Abstract
Tissue factor (TF) is an integral membrane protein essential for hemostasis. During the past several years, a number of studies have suggested that physiologically active TF circulates in blood at concentrations greater than 30 pM either as a component of blood cells and microparticles or as a soluble plasma protein. In our studies using contact pathway–inhibited blood or plasma containing activated platelets, typically no clot is observed for 20 minutes in the absence of exogenous TF. An inhibitory anti-TF antibody also has no effect on the clotting time in the absence of exogenous TF. The addition of TF to whole blood at a concentration as low as 16 to 20 fM results in pronounced acceleration of clot formation. The presence of potential platelet TF activity was evaluated using ionophore-treated platelets and employing functional and immunoassays. No detectable TF activity or antigen was observed on quiescent or ionophore-stimulated platelets. Similarly, no TF antigen was detected on mononuclear cells in nonstimulated whole blood, whereas in lipopolysaccharide (LPS)–stimulated blood a significant fraction of monocytes express TF. Our data indicate that the concentration of physiologically active TF in non–cytokine-stimulated blood from healthy individuals cannot exceed and is probably lower than 20 fM.
Introduction
Tissue factor (TF) is an integral membrane protein and is an essential component of the factor VIIa-TF complex enzyme, the initiator of blood coagulation in vivo. TF is expressed in numerous tissues of the body and is found in a variety of organs.1-3 Following mechanical or chemical damage of the vascular wall, extravascular TF is exposed to the blood and binds plasma factor VIIa. TF is also expressed and presented by monocytes and neutrophils following stimulation by inflammatory cytokines.4-6 Tumor cells also express TF, where TF is related to the metastatic potential.7-9
Tissue factor is a 263–amino acid lipoprotein with 3 major domains: (1) an amino-terminal extracellular domain, which binds with high affinity to factor VIIa; (2) a transmembrane domain, which anchors TF to the membrane surface; and (3) a cytoplasmic carboxyterminal domain, which presumably is involved in signal transduction.10-12 Binding of plasma factor VIIa to membrane TF anchors the proteolytic complex and results in an approximately 2 × 107-fold increase in the enzymatic activity of factor VIIa toward its natural substrates factor IX and factor X.13
During the past several years, a number of controversial studies related to the presence, concentration, and functional activity of TF circulating in blood have been published. Several groups of investigators reported the presence of physiologically active TF circulating in blood14-19 at concentrations as high as 37 pM.20 It has been reported that this blood-borne TF is located on blood cells and microparticles or circulates as a soluble protein. In contrast, we and several other groups did not observe TF-related activity in blood from healthy humans21-23 or in mouse blood.24 Based upon the experience accumulated in our laboratory as well as on reports from other laboratories, blood or plasma activated with (sub)picomolar concentrations of functional TF clots within several minutes.25-30 These data suggest that physiologically active TF at picomolar concentrations cannot be present in the blood or plasma of healthy individuals in vivo. Similarly conflicting conclusions have been published related to the contribution of blood-borne TF to thrombus growth. Falati et al31 suggested that vessel wall TF is an essential component of a developing thrombus and did not observe any substantial role for blood-borne TF in thrombus formation. In contrast, Bogdanov et al32 proposed that soluble blood-borne TF has prothrombotic activity and substantially contributes to thrombus growth.
The current study examines the controversy in quantitative terms regarding the presence and functional activity of blood TF. We used several methods of analysis, including 2 TF activity–sensitive in vitro models of blood coagulation developed in our laboratory25,26,33,34 and flow cytometry with a well-characterized monoclonal antibody, to detect TF antigen in blood and on monocytes and platelets from healthy individuals.
Materials and methods
Materials
Human coagulation factors VII, X, IX, and prothrombin were isolated from fresh frozen plasma using the general methods of Bajaj et al35 and were purged of trace contaminants and traces of active enzymes as described.34 Human factor V and antithrombin-III (AT-III) were isolated from freshly frozen plasma.36,37 Recombinant factor VIII and recombinant TF (residues 1-242) were gifts from Drs Shu Len Liu and Roger Lundblad (Hyland Division, Baxter Healthcare, Duarte, CA). Recombinant human factor VIIa was a gift from Dr Ula Hedner (Novo Nordisk, Bagsvaerd, Denmark). Recombinant full-length TF pathway inhibitor (TFPI) produced in Escherichia coli was a gift from Dr K. Johnson (Chiron, Emeryville, CA). Corn trypsin inhibitor (CTI) was isolated from popcorn as described elsewhere.26 Washed platelets were prepared by the procedure of Mustard et al.38 Preparation of the TF/lipid reagent was done as described elsewhere.26 PS (1,2-dioleolyl-sn-glycero-3-phospho-L-serine) and PC (1,2-dioleoyl-sn-glycero-3-phosphocholine) were purchased from Avanti Polar Lipids (Alabaster, AL) and Ca2+ ionophore A23187 was purchased from EMD Biosciences (San Diego, CA). EDTA (ethylenediaminetetraacetic acid), HEPES (4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid), IgG-FITC (fluorescein isothiocyanate–conjugated goat antimouse immunoglobulin G), and Arg-Gly-Asp-Ser (RGDS) tetrapeptide were purchased from Sigma (St Louis, MO). Phospholipid vesicles (PCPSs) composed of 25% PS and 75% PC were prepared as described.39 The chromogenic thrombin substrate Spectrozyme TH was purchased from American Diagnostica (Greenwich, CT). D-Phe-Pro-ArgCH2Cl (FPRck), monoclonal anti-TF (α–TF-5), and anti–factor XI (α–FXI-2) antibodies were produced in house. Citrated plasma was purchased from George King Bio-Medical (Overland Park, KS), α–P-selectin–PE (phycoerythrin-conjugated anti–P-selectin antibody) was purchased from BD Biosciences (San Diego, CA), and Optilyse C was purchased from Coulter Immunotech (Marseille, France). The human monocyte cell line TIB 202 was purchased from American Type Culture Collection (ATCC; Rockville, MD) and low-endotoxin bovine serum albumin was from Irvine Scientific (Santa Ana, CA).
Whole-blood clotting
Eleven healthy donors (male and female; age range, 22-55 years) were recruited and advised according to a protocol approved by the University of Vermont Human Studies Committee and their consent was obtained. All individuals exhibited normal values for the parameters of blood coagulation, plasma protein levels, and platelet count. Experiments were performed in tubes placed on a rocking table enclosed in a 37°C temperature-controlled glove box using fresh blood. For 2 individuals (nos. 1 and 6; Table 1), blood was drawn by venipuncture and immediately delivered (1.0 mL) into the tubes loaded with 0.1 mg/mL CTI and various concentrations (0-10 pM; in duplicates) of relipidated TF (PCPS/TF = 5000) in HBS (20 mM HEPES; 150 mM NaCl, pH 7.4) and 2 mM CaCl2. In all experiments, no more than 35 μL of reagent was loaded in each tube. The clotting time was observed visually by 2 observers and was called when “clumps” were noticed on the side of the tube. For the rest of the individuals, clotting time of CTI blood in the absence of exogenous TF was evaluated.
Plasma clotting
Calcium ionophore A23187–treated washed platelets (fresh or frozen) from individual no. 2 (Table 1) were added at a concentration of 2 × 108/mL to citrated pooled plasma containing 0.1 mg/mL α–FXI-2 or CTI and varying amounts of relipidated TF. Plasma clotting was initiated with 25 mM CaCl2. Clotting times were determined using the ST4 clotting instrument (Diagnostica Stago, Parsippany, NJ).
Synthetic plasma model
Procofactor solution. Washed platelets (4 × 108/mL) from individual no. 8 (Table 1) were incubated in HBS with 2 mM CaCl2 for 10 minutes at 37°C. When desired, 20 pM relipidated TF (molar ratio, PCPS/TF = 5000), 10 μM A23187, and 0.2 mg/mL α–TF-5 were added. Factor V (40 nM) and factor VIII (1.4 nM) were added prior to the initiation of the reaction.
Zymogen-inhibitor solution. Prothrombin (2.8 μM) and coagulation factors VII (20 nM), VIIa (0.2 nM), X (340 nM), IX (180 nM), and XI (60 nM), TFPI (5 nM), and AT-III (6.8 μM) were warmed in HBS with 2 mM CaCl2 at 37°C for 3 minutes. When desired, factors VII and VIIa were omitted.
The reaction was initiated by mixing equal volumes of both solutions, resulting in physiologic concentrations of the zymogens, procofactors, inhibitors, and platelets, 10 pM TF, and 0.1 mg/mL α–TF-5. Following initiation of the reaction, 10-μL aliquots were withdrawn at 1 to 10-, 12-, and 14-minute time points from the reaction mixture; quenched in 20 mM EDTA in HBS (pH 7.4) containing 0.2 mM Spectrozyme TH; and assayed immediately for thrombin activity. The hydrolysis of the substrate was monitored by the change in absorbance at 405 nm using a Molecular Devices Vmax spectrophotometer (Sunnyvale, CA). Thrombin generation was calculated from a standard curve prepared by serial dilutions of α-thrombin.
Factor X activation
Factor VIIa (0.5 nM) was incubated in HBS with 2 mM CaCl2 (pH 7.4) for 10 minutes at 37°C with either 6 × 108/mL A23187-treated platelets from a single donor or 20 pM relipidated TF in the presence of 100 μM PCPS. Factor X at 170 nM concentration was added and 20-μL aliquots were withdrawn at selected time points (0-5 minutes) and quenched into 20 mM EDTA in HBS (pH 7.4) containing 0.2 mM Spectrozyme Xa. The hydrolysis of the substrate was monitored by the change in absorbance at 405 nm using a Molecular Devices Vmax spectrophotometer. Factor Xa generation was calculated from a standard curve prepared by serial dilutions of purified factor Xa.
Flow cytometry
Monocyte stimulation. TIB 202 cells (5 × 106/mL) were cultured in serum-free media containing 0.35% low-endotoxin bovine serum albumin in the presence of 1 μg/mL lipopolysaccharide (LPS). Following overnight culture (∼16 hours), the cells were removed from the tissue culture plate by gentle trituration, aliquoted (∼3 × 105 cells/tube), and washed 2 times by centrifugation (400g, 10 minutes) followed by resuspension in HBS. The cell pellets were resuspended in immunostaining reaction mixtures (100 μL final volume) containing 10 μg/mL human Fc fragment and 0.5 μM α–TF-5. Nonspecific antibody binding was assessed by incubating with an isotype-matched irrelevant antibody at the same concentration. Following a 30-minute incubation at ambient temperature, HBS was added and the cells were subjected to centrifugation. The cell pellets were washed one time with HBS by centrifugation and resuspended with 100 μL of a 1:100 dilution of IgG-FITC. Following a 30-minute incubation at ambient temperature, HBS was added and the cells were subjected to centrifugation (400g, 10 min). The cell pellets were resuspended with 1 mL 2% paraformaldehyde and stored at 4°C until flow cytometric analyses.
Whole blood. Whole blood (donor no. 6; Table 1) containing 0.1 mg/mL CTI was incubated with Optilyse C (1:1 dilution) to fix the cells and lyse the red blood cells. In other experiments, CTI-containing blood was incubated with 50 U/mL heparin and 100 ng/mL LPS (lipopolysaccharide) for 2 hours and then diluted 1:1 with Optilyse C. Following a 15-minute incubation with Optilyse C, blood was incubated with α–TF-5 (75 μg/mL) or an isotype-matched irrelevant mouse IgG for 30 minutes. The cells were washed by centrifugation followed by resuspension in HBS. Following centrifugation, pellets were resuspended in 100 μL of IgG-FITC (1:100 dilution) and incubated for 30 minutes. The cells were diluted 1:1 with Optilyse C. Following a 15-minute incubation, 300 μL HBS was added and cells were stored at 4°C until flow cytometric analyses.
Isolated blood-cell populations. Human mononuclear cells and platelets were isolated from whole blood of donor no. 6 (Table 1) using standard techniques.38 Following appropriate stimulation, cells were immunostained for flow cytometry as described in “Whole blood.” To verify platelet activation, A23187-treated platelets were incubated with α–P-selectin–PE and analyzed by flow cytometry. Cells (10 000) were analyzed by flow cytometry on a Coulter EPICS Elite flow cytometer (Coulter, Hialeah, FL). Monocytes and platelets were identified by their forward and side scatter. The positive gate was set such that at least 98% of the cells stained with the irrelevant antibody were negative. Microparticles were defined as having the same side scatter but a smaller forward scatter as the cells from which they were derived.
Results
TF-related activity in whole blood and plasma
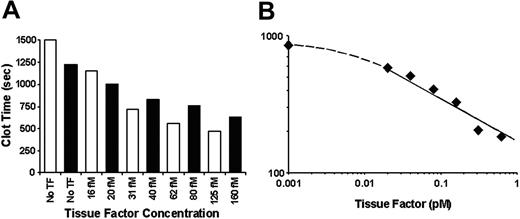
To establish if functionally active blood-borne TF is present in healthy individuals, specific experiments were designed to identify TF-related activity in blood and plasma. To prevent the influence of contact pathway–initiated coagulation, 0.1 mg/mL CTI (inhibits factor XI activation by factor XIIa40 ) was added to whole blood. In the presence of CTI, fresh nonanticoagulated blood kept at 37°C with mixing does not clot for more than 1200 seconds in the absence of exogenous TF27 (Figure 1A). The addition of as little as 16 to 20 fM TF to CTI blood resulted in accelerated clot formation (ie, this extremely low concentration of functionally active TF shortened clotting time by 220 s for donor no. 1 [Table 1; Figure 1A ▪] and by 350 s for donor no. 6 [Figure 1A □]). Titrations of TF in CTI blood resulted in shortening of the clotting time in a TF concentration–dependent manner. At the highest concentration of exogenous TF tested (10 pM), whole blood from a healthy individual clotted in 140 seconds. For all 11 individuals tested (male and female; age range, 22-55 years), the clotting time of contact pathway–inhibited blood in the absence of exogenous TF was more than 1200 seconds (20 minutes), extending in some experiments beyond 2400 seconds (40 minutes; donor nos. 10 and 11 in Table 1), suggesting that no detectible concentrations of active TF are present in the blood of healthy donors.
Titration of relipidated TF in CTI-inhibited whole blood and plasma. Increasing concentrations of relipidated TF (TF/PCPS, 1:5000) were added to whole blood containing 0.1 mg/mL CTI (A) or PFP reconstituted with A23187 (a calcium ionophore)–treated platelets at 2 × 108/mL (B). ▪ represents individual no. 1 (Table 1) and □ represents individual no. 6. Clotting time was determined either visually (whole blood) or using the ST4 clotting instrument (plasma).
Titration of relipidated TF in CTI-inhibited whole blood and plasma. Increasing concentrations of relipidated TF (TF/PCPS, 1:5000) were added to whole blood containing 0.1 mg/mL CTI (A) or PFP reconstituted with A23187 (a calcium ionophore)–treated platelets at 2 × 108/mL (B). ▪ represents individual no. 1 (Table 1) and □ represents individual no. 6. Clotting time was determined either visually (whole blood) or using the ST4 clotting instrument (plasma).
Platelet-free citrated plasma (PFP) from healthy individuals, as well as PFP reconstituted with washed platelets (2 × 108/mL), was also tested for the presence of TF activity. The activation of contact pathway coagulation was inhibited by the addition of 0.1 mg/mL CTI or antibody α–FXI-2. Under these conditions, no clot formation was observed in 1000 seconds (the upper time limit for the clotting apparatus ST-4).
To address the question of whether platelet activation induces expression/exposure of functional TF, Ca2+ ionophore A23187–treated washed platelets were added to PFP at the mean physiologic concentration (2 × 108/mL) and the clotting time was measured in the presence or absence of an inhibitory antibody (α–TF-5). In the absence of exogenous TF and α–TF-5, activated platelets and CTI-containing plasma mixture clotted in 835 seconds. The addition of α–TF-5 at 0.1 mg/mL concentration to this plasma had no effect on the clotting time, suggesting that no detectable amounts of functional TF are present on the activated platelet. To test the efficiency of the antibody against TF, α–TF-5 was added to 5 pM relipidated TF, 50 μM PCPS, and 0.1 mg/mL CTI-containing PFP. The clotting time in the absence of α–TF-5 was 190 seconds and in the presence of α–TF-5 the clotting time was more than 1000 seconds.
Relipidated TF was further titrated into activated platelet-containing plasma (Figure 1B). As mentioned in the previous paragraph, in the absence of exogenous TF, CTI-containing plasma clotted in 835 seconds. The addition of exogenous TF caused decreases in the clotting time in a TF concentration–dependent manner. At as low as 20 fM TF, the clotting time decreased to 582 seconds. At the highest TF concentration tested (5 pM), a 106-second clotting time was observed (data not shown).
One must conclude from the results of whole blood and plasma clotting experiments that the presence of functionally active TF at the high concentrations previously reported to be circulating in healthy individuals would lead to a massive thrombin generation. The coagulation response to concentrations of TF as low as 20 fM leads to the conclusion that this concentration of functional TF in nonactivated blood and activated platelets from healthy individuals is well beyond the upper limit of potential active TF concentration in blood.
Platelet-related TF activity
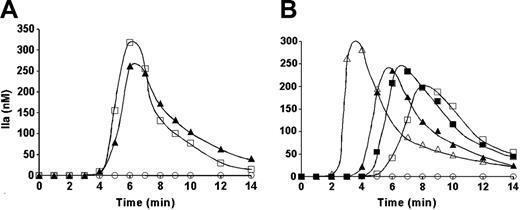
The potential of platelet-related TF activity was evaluated using synthetic plasma composed of all known elements of the extrinsic pathway (Figure 2A). Resting platelets added to the synthetic plasma did not initiate detectable levels of thrombin generation (Figure 2A ○). With A23187-treated platelets, thrombin was produced at a maximum rate of 3.1 nM/second and reached 265 nM (Figure 2A ▴). TF per se has no proteolytic activity, it only enhances that of factor VIIa toward its substrates, both natural and synthetic.13,41 Thus, if the observed thrombin generation in the presence of activated platelets is related to TF, the omission of factor VIIa and factor VII (as possible sources of factor VIIa) from the reaction mixture should prevent thrombin generation. However, neither the initiation phase of thrombin generation (4 minutes) nor the maximum rate was substantially affected by the absence of factors VII and VIIa (Figure 2A □). Thus the active agent was not TF.
Thrombin generation in synthetic plasma. Synthetic plasma was composed of factors V, VIII, VII, VIIa, IX, X, and XI, prothrombin, TFPI, and AT-III at their mean physiologic concentration. (A) Thrombin generation was initiated with 2 × 108/mL A23187-treated platelets in the absence of TF and either in the presence (▴) or in the absence (□) of factors VII and VIIa. (B) Thrombin generation was initiated either with 2 × 108/mL A23187-treated platelets in the absence (▴) or presence of 0.1 mg/mL α–TF-5 (▪) or with TF in the presence of 2 × 108/mL resting platelets and either in the absence (▵) or presence of α–TF-5 (□). ○ represents thrombin generation in the presence of resting platelets and absence of TF.
Thrombin generation in synthetic plasma. Synthetic plasma was composed of factors V, VIII, VII, VIIa, IX, X, and XI, prothrombin, TFPI, and AT-III at their mean physiologic concentration. (A) Thrombin generation was initiated with 2 × 108/mL A23187-treated platelets in the absence of TF and either in the presence (▴) or in the absence (□) of factors VII and VIIa. (B) Thrombin generation was initiated either with 2 × 108/mL A23187-treated platelets in the absence (▴) or presence of 0.1 mg/mL α–TF-5 (▪) or with TF in the presence of 2 × 108/mL resting platelets and either in the absence (▵) or presence of α–TF-5 (□). ○ represents thrombin generation in the presence of resting platelets and absence of TF.
In a further experiment, the influence of α–TF-5 on thrombin generation initiated either with relipidated TF or with A23187-treated platelets was compared (Figure 2B). In the absence of TF, no thrombin generation was observed in the presence of resting nonactivated platelets (Figure 2B ○). The addition of 10 pM relipidated TF in the presence of resting platelets caused rapid thrombin generation at a rate of 4.9 nM/second after an initiation phase of 2 minutes (Figure 2B ▵). For the same reaction conditions, the addition of α–TF-5 prolonged the initiation phase (to 5 minutes) and suppressed the maximum rate of thrombin generation to 1.4 nM/seconds (Figure 2B □). The maximum level of active thrombin formed was also reduced from 300 nM in the absence of antibody to 200 nM in the presence of antibody. The addition of A23187-treated platelets to synthetic plasma in the absence of exogenous TF (Figure 2B closed symbols) led to thrombin generation at a maximum rate of 2.7 nM/second (Figure 2B ▴). The initiation phase observed was approximately 4 minutes, and the maximum level of active thrombin formed in the reaction was 250 nM. The addition of 0.1 mg/mL α–TF-5 to the synthetic plasma with A23187-treated platelets had almost no effect on the maximum rate or level of thrombin generation (Figure 2B ▪). The initiation phase duration was slightly prolonged (by < 1 minute).
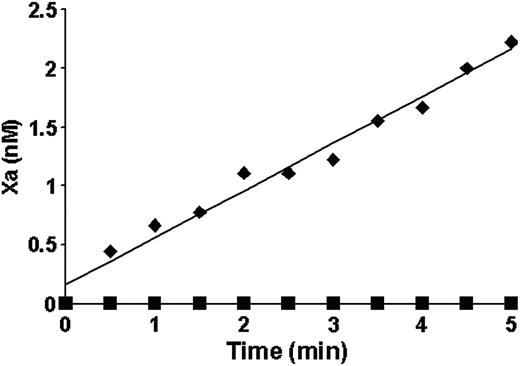
A proteolytic activity leading to thrombin generation was observed in the synthetic plasma in the presence of A23187-treated platelets. Although the data presented in Figure 2 suggest that this activity is not related to TF, a more specific reaction system was used to evaluate the ability of ionophore-treated platelets to enhance factor VIIa activity toward its natural substrate factor X. Factor VIIa and factor X were added to A23187-treated platelets at 3 × their mean physiologic concentration (6 × 108/mL). No factor Xa generation was observed over 5 minutes of the reaction (Figure 3 ▪). In a control experiment performed under the same conditions with 20 pM relipidated TF, factor X was activated at a rate of 0.4 nM/minute (Figure 3 ♦).
Factor X activation by the extrinsic Xase (♦) and A23187-treated platelets (▪). Either treated platelets at 6 × 108/mL concentration (3 × mean physiologic) or 20 pM relipidated TF were incubated with 0.5 nM factor VIIa in the presence of 100 μM PCPS. Factor X (170 nM) was added and factor Xa generation was measured in a chromogenic assay.
Factor X activation by the extrinsic Xase (♦) and A23187-treated platelets (▪). Either treated platelets at 6 × 108/mL concentration (3 × mean physiologic) or 20 pM relipidated TF were incubated with 0.5 nM factor VIIa in the presence of 100 μM PCPS. Factor X (170 nM) was added and factor Xa generation was measured in a chromogenic assay.
The search for TF antigen in whole blood and on purified cells
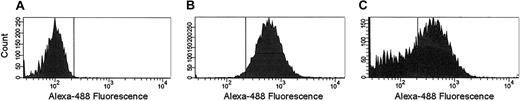
Whole blood. The majority of reports related to blood-borne TF use immunochemical methods employing monoclonal antibodies, which recognize epitope(s) on the TF molecule whether or not it is functionally active. We evaluated if TF antigen could be detected on blood cells using flow cytometry. As a negative control, cultured LPS-activated monocytes were stained using an irrelevant isotype-matched antibody (Figure 4A). As a positive control, LPS-activated monocytes were stained with α–TF-5. After a 12-hour stimulation with 1 μg/mL LPS, 98% of monocytes stained positively for TF (Figure 4B). Additionally, the majority of microparticles generated during the preparation and immunocytochemical staining of the LPS-stimulated monocytes were TF positive (Figure 4C).
Flow cytometric analyses of cultured LPS-stimulated monocytes and microparticles. Cells (5 × 106/mL) were stimulated overnight with 1 μg/mL LPS. Cells were immunostained either with an irrelevant isotype-matched mouse IgG (A) or with α–TF-5 (B). Panel C shows microparticles generated during the preparation and immunocytochemical staining of monocytes with α–TF-5.
Flow cytometric analyses of cultured LPS-stimulated monocytes and microparticles. Cells (5 × 106/mL) were stimulated overnight with 1 μg/mL LPS. Cells were immunostained either with an irrelevant isotype-matched mouse IgG (A) or with α–TF-5 (B). Panel C shows microparticles generated during the preparation and immunocytochemical staining of monocytes with α–TF-5.
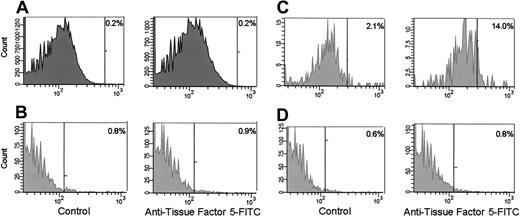
Purified cells. The monocytes (Figure 5A) and platelets (Figure 5B) present in unstimulated whole blood expressed no detectable TF. When whole blood was stimulated with 0.1 μg/mL LPS for 2 hours, 14% of monocytes were positive for TF (Figure 5C). LPS had no effect on the expression of TF by platelets (Figure 5D). To answer the question if TF antigen is expressed/exposed on activated platelets, flow cytometric analyses were performed on A23187-treated platelets (Figure 6). No TF was detected on either resting (Figure 6A) or treated platelets (Figure 6B). Under these conditions, approximately 91% of the platelets were positive for the platelet activation–dependent surface protein P-selectin (Figure 6C).
Flow cytometric analyses of monocytes and platelets from whole blood. Monocytes (A) and platelets (B) in unstimulated blood. Monocytes (C) and platelets (D) in LPS-stimulated blood. α–TF-5 was used for immunostaining. The positive analysis regions (vertical bars) were defined such that less than 99% of the cells stained with an irrelevant, isotype-matched mouse IgG were positive. The percentages shown are the percent of positive cells in these analysis regions.
Flow cytometric analyses of monocytes and platelets from whole blood. Monocytes (A) and platelets (B) in unstimulated blood. Monocytes (C) and platelets (D) in LPS-stimulated blood. α–TF-5 was used for immunostaining. The positive analysis regions (vertical bars) were defined such that less than 99% of the cells stained with an irrelevant, isotype-matched mouse IgG were positive. The percentages shown are the percent of positive cells in these analysis regions.
Flow cytometric analyses of resting and A23187-treated platelets. Resting (A) or A23187-treated (B) platelets were immunostained with α–TF-5. A23187-treated platelets were also treated with an anti–P-selectin antibody (C). The positive analysis regions (horizontal bars) were defined such that less than 99% of the cells stained with an irrelevant isotype-matched mouse IgG were positive. The percentage of positive cells in each of these analysis regions is shown.
Flow cytometric analyses of resting and A23187-treated platelets. Resting (A) or A23187-treated (B) platelets were immunostained with α–TF-5. A23187-treated platelets were also treated with an anti–P-selectin antibody (C). The positive analysis regions (horizontal bars) were defined such that less than 99% of the cells stained with an irrelevant isotype-matched mouse IgG were positive. The percentage of positive cells in each of these analysis regions is shown.
Discussion
The conflicting reports related to the presence, source, and function of blood-borne tissue factor16,17,20,21,31,32,42-44 stimulated the current study. We looked for TF-related activity and antigen in blood, plasma, and platelets. The data presented in this study indicate that less than 20 fM functionally active TF can be present in unstimulated blood and plasma from healthy individuals. In contrast, immunoassay showed that this plasma contains approximately 0.5 pM TF-like antigen.45 No TF-related activity was observed using activated platelets either in the clotting, the synthetic plasma, or the extrinsic Xase assays, which allow for the detection of TF at (sub)picomolar concentrations.
TF antigen was not detected on blood mononuclear cells in the absence of intentional LPS stimulation. In contrast to previous studies,16,43 no TF antigen was detected on platelets present in unstimulated and LPS-stimulated blood or on washed and activated platelets. These observations, as well as those previously published by our23,25,26,33,34 and several other laboratories,21,46 suggest an absence of measurable amounts of active TF in blood and plasma from healthy individuals (> 20 fM) and are in contrast to other studies indicating the presence of picomolar amounts of TF in plasma.14-20
The origins of the discrepancies in detection of blood-borne TF are of interest. The methods used for the quantification of TF in blood and plasma can be divided into 2 groups: (1) immunoassays and (2) activity-based assays. The first group of assays is based upon the recognition of specific TF epitopes by anti-TF antibodies.16,18,31,47-49 Although routinely monoclonal antibodies are used in immunoassays of TF, it is likely that these antibodies will recognize not only full-length functional protein but also truncated and inactive forms including TF degradation products.50 It is not likely that these soluble TF fragments or product are functionally active. Additionally, it is also possible that the antibodies used were not entirely specific for TF antigen and can cross-react with other proteins. These considerations may explain an apparent discrepancy between the levels of TF-like antigen detected in plasma from healthy individuals by the immunoassay and the absence of functional TF activity in the same plasma.
The most commonly used TF activity–based assay evaluates factor Xa generation in the presence of factor VIIa.17,44,51-53 In these assays, supraphysiologic concentrations of factor VIIa are used, frequently exceeding those circulating in vivo by 2 orders of magnitude.52,53 At these high factor VIIa concentrations, the soluble form of TF (an extracellular domain of TF) will bind factor VIIa and display a limited proteolytic activity. At a physiologic factor VIIa concentration (∼ 0.1 nM), however, the soluble form of TF displays a negligible activity54 and is not likely to trigger blood coagulation.
It is important to note that there is a large discrepancy between the reported plasma concentration of soluble TF and that required to achieve very limited clotting activity as described by Bogdanov et al.32 The reported concentration of alternatively spliced TF (soluble form of TF) in plasma is approximately 0.5 pM (consistent with our antigen measurements45 ). In that study, approximately 40 nM of this TF form was used to decrease the plasma clotting time from 233 to 150 seconds (ie, 80 000-fold higher than the reported physiologic concentration). The clotting activity of this TF species is similar to that observed for the extracellular domain of TF.54 A similar effect on the clotting time was observed using only 2 pM transmembrane domain–containing TF (Figure 1B).54 Thus, the soluble form of TF was used at concentrations exceeding those reported as present in vivo by almost 5 orders of magnitude to show a limited effect on clot formation.
The suggestion that soluble blood-borne TF has an effect on the pathology of coronary arteries when recruited to thrombi is speculative.32 There are no data indicating that soluble TF accumulated in thrombi has functional activity; it might be hypothesized that this form of TF can act as an inhibitor of coagulation because by binding factor VIIa, soluble TF will form an inactive complex enzyme. As a consequence, the concentration of factor VIIa available for the complex formation with the functional, membrane-bound full-length TF will decrease.
In a publication by Falati et al,55 the authors report accumulation of blood-borne (microparticle) TF in the platelet thrombus and suggest that this TF triggers the initiation of blood coagulation. Based upon the data presented in the current study, the concentration of the functionally active TF in blood of healthy individuals cannot exceed a few fM, whereas 5 pM active TF is required to provide approximately 5-minute clotting time in the contact pathway–inhibited whole blood.27 This clotting time is similar to that observed in the “Simplate” bleeding assay.56 Thus, the concentration of the blood-borne TF at the site of the vascular injury would have to increase by approximately 3 orders of magnitude to provide normal hemostasis. As a consequence, a life-threatening blood loss would occur during the time required to reach such a blood-borne TF concentration. Additionally, it is possible that the observed blood-borne TF antigen was generated by mechanical injuries inflicted on the mice during the preparations of the animals for the experiment.55
A TF-independent initiation of thrombin generation by activated platelets was observed in both synthetic and citrated plasma. This platelet-related initiating activity was less pronounced in citrated plasma (∼ 14-minute clotting time) than in the synthetic plasma (∼ 4-minute “clotting” time). The difference in activity in 2 reaction systems is caused, most likely, by the absence of natural serine protease inhibitors (α1-antitrypsin, α2-macroglobulin, heparin cofactor II, etc) in the synthetic plasma, whereas these inhibitors are present in citrated plasma. The most probable initiator of thrombin generation in these 2 reaction systems is the factor XI–like protein reported to be present in platelets and released in an active form during platelet activation.57 In a previously published study, a pronounced effect of this activity on thrombin generation in the synthetic plasma was observed.58 Additionally, upon activation, platelets expose membrane binding sites for the enzymatic reactions leading to thrombin generation and, as a consequence, accelerate that process.59 A different activity of ionophore-treated platelets in citrated and synthetic plasma allows an assumption that the observed activity in the former reaction system is mostly related to the exposed membrane binding sites, whereas in the synthetic plasma the prevailing source of activity could be related to the factor XI–like protein. An experimental confirmation of the hypotheses presented in this paragraph is a subject of future studies.
Prepublished online as Blood First Edition Paper, December 16, 2004; DOI 10.1182/blood-2004-09-3567.
Supported by P01 46703 from the National Institutes of Health.
Presented in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 6-9, 2003.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs S. L. Liu, R. Lundblad, U. Hedner, and K. Johnson for providing us with recombinant proteins (factor VIII, TF, factor VIIa, and TFPI) and M. T. Gissel and M. F. Whelihan for their technical assistance.