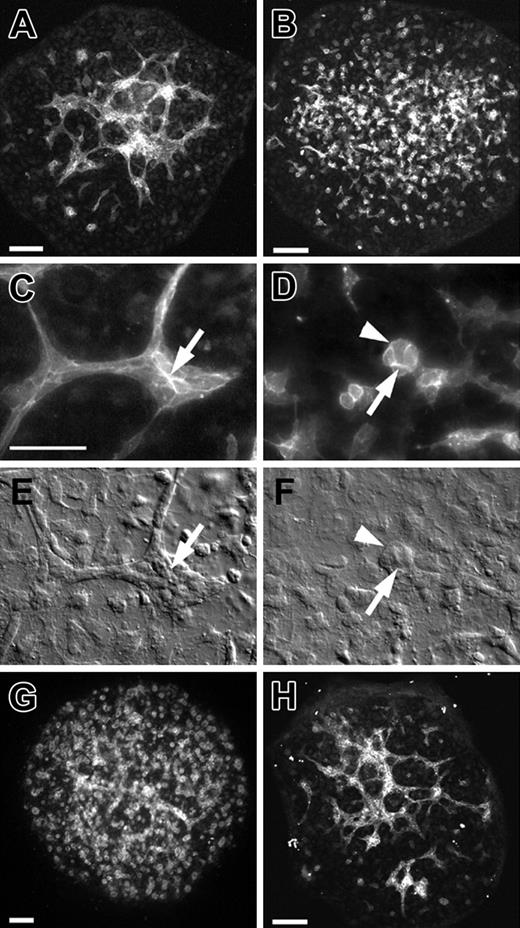
In addition to establishing the role of VE-cadherin in preventing the disassembly of newly formed blood vessels, we also evaluated its potential role in the process of de novo blood vessel formation. To do this we used the 7.8-dpc allantois explant culture model in which prevascularized allantois explants are obtained from 7.8-dpc embryos and cultured for 18 hours. During this culture period, blood vessels form de novo (Figure 6A). As shown in Figure 6B, introduction of BV13 at the initiation of the culture period had profound consequences on vascular morphogenesis. In contrast to control explants (Figure 6A) in which interconnected PECAM+ networks of vessels were evident, only clusters of PECAM+ cells could be observed in the BV13-treated cultures (Figure 6B). High-magnification fluorescence imaging of the BV13-treated explants shows that the clusters are composed of small numbers of cells with PECAM immunolabeling in cell-cell junctions (compare arrows in control, Figure 6C, to BV13 treated, Figure 6D). The effects of BV13 on vascular morphology can also be observed using differential interference contrast (DIC) microscopy (compare arrows in control, Figure 6E, to BV13-treated, Figure 6F). In separate experiments, we showed that Fab fragments of BV13 elicited a response similar to that of the intact antibody (Figure 6G). By contrast, the monoclonal antibody BV14, which unlike BV13 does not to perturb vascular permeability,7,14 had little or no effect on vasculogenesis in cultured 7.8-dpc explants (Figure 6H).
VE-cadherin function-blocking antibody treatment disrupts vascular morphogenesis in the 7.8-dpc allantois explant culture model. (A-D) PECAM-immunolabeled allantois explants from 7.8-dpc embryos following 18 hours of culture in control medium (A,C) or BV13 monoclonal antibody (24 μg IgG/mL; B,D). Arrows (C, control; D, BV13-treated) indicate PECAM deposition at cell-cell junctions. (E-F) DIC images of the same regions of the explants depicted in panels C and D, respectively. Arrowheads in panels D,F indicate a PECAM+ cluster of cells resulting from BV13 treatment. (G-H) PECAM-immunolabeled allantois explants from 7.8-dpc embryos following 18 hours of culture in Fab fragments of BV13 antibody (24 μg IgG/mL; G) or BV14 antibody (24 μg IgG/mL; H). Bars equal 50 μm. Panels A, B, G, and H are confocal images; C and D, epifluorescence images; and E and F, DIC images.
VE-cadherin function-blocking antibody treatment disrupts vascular morphogenesis in the 7.8-dpc allantois explant culture model. (A-D) PECAM-immunolabeled allantois explants from 7.8-dpc embryos following 18 hours of culture in control medium (A,C) or BV13 monoclonal antibody (24 μg IgG/mL; B,D). Arrows (C, control; D, BV13-treated) indicate PECAM deposition at cell-cell junctions. (E-F) DIC images of the same regions of the explants depicted in panels C and D, respectively. Arrowheads in panels D,F indicate a PECAM+ cluster of cells resulting from BV13 treatment. (G-H) PECAM-immunolabeled allantois explants from 7.8-dpc embryos following 18 hours of culture in Fab fragments of BV13 antibody (24 μg IgG/mL; G) or BV14 antibody (24 μg IgG/mL; H). Bars equal 50 μm. Panels A, B, G, and H are confocal images; C and D, epifluorescence images; and E and F, DIC images.
Initial events of vasculogenesis including the formation of nascent endothelial tubes are not dependent on VE-cadherin activity
If the phenotype observed in BV13-treated allantoides (ie, clusters of PECAM+ cells) was due to blood vessel disassembly resulting from the loss/lack of VE-cadherin function, we reasoned that the antibody should, therefore, not perturb earlier events in vasculogenesis (ie, the formation of nascent blood vessels). To test this hypothesis, 7.8-dpc allantois explants were cultured in the presence of BV13 antibody for varying periods of time. As shown in Figure 7C, blood vessel formation in 7.8-dpc allantois explants was not perturbed by treatment with BV13 for 9 hours from initiation of the culture. By contrast, when 7.8-dpc allantois explants were cultured for 9 hours in the absence of antibody and then exposed to BV13 for an additional 9 hours, PECAM+ clusters of cells replaced the normal reticulated vascular networks (Figure 7D).
Initial events of vasculogenesis including the formation of nascent endothelial tubes are not dependent on VE-cadherin activity. (A-D) Confocal images of PECAM-immunolabeled 7.8-dpc allantoides cultured for 18 hours. (A-B) Cultured allantoides that were either untreated (A) or treated with BV13 monoclonal antibody (24 μg IgG/mL; B) for 18 hours. (C) An allantois that was exposed to BV13 for the first 9 hours of culture and then cultured for 9 hours in the absence of BV13. (D) An allantois that was cultured in the absence of BV13 for 9 hours and then cultured for 9 hours in the presence of BV13. Comparison of panels A and C demonstrates that the presence of the BV13 antibody had no discernible effect on de novo blood vessel formation. In contrast, following BV13 treatment, the blood vessels formed in the first 9 hours of culture (C) disassemble, forming disconnected clusters of PECAM+ cells (D). Bars equal 100 μm.
Initial events of vasculogenesis including the formation of nascent endothelial tubes are not dependent on VE-cadherin activity. (A-D) Confocal images of PECAM-immunolabeled 7.8-dpc allantoides cultured for 18 hours. (A-B) Cultured allantoides that were either untreated (A) or treated with BV13 monoclonal antibody (24 μg IgG/mL; B) for 18 hours. (C) An allantois that was exposed to BV13 for the first 9 hours of culture and then cultured for 9 hours in the absence of BV13. (D) An allantois that was cultured in the absence of BV13 for 9 hours and then cultured for 9 hours in the presence of BV13. Comparison of panels A and C demonstrates that the presence of the BV13 antibody had no discernible effect on de novo blood vessel formation. In contrast, following BV13 treatment, the blood vessels formed in the first 9 hours of culture (C) disassemble, forming disconnected clusters of PECAM+ cells (D). Bars equal 100 μm.
Discussion
After globally assessing embryonic blood vessel formation in VE-cadherin null embryos, we established that the regional vascular abnormalities could be correlated with the age of the given vascular network. The temporal order of the formation of discrete vascular networks/blood vessels in normal mouse embryos is yolk sac and allantoic vessels, the endocardium, dorsal aortae/sinus venosus, and the cranial vascular network.11 Vessels that were first to form in VE-cadherin null embryos (ie, yolk sac and allantois vessels) were the first to display morphologic abnormalities. Our findings in this regard are consistent with observations by Gory-Faure et al,6 who also noted aberrant yolk sac vessels in VE-cadherin null embryos at a stage in which other vessels (ie, dorsal aortae) appeared normal. However, although our observations are similar to those of Gory-Faure et al,6 our conclusions are different. In contrast to Gory-Faure et al,6 we contend that vascular defects in the extraembryonic vasculature at a time when intraembryonic vessels appear normal is due to the fact that the extraembryonic vasculature is the first to form and it is the first to reach the stage in vascular morphogenesis when VE-cadherin function is essential.
To delineate the stage in the process of neovascularization in which VE-cadherin function is essential, we used the allantois culture model in which nonvascularized 7.8-dpc allantoic mesoderm undergoes de novo blood vessel formation in culture. We showed that treatment of allantois explants for 9 hours with the function-blocking VE-cadherin antibody, BV13, did not affect the initial events in vasculogenesis: angioblast formation, angioblast coalescence, and the formation of networks of PECAM+ cells. These findings are consistent with those of both Carmeliet et al5 and Gory-Faure et al,6 who showed lumenized blood vessels in VE-cadherin null embryos at 8.5 dpc. Therefore, we conclude that the events of de novo blood vessel formation up to the point at which a vascular epithelium forms (ie, nascent vessels with lumens) are not dependent on VE-cadherin.
The stage of blood vessel formation at which VE-cadherin function is essential became evident when the duration of BV13 antibody treatment of 7.8-dpc allantois explants was extended (> 9 hours). Specifically, such treatments led to the replacement of normal reticulated networks with isolated clusters of PECAM+ cells. These findings are consistent with the formation of abnormal clusters of PECAM+ cells in VE-cadherin null allantois explants cultured for more than 9 hours. Based on these findings, we conclude that VE-cadherin activity is critical to the stabilization of nascent vessels or to prevent nascent vessels from undergoing an epithelial to mesenchymal transformation (ie, disassembly). If correct, then the cells forming the clusters of PECAM+ cells observed in response to BV13 or VE-cadherin gene deletion were once endothelial cells incorporated into an endothelium. In support of this hypothesis, we observed that treatment of allantois explants having established vessels (ie, 8.5 dpc) with VE-cadherin function-blocking antibodies also resulted in the formation of isolated clusters of PECAM+ cells. The fact that an antibody to VE-cadherin induced disassembly of blood vessels without altering endothelial cell numbers suggests that the mechanism for disassembly is not the result of cell death (ie, anoikis), although cell death may be the eventual outcome as described by Carmeliet et al.5 Together these findings indicate that lack of VE-cadherin activity leads to disassembly of embryonic blood vessels, implying that VE-cadherin is required for maintaining a vascular epithelium. Given the fact the angiogenic sprouts are in many respects similar to embryonic vessels formed as part of vasculogenesis (ie, they are nascent endothelial tubes), the disassembly effect that we observed may be the underlying basis for the capacity of BV13 and other VE-cadherin function-blocking antibodies to inhibit tumor vascularization in vivo.7,9,14 In this context, our findings that BV13 but not BV14 promotes the disassembly of vessels serves to support the conclusions of Corada et al,7 that the mechanism by which these two antibodies inhibit tumor neovascularization differ.
The clusters of PECAM+ cells that form in response to either genetic VE-cadherin deficiency or functional inhibition by VE-cadherin antibody are similar to the clusters of PECAM+ cells that result from coalescence of angioblasts during normal vasculogenesis11 and the clusters of PECAM+ cells that result from inhibition of vascular endothelial growth factor (VEGF) signaling during vasculogenesis. For example, administration of either neutralizing antibodies against recombinant mouse VEGF164 or the soluble form of VEGF receptor-1 (sFlt1) to avian embryos or cultured murine allantois explants results in PECAM+ cell clusters.12,15 The similarity between the response to VE-cadherin deficiency and suppression of VEGF signaling suggests a link that is critical to vasculogenesis. Numerous studies support this possibility, including reports that VEGF receptor-2 (Flk1) and VE-cadherin can be coprecipitated from endothelial cell preparations,5,16,17 VE-cadherin deficiency reduces the half-life of Flk1,18 VEGF can induce VE-cadherin tyrosine phosphorylation in endothelial cells,19 and VE-cadherin–deficient endothelial cells are unresponsive to VEGF signaling.5 However, the coalescence of angioblasts that normally occurs during early vasculogenesis, as well as the transition of angioblast aggregates to a vascular epithelium, involves cells that express Flk1 but not VE-cadherin.11 In the yolk sac, allantois and embryo proper, VE-cadherin expression is up-regulated between 8 and 8.5 dpc,20 stages when PECAM+ endothelia are detectable in each of these regions. Therefore, we can exclude a role for an integrated Flk1–VE-cadherin signaling pathway up to the point in which lumenized blood vessels are formed, the pre-epithelial phase of vasculogenesis. With the onset of VE-cadherin expression, integration of Flk1 signaling and VE-cadherin function is possible. The functional significance of this integration is likely to involve regulation of cell-cell interaction and permeability in response to VEGF.
The VE-cadherin knockout mouse teaches us that blood vessels can form in the absence of VE-cadherin.5,6 Herein we show that perturbation of VE-cadherin leads to disassembly of nascent vessels. These findings seem incongruent unless one evokes a role for VE-cadherin in which it serves to regulate the disassembly of vessels. In other words, its expression in established blood vessels imparts a mechanism by which cell-cell adhesion can be diminished to allow permeability. Such a role is also consistent with the mechanism of action of VEGF to regulate vascular permeability. Therefore, the integration of Flk1 signaling and VE-cadherin function confers on the endothelium the ability to modulate cell-cell adhesion in response to VEGF.
Assigning a role for VE-cadherin in either vasculogenesis or angiogenesis is dependent on one's definition of these processes. Although there is a relatively clear understanding of many of the steps in the process of vasculogenesis (ie, the birth of endothelial progenitors [angioblasts], coalescence of angioblasts into cordlike structures, and lumenization to form a vascular endothelium), a clear end point of the process is lacking. One end point may be when mural cells have invested the blood vessel. In the allantois, smooth muscle cell antigens (ie, smooth muscle α actin) are not detectable until 9.0 dpc (P.A.F., unpublished observations, September 2003). The fact that vascular anomalies are apparent in the allantoides of 8.5-dpc VE-cadherin nulls and the fact that VE-cadherin function-blocking antibodies have dysmorphogenic effects on the vasculature of 7.8-dpc allantoic explants indicates that VE-cadherin expression and therefore function is essential at the point following the formation of vascular endothelium but prior to mural cell investment. Based on this point of view, VE-cadherin is critical for vasculogenesis.
Prepublished online as Blood First Edition Paper, December 16, 2004; DOI 10.1182/blood-2004-06-2244.
Supported by grants from the National Institutes of Health (HL57375, HL52813, HL61873), the Department of Defense (DAMD 17-00-1-0338), and United Negro College Fund (UNCF)–MERCK (01013119-01).
C.V.C. and P.A.F. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Haiqun Zeng for her expert technical assistance with fluorescence-activated cell scanning.