Abstract
Acquired hemophilia (AH) is an extremely rare condition in which autoantibodies (inhibitors) against clotting factor VIII induce acute and life-threatening hemorrhagic diathesis because of abnormal blood clotting. The mortality rate of AH is as high as 16%, and current treatment options are associated with adverse side effects. We investigated a therapeutic approach for AH called the modified Bonn-Malmö Protocol (MBMP). The aims of MBMP include suppression of bleeding, permanent elimination of inhibitors, and development of immune tolerance, thereby avoiding long-term reliance on coagulation products. The protocol included immunoadsorption for inhibitor elimination, factor VIII substitution, intravenous immunoglobulin, and immunosuppression. Thirty-five high-titer patients with critical bleeding who underwent MBMP were evaluated. Bleeding was rapidly controlled during 1 or 2 apheresis sessions, and no subsequent bleeding episodes occurred. Inhibitor levels decreased to undetectable levels within a median of 3 days (95% confidence interval [95% CI], 2-4 days), factor substitution was stopped within a median of 12 days (95% CI, 11-17 days), and treatment was completed within a median of 14 days (95% CI, 12-17 days). Long-term follow-up (7 months-7 years) showed an overall response rate of 88% for complete remission (CR). When cancer patients were excluded, the CR rate was 97%.
Introduction
The development of autoantibodies (inhibitors) against blood clotting factors in nonhemophilic patients is a rare but clinically serious condition that can lead to life-threatening hemorrhage because of the failure of the blood clotting system. Several clinical studies have reported mortality rates of 16% to 22% for patients with acquired hemophilia (AH), depending on age, inhibitor titer levels, and individual response to treatment.1,2 AH is an autoimmune disorder because the antibody is directed against a self-protein, and clinical symptoms can be transmitted by passive interindividual antibody transfer.3
The estimated incidence of AH is 0.2 to 1 cases per million people per year.4 The immunologic background of the disease is still unknown, at least in part because of its low frequency. In approximately half the cases, other conditions coincide with AH, such as systemic lupus erythematosus, rheumatoid arthritis, Sjögren syndrome, viral infection, cancer, and pregnancy.4,5 Cross-reactivity to a variety of coagulation factors because of immunization against an external antigen has been discussed as a possible underlying mechanism.2
The most common inhibitors in AH are directed against factor VIII (FVIII), though inhibitors of other coagulation factors, including factor V6 and factor IX,7 have also been described. FVIII inhibitors are primarily oligoclonal or polyclonal immunoglobulin G1 (IgG1) or IgG4 immunoglobulins. The main antigenic regions are the A2 and C2 domains of the FVIII molecule. Bound inhibitors block the binding of FVIII to phospholipids, to the von Willebrand factor, and to cofactors of the FVIII molecule. Most FVIII inhibitors have complex type 2 kinetics, with a rapid and nonlinear inactivation of FVIII. A result of this type of kinetics is that it is difficult to saturate the inhibitors by adding antigens. Therefore, FVIII substitution therapy is often unsuccessful in the presence of high-titer inhibitors.3,4,8 High-dose FVIII substitution, which acts by eliminating the inhibitor in hemophilia A, was unsuccessful in AH, particularly in high-titer patients (more than 5 BU/mL).9,10
Various immunosuppressive treatment regimens, which are beneficial in other autoimmune diseases, often fail in AH.2 AH has been treated with FVIII and cyclophosphamide for approximately 30 years. In a randomized trial of 14 patients, various immunosuppressive regimens with prednisolone, cyclophosphamide, or both reduced the inhibitor titers to undetectable levels in 68% of patients with low-titer inhibitors (less than 5 BU/mL).11,12
The response to chemotherapy, especially in high-titer patients, may require several months and appears to be not convincing. A recently published meta-analysis by Delgado et al1 of 245 inhibitor patients focused on the response to various treatments and prognostic factors. Patients in whom the inhibitor could not be eliminated had a mortality rate of 42%, providing a strong argument for the necessity of rapid and long-term inhibitor elimination as part of AH treatment. The meta-analysis showed an overall mortality rate of up to 16% for various treatment regimens and a high rate of complications, which were mainly associated with infections related to chemotherapy-induced neutropenia. Indeed, today's leading cause of death in patients with AH is not the bleeding itself but the complications that occur as a result of extended bleeding and of its treatment, such as surgery for compartment syndrome, transfusion-related reactions, and infections caused by prolonged chemotherapy, particularly in elderly patients.13
Patient age is another important risk factor in outcome. In the meta-analysis of Delgado et al,1 50% of the patients with AH were older than 65 years. This group had a mortality rate of 18%, an observation that has also been described by other authors.14 Death in these patients is mainly caused by infection, a common complication of long-term immunosuppression. Rapid elimination of the inhibitor might, therefore, prevent this complication.
In most patients with AH, intravenous administration of immunoglobulin (ivIg) leads to a temporary displacement of inhibitors. Two possible mechanisms that have been proposed are inhibitor neutralization caused by the presence of anti-idiotype antibodies in the pooled immunoglobulin preparation and attenuation of inhibitor synthesis. However, not all patients respond to ivIg, and inhibitor titers rarely decrease to undetectable levels in responders.15
Despite the low estimated incidence of the disease, it is a devastating disorder, and the costs of treatment are often immense. Therefore, there is considerable interest in improving and optimizing existing treatment regimens. The primary objectives for treating patients with AH should be the safe and rapid elimination of the inhibitor and the development of long-term immune tolerance induction (ITI). However, in most studies, AH patients are treated with numerous treatment regimens and variable factor substitutions, making it impossible to determine the immunomodulatory potency of each treatment modality on inhibitor eradication.
Our modified Bonn-Malmö Protocol (MBMP) involves the combination of 4 therapeutic steps: (1) immunoadsorptive inhibitor removal, (2) high-dose FVIII administration, (3) ivIG, and (4) immunosuppressive medication. Therefore, MBMP incorporates elements of the previously reported Bonn Protocol,9,16 which emphasizes ITI, and the Malmö Protocol, which focuses on immunoadsorption and immunosuppression.17 The present study reports on the largest patient population with AH (n = 35) treated with one consistent protocol at a single center.
Patients, materials, and methods
Patients
Between 1993 and 2004, a series of 35 patients with AH were treated and documented in our center. Patients included in the study were characterized by high-titer inhibitor to FVIII (greater than 5 BU/mL)9,10 and the occurrence of at least one acute bleeding episode. The Ethical Committee of the Medical Faculty at the University of Bonn approved the novel treatment protocol. All patients gave informed consent in writing.
From 1993 to 1995, diagnosis was confirmed by an inhibitor assay using the Bethesda method. From 1995 onward, inhibitor analysis was performed with the Bethesda assay, as modified by Njimegen.18 Differential diagnosis in relation to the lupus erythematosus–associated inhibitor was established with the dilute Russell viper venom test, lupus-activated partial thromboplastin time, plasma dilution test, and determinations of factors II, V, VII, IX, X, and XI. The FVIII levels were determined by 2 methods: the 1-stage clotting assay and the chromogenic FVIII assay. At the beginning of the MBMP treatment, FVIII was less than 1% of the normal range (70-140%) in all patients.
Recombinant FVIIa (rFVIIa) (Novo-7; Novo Nordisk A/S, Bagsvaerd, Denmark) was substituted in 5 patients who received diagnoses after 1995 (Table 1; patients 10, 30, 31, 32, 35) to achieve an immediate reduction in bleeding diathesis during each patient's transfer to our hospital.
Complete remission (CR) was defined as normal FVIII activity (70-140%) without factor substitution and undetectable inhibitor titer levels during a minimum follow-up period of 12 months. Partial remission (PR) was defined as attainment of FVIII recovery by up to 30%, reduction of the inhibitor titer to less than 5 BU/mL, or both.
MBMP treatment
Thirty-five patients with AH were treated with the MBMP. This treatment included (1) large-volume immunoadsorption (IA) 2.5 to 3 times the total plasma volume on days 1 to 5; (2) ivIg substitution (0.3 g/kg body weight [BW]/d, on days 5-7); (3) immunosuppression therapy with cyclophosphamide (1-2 mg/kg BW/d) and prednisolone (1 mg/kg BW/d) from day 1 until remission (dose reduction); and (4) administration of FVIII (100 U/kg BW, and, in exceptional cases [body mass index (BMI) greater than 40] up to 200 U/kg BW) every 6 hours. Optional dose reductions were executed on the basis of clinical signs and the level of recovery achieved (50%-80% FVIII residual activity after 4-6 hours) throughout the treatment cycle. Treatment cycles (days 1-7) were repeated several times, depending on the clinical response and the coagulation factor activity.
Immunoadsorption was accomplished by apheresis of sheep-derived polyvalent antihuman immunoglobulin bound to Sepharose CL 4B (Amersham Pharmacia, Biotech AB, Uppsala, Sweden), using a dual-column system (immunoglobulin; Miltenyi Biotec GmbH, Plasmaselect Division, Bergisch Gladbach, Germany). Blood was drawn from an antecubital vein on one arm at a rate of up to 70 mL/min and was returned after processing through an antecubital vein on the other arm. Alternatively, if antecubital vein access was inadequate, a biluminal central venous catheter was placed. Plasma was continuously separated at a flow rate of up to 80 mL/min using either of 2 apheresis systems (Cobe Spectra; Cobe Labs, Lakewood, CO) (Autopheresis-C Therapeutic Plasma Systems; Baxter Healthcare, Round Lake, IL), with acid–citrate–dextrose (ACD-A; Baxter Healthcare) as an anticoagulant diluted 1:30 or 1:40, respectively, in either system. Separated plasma was passed through the columns. Adsorptive capacity of the columns was 1.25 g for all IgG subclasses.
The target of processing was 2.5 times the plasma volume, and apheresis was continued for 5 days in each treatment cycle. After apheresis, either plasma-derived or recombinant human FVIII was administered, typically 100 IU/kg every 6 hours, or, in an exceptional case (BMI greater than 40), up to 200 IU/kg every 6 hours. The FVIII dosage could be reduced in patients with satisfactory clinical response and 4- to 6-hour recoveries of 50% to 80% throughout the treatment cycle. The magnitude of such dose reductions was equal to 20% of the coagulation factor dose administered on the preceding day.
Treatment-related side effects from concomitant chemotherapy were scaled as follows: 0 indicates no side effects; 1, mild side effects (nausea, hair loss, loss of appetite); 2, severe side effects (fever, infection, alopecia, neutropenia, thrombopenia); and 3, sepsis (if this occurred, MBMP was immediately halted).
Conventional treatment
Three patients received the following treatments between January 1993 and February 1996: cyclophosphamide (n = 3), corticosteroids (n = 3), immunoglobulin (n = 3), azathioprine (n = 3), vincristine (n = 1), FVIII inhibitor bypassing treatment (n = 2) (FEIBA; Baxter Immuno AG, Vienna Austria), and FVIII substitution (n = 3). MBMP treatment started in March 1996, and follow-up continued until March 2004.
Data analysis
All statistical analyses were performed using the Statistical Package for Social Sciences SPSS, version 11.0 (SPSS, Chicago, IL). Nonparametric statistics, the Spearman rank correlation (rs), and the Mann-Whitney U test were used.
Primary study end points were time of apheresis at which activity of the inhibitor was first undetectable, discontinuance of factor substitution on relevant normal FVIII activity levels for 24 to 48 hours after a single 30 IU/kg FVIII dose, and termination of the MBMP treatment without requirement for further apheresis. Kaplan-Meier analysis was performed to evaluate the time at which these end points were reached. Median time to reach these end points was calculated on the basis of the associated 95% confidence interval (95% CI).
Results
Patient characteristics
On admission, all patients exhibited life-threatening bleeding that required blood transfusions and intensive care monitoring. The FVIII level at initial diagnosis and at the beginning of the MBMP was less than 1% of normal (normal range, 70-140%). The types of bleeding observed included muscle bleeding (n = 25) associated with compartment syndrome (n = 4), retroperitoneal bleeding (n = 6), retropharyngeal bleeding necessitating artificial respiration (n = 3), and hematuria (n = 2).
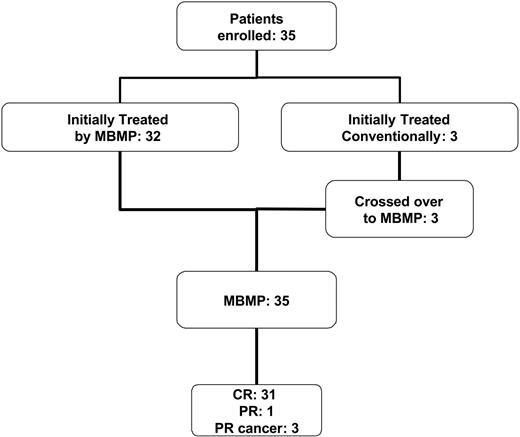
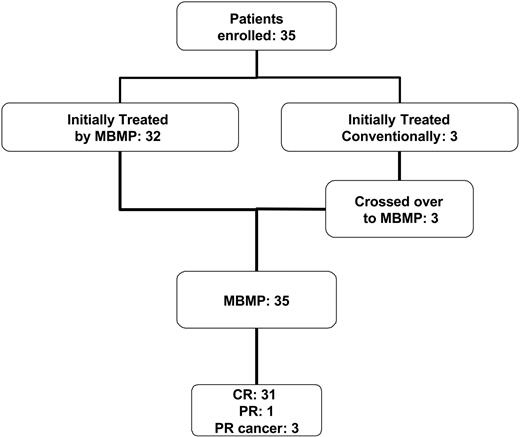
An overview of all enrolled patients is given in Figure 1. Thirty-five patients (19 women, 16 men) with AH caused by high-titer inhibitor levels (greater than 5 BU) received diagnoses in our hospital. Three patients initially received conventional therapies and subsequently crossed over to the MBMP treatment. The mean age of the patients was 61 years ± 16.7 years (mean ± SD; median, 65 years; range, 28-81 years).
Number of patients undergoing the MBMP and treated by conventional therapy. Schematic summary of the number of enrolled patients, the number of patients who received MBMP, conventional treatment, or both, and the clinical outcome.
Number of patients undergoing the MBMP and treated by conventional therapy. Schematic summary of the number of enrolled patients, the number of patients who received MBMP, conventional treatment, or both, and the clinical outcome.
The mean inhibitor titer in the MBMP-treated patients was 369 BU ± 816.3 BU (median, 146 BU; range, 6-3600 BU). Patients with paraneoplastic syndrome (n = 3) had significantly lower inhibitor levels (mean, 18.7 BU ± 14.8 BU; median, 18.7 BU; range, 6-35 BU; P = .021) compared with patients without cancer who underwent the MBMP (mean, 402 BU ± 847 BU; median, 156 BU; range, 20-3600 BU).
Of the 35 patients who underwent the MBMP, 33 completed the treatment. In 2 patients, the MBMP was interrupted in the 3rd and 12th treatment cycles, respectively, because of comorbidities (epileptic seizure, obesity).
Underlying diseases were detected in 11 patients (28.9%). In 4 women (10.5%), the inhibitor was diagnosed peripartum (within 3 months of childbirth). Four patients (10.5%) had another autoimmune disease (mixed connective tissue disease, n = 1; psoriasis, n = 1; polymyalgia rheumatica, n = 1; Sjögren syndrome, n = 1), and in 3 patients (7.8%) the inhibitor occurred as paraneoplastic syndrome (lung cancer, n = 1; paraproteinemia, n = 2; lymphoma, n = 1).
Immunoadsorption
A total of 695 immunoadsorption procedures (apheresis) were carried out, with an average of 19.6 (range, 3-62) apheresis sessions per patient. The extracorporeal treatment was well tolerated. Mild side effects, such as hypotension, hypesthesia from citrate anticoagulation (citric reactions), and allergic reactions occurred in less than 1% of all apheresis sessions that did not require the interruption of treatment. A median plasma volume of 5034 mL (range, 3500-9500 mL) was used, resulting in an average inhibitor reduction of up to 75%. The median reduction during one apheresis session of immunoglobulin was 407 mg/dL for IgG (range, 156-1000 mg/dL), 50 mg/dL for IgA (range, 0-157 mg/dL), and 19 mg/dL for IgM (range, 0-328 mg/dL).
In patients without paraneoplastic syndrome, the number of apheresis sessions correlated with the inhibitor level (rs = 1; P = .005).
Factor substitution
The total amount of factor substitution during MBMP (all patients) and conventional treatment (patients 33, 34, 35) is summarized in Table 1.
The average amount of FVIII that had to be substituted during the MBMP to achieve CR in patients was 0.26 × 106 IU ± 0.35 × 106 IU (median, 0.14 × 106 IU; range, 0.024- 1.87 × 106 IU). Patients who achieved PR received an average of 0.39 × 106 IU ± 0.26 × 106 IU (median, 0.41 IU × 106; range, 0.1-0.7 × 106 IU) FVIII concentrate. FVIII substitution therapy correlated with the inhibitor titer and with the patient's plasma volume (rs = 1, P = .002; rs = 1, P = .03). Five of the MBMP patients required supplementary therapy with rFVIIa. A mean of 14 × 103 kIU rFVIIa ± 10.1 × 103 (median, 12.5 × 103 kIU; range, 2.2-30.0 × 103 kIU) was administered to 5 patients (patients 10, 30, 31, 32, 35) for a median duration of 3 days (range, 2-5 days). Two patients undergoing conventional therapy (patients 34, 35) received 0.7 × 106 IU and 4.2 × 106 IU of FEIBA.
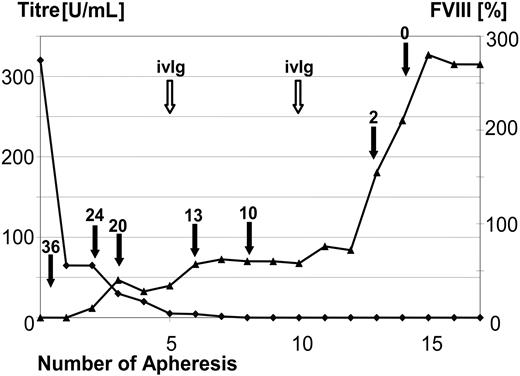
The time course of the development of the FVIII activity and the administered dosages of FVIII for one representative patient are shown in Figure 2. This 68-year-old patient (patient 14) had a pretreatment FVIII inhibitor titer of 327 BU, and the inhibitor was eliminated before the third treatment cycle.
MBMP treatment eliminated the inhibitor and allowed coagulation factor administration to be discontinued. On the left y-axis (▴), inhibitor titer (BU/mL) is shown over the course of apheresis procedures in a representative patient (patient 14). On the right y-axis ( ), the change in measured FVIII activity is shown over the course of apheresis procedures. The dose of administered FVIII IU × 1000 (filled arrow) and the time points of immunoglobulin substitution (open arrow) are marked. In this 68-year-old patient with a pretreatment inhibitor titer of 327 BU FVIII, the inhibitor was completely eliminated before the third treatment cycle (after the 14th apheresis session).
), the change in measured FVIII activity is shown over the course of apheresis procedures. The dose of administered FVIII IU × 1000 (filled arrow) and the time points of immunoglobulin substitution (open arrow) are marked. In this 68-year-old patient with a pretreatment inhibitor titer of 327 BU FVIII, the inhibitor was completely eliminated before the third treatment cycle (after the 14th apheresis session).
MBMP treatment eliminated the inhibitor and allowed coagulation factor administration to be discontinued. On the left y-axis (▴), inhibitor titer (BU/mL) is shown over the course of apheresis procedures in a representative patient (patient 14). On the right y-axis ( ), the change in measured FVIII activity is shown over the course of apheresis procedures. The dose of administered FVIII IU × 1000 (filled arrow) and the time points of immunoglobulin substitution (open arrow) are marked. In this 68-year-old patient with a pretreatment inhibitor titer of 327 BU FVIII, the inhibitor was completely eliminated before the third treatment cycle (after the 14th apheresis session).
), the change in measured FVIII activity is shown over the course of apheresis procedures. The dose of administered FVIII IU × 1000 (filled arrow) and the time points of immunoglobulin substitution (open arrow) are marked. In this 68-year-old patient with a pretreatment inhibitor titer of 327 BU FVIII, the inhibitor was completely eliminated before the third treatment cycle (after the 14th apheresis session).
Clinical outcome, treatment efficiency, and side effects
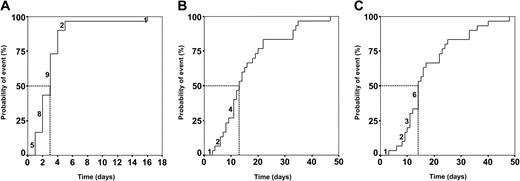
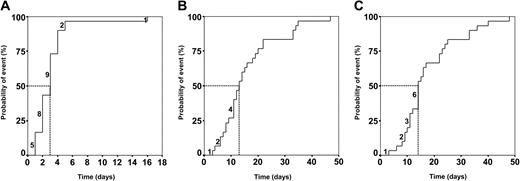
Figure 3 indicates the time points at which undetectable inhibitor levels were achieved (Figure 3A), coagulation factor concentrates could be discontinued (Figure 3B), and stable inhibitor elimination was achieved (Figure 3C). The median number of apheresis days required to reach these end points was 3 days (95% CI, 2-4 days), 12 days (95% CI, 11-17 days), and 14 days (95% CI, 12-17 days), respectively.
Treatment end points were rapidly reached in the MBMP group. Kaplan-Meier plots. (A) Median time to reduce inhibitor to undetectable levels, 3 days (95% CI, 2-4 days). (B) Median time of factor substitution, 12 days (95% CI, 7-17 days). (C) Median time of treatment, 14 days (95% CI, 11-17 days). Abscissa shows time in apheresis days. Numbers above the Kaplan-Meier curves represent patients concluding the MBMP within the corresponding time period. Vertical excursions of the curves signify the occurrence of events. Dotted line indicates the median.
Treatment end points were rapidly reached in the MBMP group. Kaplan-Meier plots. (A) Median time to reduce inhibitor to undetectable levels, 3 days (95% CI, 2-4 days). (B) Median time of factor substitution, 12 days (95% CI, 7-17 days). (C) Median time of treatment, 14 days (95% CI, 11-17 days). Abscissa shows time in apheresis days. Numbers above the Kaplan-Meier curves represent patients concluding the MBMP within the corresponding time period. Vertical excursions of the curves signify the occurrence of events. Dotted line indicates the median.
In 4 patients (patients 29, 31, 32, 35), MBMP induced PR with a median decrease of the inhibitor levels to 2.3 BU (range, 1-4.5 BU), resulting in a median FVIII recovery of 30% (range, 27-35%). In 2 patients, the MBMP was interrupted as a consequence of other concomitant diseases. An 81-year-old woman (patient 29) with grand mal epilepsy and renal dysfunction developed neutropenia, and MBMP treatment had to be interrupted several times. This patient died 5 months later of an epileptic seizure and stroke. The second case was a 38-year-old man (patient 30) who had an excessive plasma volume as a result of obesity (BMI, 49). This patient had an inhibitor titer of 3550 BU. After 62 immunoadsorption cycles, he still had a low inhibitor titer of up to 2.5 BU, but he experienced remission 6 months later.
In 3 patients (patients 31, 32, 35), a malignant disorder with a poor prognosis was diagnosed during the course of the MBMP. The improvement in blood clotting because of the MBMP permitted patients to undergo diagnostic steps for tumor staging, including pleurodesis (patient 35), lymph node biopsy (patient 32), and mediastinoscopy (patient 31) without bleeding events.
The overall CR rate was 88% (31 of 35 patients). When patients with underlying cancer were excluded, the CR rate was 97% (31 of 32 patients).
In all 35 patients treated with MBMP, the acute bleeding episodes were rapidly controlled within the first 2 to 3 apheresis procedures, and no subsequent episodes were encountered. This was also the case for the 3 patients who received conventional treatment between 1993 and 1995. During conventional treatment, they experienced a median of 17 events (range, 12-27 events) (Table 1). Of the 35 patients, 30 tolerated the MBMP treatment very well, with only 1 of those experiencing moderate side effects (nausea and slight hair loss). The remaining 5 patients exhibited severe side effects (infection, n = 3; neutropenia, n = 1; mucositis, n = 1) that were successfully managed by antibiotics without any interruption of MBMP.
Patients who achieved CR because of MBMP had a mean hospital stay of 23 ± 16.8 days (median, 17 days; range, 8-68 days). Patients who achieved PR because of MBMP were hospitalized for 49 days (range, 23-80 days). The 3 patients who received conventional treatment before undergoing MBMP treatment had spent a median of 102 days (range, 25-155 days) in the hospital (Table 1).
During a long-term follow-up (median, 44 months; range, 5-86 months), there was no evidence of any inhibitor relapse in 33 patients. The remaining 2 patients experienced a period in which FVIII declined to 10% and to 50%, respectively, without any bleeding events 10 and 12 months after the initial MBMP treatment. Both patients (patients 33, 34) received conventional therapy before the MBMP treatment. Relapses were managed by apheresis for 5 to 6 days and by immunosuppression therapy. These interventions succeeded in restoring normal FVIII levels, and the clinical condition of the patients remained stable over a follow-up period of 4.5 years. None of the patients died as a direct consequence of bleeding events. However, 2 patients with paraneoplastic disease (lung cancer, patient 35; lymphoma combined with paraproteinemia, patient 31) died of malignant disease 15 and 12 months, respectively, after the beginning of the MBMP.
Discussion
This report describes the successful MBMP treatment of 35 patients with AH. All patients exhibited high inhibitor titers and critical bleeding at presentation. AH is a rare condition, but high-titer patients with AH are seen even less frequently. Because our center is well known and has a good reputation for treating patients with hemophilia, physicians in the surrounding area frequently refer difficult cases to us. This has allowed us to document, for the first time, the treatment of such a large population of these high-titer patients.
Our “difficult-to-treat” patients achieved CR within 2.5 weeks (12-17 days). Various conventional treatment regimens are reported to require 3 to 12 weeks until CR in a population of mainly low-titer patients. The mean time to achieve cessation of bleeding is reported to be 3 weeks for various conventional treatment regimes compared with 3 days with our protocol.19-22
We attribute the high efficacy of the MBMP to the immunoadsorption that was included in our AH treatment protocol. The intensive large-volume immunoadsorption removes high levels of autoantibodies and circulating immune complexes from patient's plasma. Overnight redistribution results in mobilization of the inhibitor from the extravascular space and allows its removal in the subsequent apheresis sessions.
The major advantage of immunoadsorption compared with other extracorporeal treatments, such as plasma exchange, is its high efficiency in removing pathogenic antibodies without a simultaneous loss of coagulation factors. As Fischer et al23 reported recently, plasma exchange can lead to fatal bleeding. Even though these acute bleedings can be controlled with FEIBA, rFVIIa, and porcine FVIII, these approaches do not eliminate the inhibitor. These are symptomatic treatments that require the additional use of expensive clotting factors. Furthermore, we assume that recurring bleeding events increase the inhibitor titer, thereby delaying inhibitor elimination.8
After apheresis, FVIII substitution is an essential element of the MBMP. FVIII infusions did not cause an increase in pathogenic antibodies in the MBMP-treated patients. Several possible mechanisms could explain this phenomenon. First, high concentrations of FVIII may selectively stimulate autoreactive cell clones and make them more sensitive to cytotoxic agents.1,11 Second, immunoadsorption allows high levels of FVIII recovery, which may then trigger the elimination of autoreactive cell clones by Fas-mediated cytotoxicity.24 Third, the presence of low-titer anti-FVIII and anti-idiotypic antibodies in healthy persons has been well established.25,26 Finally, the concentration of natural inhibitors in normal plasma is low and is regulated by natural anti-idiotype antibodies. Although the role of natural inhibitors in maintaining hemostasis and tolerance is not clearly understood, it is possible that they may regulate plasma FVIII levels. In contrast to the highly specific inhibitor antibodies, these antibodies (known as natural antibodies) are polyclonal and polyreactive, and they recognize several types of different antigenic structures. Based on these findings, we postulate that the FVIII substitution in the MBMP allows sufficient biosynthesis of anti-idiotypic antibodies controlling the inhibitor through idiotypic suppression, ensuring permanent inhibitor control.27
Considering these potential immune-stimulating aspects of the MBMP, the beneficial effects of concomitant immunosuppressive therapy may be questioned. Nevertheless, based on our experience, the immunosuppression is obligatory for successful elimination of the inhibitor. Autoreactive cell clones stimulated by the presence of FVIII are likely to have a higher turnover and to become more sensitive to immunosuppressive drugs. Modifications of the MBMP, such as excluding or reducing the FVIII substitution component, may initially save costs but will ultimately lead to higher total costs because of the necessity of bypassing agents in controlling bleeding.
The rapid response to MBMP permits the immunosuppressive drugs to be quickly reduced, thereby lowering the cumulative dose. This issue is of particular importance given the potential risk for leukemia and the procarcinogenic activity of cyclophosphamide doses up to 25 g, especially in younger patients.28-31 As Delgado et al1 recently reported, side effects are frequently observed after an average duration of 3.5 months of chemotherapy, especially in elderly patients. Reducing the levels of immunosuppressive drugs will result in fewer adverse side effects.
Improvements in the MBMP in future should include a more specific knockout of autoreactive cell clones. Rituximab (Mabthera), a chimeric monoclonal antibody that targets the CD20 antigen, is a potential candidate to substitute the current immunosuppression. It has already been reported to be used in AH in several case reports and in 1 prospective clinical study.32-34 Rituximab induces apoptosis primarily in pre–B-cell clones showing no immediate effect on the antibody-bearing plasma cells.35,36 The blockade of B-cell proliferation allows a reestablishment of an intact B-cell pool an average of 3 to 6 months after treatment.35,36 Stasi et al32 describe, in their prospective study of 10 patients, an elimination of the inhibitor within 3 to 12 weeks from the start of rituximab. Critical bleeding events were managed with plasmapheresis, rFVIIa, or both before or during rituximab treatment. CR was seen in 8 patients with low titers. Three of these patients had relapses after restoration of the immune system. In 2 high-titer patients, rituximab alone failed to eliminate the inhibitor but was successful in a second rechallenge in combination with cyclophosphamide. Detailed data on the long-term follow-up of these patients (more than 5 months) would be of considerable interest, especially considering that restoration of the B-cell pool requires a minimum of 3 to 6 months.
In 35 patients who received MBMP treatment, 31 exhibited stable elimination of inhibitor. The remaining 4 patients achieved PR; 3 of them had cancer. The spontaneous formation of inhibitors in patients with malignancy represents a clinical and diagnostic dilemma for the physician. According to Sallah and Wan,5 50% of cancer patients die as a result of bleeding rather than of the cancer itself. In 2 of our patients, IgGκ paraproteinemia was diagnosed, indicating that the inhibitor arose from a monoclonal cell clone. Immunoadsorption can reduce inhibitor mass, but treatment with cyclophosphamide as a part of MBMP was insufficiently potent to suppress a monoclonal cell clone. Finally, the MBMP enabled PR, allowing FVIII recovery of up to 30% in these patients, which permitted invasive diagnostics to determine exact tumor staging.
Sallah and Wan5 found significantly lower inhibitor titers in cancer patients compared with patients without cancer, a finding that was confirmed in our study. In our cancer patients, a discrepancy was seen between inhibitor titers and bleeding severity. Bleeding events are often fulminant in cancer patients, though inhibitor titers are low.14 In the study of Sallah and Wan,5 CR was achieved in only up to 70% of cancer patients. Cancer treatment alone eradicated the inhibitor in 22% of the patients. Those who continued to exhibit the inhibitor developed a more severe disease state. According to Sallah and Wan,5 successful inhibitor elimination was limited to patients who were diagnosed with early and potentially treatable tumors (65% of patients). Furthermore, a strong correlation between type of malignancy and inhibitor remission has been observed. For example, higher response rates occurred in patients with immunohematologic disorders than in those with solid tumors.
In cancer patients, the inhibitor exists as a paraneoplastic syndrome subject to an immunologic mechanism that differs from that seen in patients without cancer. In these patients, chemotherapy with or without surgery may have a profound influence on eliminating the inhibitor. The MBMP approach is applied symptomatically here without expecting or intending attainment of CR. Therefore, the results were calculated separately by excluding and including these patients.
During the long-term follow-up of all patients, only 2 had an episode of reduced FVIII activity without bleeding. These relapses were successfully managed by the MBMP. Both patients had been pretreated with diverse conventional regimens, suggesting that failed treatment regimens delay the elimination of autoreactive cell clones and propose MBMP as the first-line choice.
Our detailed documentation of the clinical parameters (severity/number of bleeding events, use of clotting factors, days of hospitalization) provides enough transparency to enable a cost-benefit analysis to compare MBMP with other treatment strategies. The average consumption of 0.26 × 106 IU FVIII accounted for the major share of the total MBMP treatment costs. The cost of an average dose of 14 × 103 kIU supplementary rFVIIa, and the costs of approximately $1000 US for each immunoadsorption, should be included. Even in high-titer patients with AH, these costs occur only once, whereas with conventional treatments, recurring costs accumulate because of additional bleeding events and longer hospital stays.
Our treatment approach has already been implemented successfully by other institutions.37 Our findings show that MBMP offers a new and safe approach to retain hemostasis and to cure patients with high-titer AH, especially those with critical bleeding. Considering the high efficacy of the MBMP, there are legitimate reservations about carrying out randomized trials. Because of the low incidence of AH and the resultant lengthy recruitment process, randomized clinical trials would delay the introduction of successful treatment by many years.
In conclusion, AH is a rare, but highly interesting, disorder. In contrast to most other autoimmune diseases, the pathogenic antibody involved in AH is identified and quantifiable. Furthermore, this pathogenic antibody can be removed from the patient's plasma, and the antigen can be identified and made available for infusion therapy. These features provide a unique opportunity to study the relationship between natural autoreactivity, autoimmunity, and antigen-driven immune responses to a single protein in vivo. Therefore, the treatment of AH can perhaps facilitate understanding of the pathogenesis and the treatment of autoimmune diseases in general.
Prepublished online as Blood First Edition Paper, November 12, 2004; DOI 10.1182/blood-2004-05-1811.
H.Z. and G.U.-M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


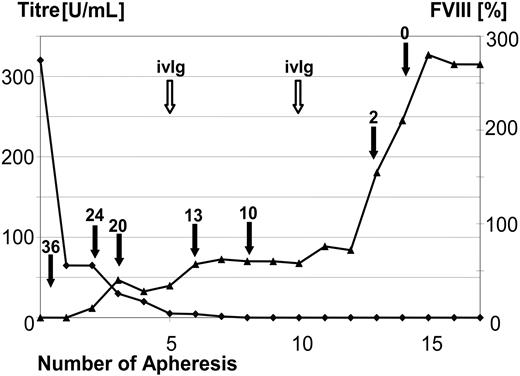
 ), the change in measured FVIII activity is shown over the course of apheresis procedures. The dose of administered FVIII IU × 1000 (filled arrow) and the time points of immunoglobulin substitution (open arrow) are marked. In this 68-year-old patient with a pretreatment inhibitor titer of 327 BU FVIII, the inhibitor was completely eliminated before the third treatment cycle (after the 14th apheresis session).
), the change in measured FVIII activity is shown over the course of apheresis procedures. The dose of administered FVIII IU × 1000 (filled arrow) and the time points of immunoglobulin substitution (open arrow) are marked. In this 68-year-old patient with a pretreatment inhibitor titer of 327 BU FVIII, the inhibitor was completely eliminated before the third treatment cycle (after the 14th apheresis session).