Abstract
BCR-ABL oncoprotein-expressing cells are associated with a relative increase of intracellular reactive oxygen species (ROS), which is thought to play a role in transformation. Elevated ROS levels in BCR-ABL-transformed cells were found to be blocked by the mitochondrial complex I inhibitor rotenone as well as the glucose transport inhibitor phloretin, suggesting that the source of increased ROS might be related to increased glucose metabolism. The glucose analog 2-deoxyglucose (2-DOG) reduced ROS to levels found in non-BCR-ABL-transformed cells and inhibited cell growth alone or in cooperation with imatinib mesylate (Gleevec). A mutant of BCR-ABL that is defective in transformation of myeloid cells, Tyr177Phe, was also found to be defective in raising intracellular ROS levels. Glucose metabolism in BCR-ABL-transformed cells is likely to be mediated by activation of the phosphatidylinositol-3′-kinase (PI3K) pathway, which is regulated through this site. Inhibition of PI3K or mTOR led to a significant decrease in ROS levels. Overall, our results suggest that elevated levels of ROS in BCR-ABL-transformed cells are secondary to a transformation-associated increase in glucose metabolism and an overactive mitochondrial electron transport chain and is specifically regulated by PI3K. Finally, these results hint at novel targets for drug development that may aid traditional therapy. (Blood. 2005; 105:1717-1723)
Introduction
The breakpoint cluster region-abelson (BCR-ABL) fusion protein in CML (chronic myelogenous leukemia), generated by the Philadelphia (Ph) chromosome translocation t(9;22)(q34;q11), has elevated ABL tyrosine kinase activity that is critical for transformation of hematopoietic cells. BCR-ABL-transformed cells show reduced growth factor requirements, enhanced viability, and altered adhesion as a result of constitutive activation of signaling pathways such as p21RAS, STAT5, and phosphatidylinositol-3′-kinase (PI3K). Advances in the development of small molecule drugs led to the discovery of the ABL tyrosine kinase inhibitor imatinib mesylate (Gleevec). However, BCR-ABL has been associated with genomic instability, and progression of the disease correlates with additional cytogenetic alterations that are likely to contribute to resistance to imatinib mesylate and the failure of traditional therapy. Allogeneic bone marrow transplantation (BMT) is the only known curative therapy for CML, and there are many studies currently focused on combining imatinib mesylate with other therapies, including BMT. There is a clear need to identify novel therapeutic targets for the treatment of CML (see Sattler and Griffin1 for review).
We have previously demonstrated that transformation by BCR-ABL is associated with an increase of intracellular reactive oxygen species (ROS).2 In this context the term ROS refers to sequential intermediates generated by univalent reductions of molecular oxygen through stepwise electron transfer from the superoxide radical (O2-), to hydrogen peroxide (H2O2), and then to the hydroxyl radical (OH), which can be reduced to water. Indirect evidence for elevated levels of ROS in primary CML cells has previously been provided by Huang et al.3 It was suggested that specific inhibition of superoxide dismutase would lead to cell death caused by toxic levels of superoxide in leukemic cells with high ROS but not in normal cells with low ROS levels. ROS are thought to be involved in the pathogenesis of many different types of cancer. ROS can play a role in processes that promote cell growth and regulate other biologic functions such as gene expression or migration of cells.4 Interestingly, for example, Suh et al5 have demonstrated that increased production of ROS can lead to a transforming phenotype. Overexpression of the O2--generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase Mox1 in NIH3T3 fibroblasts increases cell growth and induces tumors in athymic mice. ROS are also formed in normal cells in response to a variety of stimuli, including UV irradiation or hematopoietic growth factors, including thrombopoietin (TPO), steel factor (SF), interleukin-3 (IL-3), granulocyte-macrophage colony stimulating factor (GM-CSF), and others.4,6 The platelet-derived growth factor (PDGF)-dependent increase of H2O2 was shown to induce tyrosine phosphorylation, mitogen-activated protein (MAP) kinase stimulation, DNA synthesis, and chemotaxis.7 It has been suggested in these cases that ROS may act as second messengers to regulate activities of redox-sensitive enzymes, including protein kinases and protein phosphatases. Of particular interest is the fact that protein tyrosine phosphatases (PTPases) are highly sensitive to oxidation because of a critical thiol group in the active site. All PTPases contain a highly conserved 11-amino acid sequence that contains a critical cysteine residue: (Ile/Val)-His-Cys-Xxx-Ala-Gly-Xxx-Xxx-Arg-(Ser/Thr)-Gly. Oxidation or mutation of this cysteine residue inactivates the phosphatase activity.8,9 Previous results have, in fact, directly demonstrated that H2O2 can efficiently reduce the cellular PTPase activity and increase tyrosine phosphorylation of cellular proteins. For example, H2O2 can specifically inhibit the protein tyrosine phosphatase activity of LAR and PTP1.10 Overall, there is a fine balance between phosphatases and kinases in the homeostatic mechanism of the cell, and it is likely that changes in the oxidative state in the cell lead to an imbalance that may amplify the signal sent from an activated tyrosine kinase. In addition to PTPases, there are other redox-sensitive molecules that can be regulated by ROS. For example, oxidation of Cys118 in RAS is known to activate its guanosine triphosphatase (GTPase) activity.11 Another example includes redox-dependent inactivation of forkhead family transcription factors in a p66Shc-dependent manner.12 However, the exact mechanism of ROS action has not been entirely elucidated, and it is likely that further characterization of redox-sensitive proteins will be helpful in understanding the signaling involving ROS and in particular its contribution to transformation. It is possible that in vivo ROS simply synergize with cellular functions instead of regulating them.
The goal of this study was to use in vitro models to determine mechanisms involved in the induction of ROS in cells transformed by BCR-ABL. Elevated ROS levels were found to depend on the BCR-ABL-dependent increase in glucose metabolism and activity of the mitochondrial electron transport chain. Pharmacologic inhibition of the glucose pathway led to reduced tyrosine phosphorylation of cellular proteins, which could be overcome by the addition of exogenous ROS in the form of hydrogen peroxide. We have also identified Tyr177 in BCR-ABL as a major regulatory site for ROS induction, likely through a PI3K/mTOR-dependent mechanism. Finally, activation of PI3K was found to be sufficient for elevated levels of ROS by itself. These results hint at the potential of targeting ROS-generating pathways for therapeutic use in cancers associated with BCR-ABL.
Materials and methods
Cells
The murine pre-B cell line BaF3 was grown in RPMI 1640 containing 10% fetal calf serum and 10% WEHI-3B conditioned medium as a source of murine interleukin-3, and the human megakaryocytic cell line MO7e was grown in Dulbecco modified Eagle medium (DMEM) containing 20% fetal calf serum and 20 ng/mL granulocyte-macrophage colony-stimulating factor (Immunex, Seattle, WA). BaF3 and MO7e cells transfected with a BCR-ABL cDNA were grown in the absence of growth factors. The CML (K562, KU812, BV173, MEG01) or leukemia and lymphoma cell lines (DHL4, DHL6, Jurkat, MM1S) were grown in RPMI 1640 (Mediatech/Cellgro, Herndon, VA) containing 10% fetal calf serum (Harlan Bioproducts, Indianapolis, IN). Small cell lung cancer cell lines (H345, H69) were grown in RPMI 1640 supplemented with 1 mM sodium pyruvate (Gibco/Invitrogen, Carlsbad, CA) containing 10% fetal calf serum, and breast cancer cell lines (MDA-MB-231, HTB-30) were grown in McCoy 5A medium (Mediatech/Cellgro) containing 10% fetal calf serum. Cells from patients in CML blast crisis with informed consent and institutional review board (IRB)-approved protocol were obtained through the Connell and O'Reilly Families Cell Manipulation Core at the Dana-Farber Cancer Institute (Boston, MA). Cell growth and viability were determined using an methyl thiazolyl tetrazolium (MTT) assay (In Vitro Toxicology Assay Kit; Sigma, St Louis, MO) or trypan blue exclusion after treatment with glucose, 2-deoxyglucose (2-DOG) or 3-O-methylglucose (3-OMG) (all reagents from Sigma).
Inducible expression system for constitutively active phosphatidylinositol-3′-kinase
The cDNA for bovine p110 phosphatidylinositol-3′-kinase (p110PI3K) fused with an amino-terminal myristoylation sequence and tagged with a carboxy-terminal histidine sequence was kindly provided by Dr Radha P. Narsimhan (Dana-Farber Cancer Institute). The 3.4-kb fragment was subcloned into the BamHI/HindIII restriction sites of the pTRE2 expression vector (Clontech, Palo Alto, CA) to generate the pTRE2.myr-PI3K plasmid. The pTRE2.myr-PI3K plasmid was then cotransfected with the pTK-Hyg plasmid (Clontech) into Ton.BaF.1 cells (kindly provided by Dr George Q. Daley, MIT, Cambridge, MA) containing the reverse Tet transactivator. Cell lines were then selected for hygromycin B resistance in the presence of interleukin-3. Individual subclones were tested for the doxycycline-inducible phosphorylation of AKT as a function of inducible expression of constitutively active p110PI3K in the absence of interleukin-3.
Apoptosis assays
The activity of caspase-3 was measured in cell lysates (CaspACE Assay System; Promega, Madison, WI), and Annexin V-positive staining was determined by fluorescence activated cell sorting (FACS) analysis (Annexin-V-Fluos Staining Kit; Roche Diagnostics, Indianapolis, IN) according to the manufacturer's directions in cells that were treated with glucose, 2-deoxyglucose, 3-O-methylglucose, or left untreated (control).
Cell-cycle analysis
Cells were stained with propidium iodide (50 μg/mL; Sigma) in NP-40 (0.1% vol/vol; Calbiochem, San Diego, CA) and sodium citrate (0.1% vol/vol; Fisher Scientific, Pittsburgh, PA) in the dark at 4°C for at least 15 minutes. Cell-cycle parameters were determined by FACS analysis using a Beckman-Coulter Epics XL flow cytometer (Beckman-Coulter, Miami, FL). The DNA analysis program MultiCycle for Windows (Phoenix Flow Systems, San Diego, CA) was used to determine cell-cycle distribution.
Measurement of intracellular ROS
Fluorescence image analysis was used to determine the relative levels of ROS in response to various stimuli. Cells were treated before FACS analysis, and the relative levels of intracellular ROS were analyzed using the cell-permeable redox-sensitive fluorochrome DCF-DA (2′,7′-dichlorofluorescin diacetate; Fisher Scientific/Acros Organics). Cells were incubated with DCF-DA (5 μM) for 5 minutes at 37°C and subsequently washed twice in cold phosphate-buffered saline (PBS) before analysis using a Coulter Epics XL flow cytometer (Beckman Coulter).
Immunoblotting
Proteins were extracted from whole cells by lysing them in a Tris (tris(hydroxymethyl)aminomethane) buffer (50 mM, pH 8.0; Invitrogen, Carlsbad, CA) containing NaCl (150 mM; Fisher Scientific), NP40 (1% vol/vol; Calbiochem), deoxycholic acid (0.5% wt/vol; Fisher Scientific), sodium dodecylsulfate (0.1% wt/vol; Bio-Rad, Hercules, CA), NaF (1 mM; Sigma), Na3VO4 (1 mM; Sigma), and glycerol (10% vol/vol; Invitrogen) supplemented with leupeptin (5 μg/mL; Roche), aprotinin (10 μg/mL; Roche), and phenylmethylsulfonyl fluoride (1 μM; Sigma). Polyclonal rabbit antibodies against p85 phosphatidylinositol-3′-kinase (Upstate Biotechnology, Lake Placid, NY) or phosphorylated p70S6K or AKT (Cell Signaling, Beverly, MA) and mouse monoclonal antibodies against phosphotyrosine (kindly provided by Dr T. Roberts, Dana-Farber Cancer Institute) were used for immunoblotting or immunoprecipitation.
Results
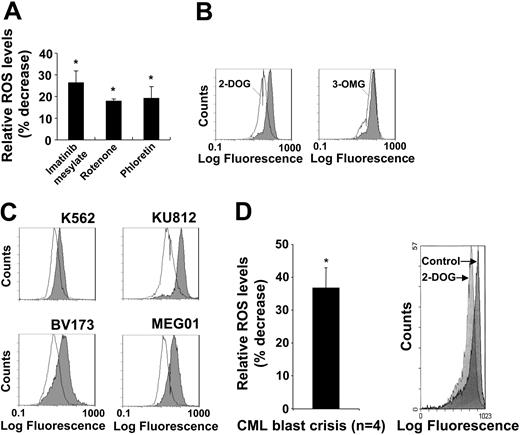
Enhanced glucose transport is required for BCR-ABL-dependent ROS increase. We have previously shown that BCR-ABL kinase activity and an active electron transport chain are required for optimal ROS production, suggesting an involvement of glycolysis. We therefore asked whether inhibition of glucose transporter activity with phloretin would regulate intracellular ROS. As positive controls, the BCR-ABL tyrosine kinase activity in MO7e.BCR-ABL was inhibited with imatinib mesylate (26.4% decrease) and the electron transport with complex I inhibitor rotenone (17.9% decrease). Phloretin (19.2% decrease) was found to be as effective as either imatinib mesylate or rotenone in inhibiting intracellular ROS levels (Figure 1A). This is consistent with a model in which glucose metabolism drives the mitochondrial electron transport chain as a source of ROS. To further determine the role of glucose in this process, we used the glucose analogs 2-DOG and 3-OMG. Both analogs can be taken up into the cell through the glucose transporter, but 2-DOG can be further phosphorylated by hexokinase to 2-deoxyglucose-6′-phosphate. In BCR-ABL-transformed MO7e cells, 3-OMG did not have an effect on intracellular ROS, whereas 2-DOG reduced ROS to levels similar to those observed after imatinib mesylate inhibition of BCR-ABL (not shown). In BaF3 cells, BCR-ABL consistently induced a greater increase in ROS compared with the MO7e cell system, and consequently 2-DOG led to a stronger decrease in ROS levels in BaF3.BCR-ABL cells (51.2% decrease) (Figure 1B, left panel). In addition, 3-OMG induced a marginal and potentially insignificant reduction in ROS (Figure 1B, right panel), indicating that 2-DOG is more effective than 3-OMG in reducing ROS levels. We next measured intracellular ROS levels in response to 2-DOG in the Philadelphia chromosome-positive cell lines K562, KU812, BV173, and MEG01, which were derived from patients with CML in blast crisis. Consistent with the above data, 2-DOG reduced ROS levels in all 4 cell lines (Figure 1C).
The glucose pathway is involved in regulating intracellular ROS levels. Intracellular ROS levels were measured by DCF-DA staining (A-C). (A) MO7e.BCR-ABL cells were treated for 18 hours with imatinib mesylate (1 μM) or for 1 hour with either rotenone (1 μM) or phloretin (100 μM) as indicated (n = 3). (B) BaF3.BCR-ABL cells were treated for 18 hours with 2-DOG (4.5 mg/mL) or 3-OMG (4.5 mg/mL) as indicated and compared with cells in the presence of 4.5 mg/mL glucose (shaded histograms). (C) The Philadelphia chromosome-positive cell lines K562, KU812, BV173, and MEG01 were left untreated (shaded histograms) or treated with 2-deoxyglucose (open histograms). (D) Cells from a patient in CML blast crisis were left untreated or treated for 6 hours with 2-deoxyglucose (2 mg/mL), and the change in intracellular ROS was calculated relative to untreated cells (n = 4). The right panel shows the plot of a typical experiment, comparing untreated cells (control) with 2-deoxyglucose (2-DOG)-treated cells. Error bars indicate standard error of the mean. *Significant differences (P < .05) were observed between treated and control cells.
The glucose pathway is involved in regulating intracellular ROS levels. Intracellular ROS levels were measured by DCF-DA staining (A-C). (A) MO7e.BCR-ABL cells were treated for 18 hours with imatinib mesylate (1 μM) or for 1 hour with either rotenone (1 μM) or phloretin (100 μM) as indicated (n = 3). (B) BaF3.BCR-ABL cells were treated for 18 hours with 2-DOG (4.5 mg/mL) or 3-OMG (4.5 mg/mL) as indicated and compared with cells in the presence of 4.5 mg/mL glucose (shaded histograms). (C) The Philadelphia chromosome-positive cell lines K562, KU812, BV173, and MEG01 were left untreated (shaded histograms) or treated with 2-deoxyglucose (open histograms). (D) Cells from a patient in CML blast crisis were left untreated or treated for 6 hours with 2-deoxyglucose (2 mg/mL), and the change in intracellular ROS was calculated relative to untreated cells (n = 4). The right panel shows the plot of a typical experiment, comparing untreated cells (control) with 2-deoxyglucose (2-DOG)-treated cells. Error bars indicate standard error of the mean. *Significant differences (P < .05) were observed between treated and control cells.
Finally, the potential glucose-dependent generation of ROS was tested in primary cells from patients in CML blast crisis. 2-DOG treatment (6 hours, 2 mg/mL) was found to significantly reduce the intracellular ROS levels by 39% (n = 4) compared with untreated cells (Figure 1D). These findings are comparable with the above data obtained in cell lines, suggesting that 2-DOG inhibits a ROS-inducing pathway in BCR-ABL-transformed cells. Interestingly, prolonged treatment (18 hours) of the CML cells or higher concentrations of 2-DOG (4.5 mg/mL) led to significant apoptosis and a secondary increase in ROS levels (data not shown).
2-DOG cooperates with imatinib mesylate in cell line models
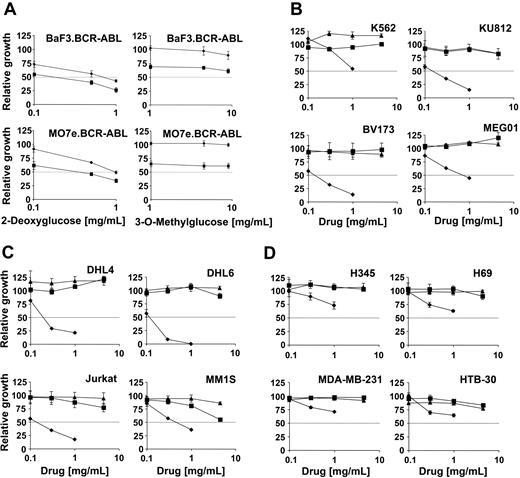
ROS have been suggested to contribute to normal signaling as well as transformation by oncogenic tyrosine kinases. We have previously shown that the tyrosine kinase inhibitor imatinib mesylate leads to inhibition of ROS in BCR-ABL-expressing hematopoietic cells. We therefore also sought to determine whether 2-DOG could cooperate with imatinib mesylate in inhibiting cell growth. BaF3 and MO7e cells transformed by the BCR-ABL oncogene were treated with 0-1 mg/mL 2-DOG (Figure 2A, left panels) or 0 to 4.5 mg/mL 3-OMG (Figure 2A, right panels) alone or in combination with imatinib mesylate. The concentration of imatinib mesylate (0.5 μM) was chosen to induce an approximate 50% reduction in cell growth. The solvent dimethyl sulfoxide (DMSO) did not affect cell growth of BCR-ABL-transformed BaF3 or MO7e cells (data not shown). 2-DOG led to a dose-dependent reduction in cell growth, which was further reduced by 0.5 μM imatinib mesylate treatment. Interestingly, imatinib mesylate (0.5 μM) reduced cell growth similarly in the absence of 2-DOG as in the presence of 1 mg/mL 2-DOG. This suggests that these 2 drugs cooperate, and it is also possible that imatinib mesylate and 2-DOG inhibit overlapping pathways. In these cell line models the related glucose analog 3-OMG did not lead to a significant reduction in cell growth alone or in combination with imatinib mesylate, in contrast to 2-DOG.
The glucose analogs 2-deoxyglucose and 3-O-methylglucose inhibit cell growth in hematologic and nonhematologic malignancies. Relative growth of various cell lines in response to 2-deoxyglucose or 3-O-methylglucose or imatinib mesylate was calculated as a percentage compared with cells left untreated. (A) Cell growth of the BCR-ABL-expressing hematopoietic BaF3 and MO7e cell lines was determined. Cells were treated for 1 day with the indicated amounts of 2-deoxyglucose (left panels) or 3-O-methylglucose (right panels) in the absence (♦) or presence (▪) of imatinib mesylate (0.5 μM). (B-D) Cell growth of (B) Ph+ cell lines, (C) leukemia and lymphoma cell lines, and (D) solid-tumor cell lines was determined as indicated. Cells (n = 4) were treated for 3 days with either glucose (▴), 3-O-methylglucose (▪), or 2-deoxyglucose (♦) (B-C). Error bars indicate standard error of the mean.
The glucose analogs 2-deoxyglucose and 3-O-methylglucose inhibit cell growth in hematologic and nonhematologic malignancies. Relative growth of various cell lines in response to 2-deoxyglucose or 3-O-methylglucose or imatinib mesylate was calculated as a percentage compared with cells left untreated. (A) Cell growth of the BCR-ABL-expressing hematopoietic BaF3 and MO7e cell lines was determined. Cells were treated for 1 day with the indicated amounts of 2-deoxyglucose (left panels) or 3-O-methylglucose (right panels) in the absence (♦) or presence (▪) of imatinib mesylate (0.5 μM). (B-D) Cell growth of (B) Ph+ cell lines, (C) leukemia and lymphoma cell lines, and (D) solid-tumor cell lines was determined as indicated. Cells (n = 4) were treated for 3 days with either glucose (▴), 3-O-methylglucose (▪), or 2-deoxyglucose (♦) (B-C). Error bars indicate standard error of the mean.
We also compared the effect of 2-DOG, 3-OMG and glucose on BCR-ABL-transformed cell lines (K562, KU812, BV173, MEG01) (Figure 2B) and on cells transformed by a mechanism different from a tyrosine kinase fusion protein, including leukemia (Jurkat), lymphoma (DHL4, DHL6), and multiple myeloma (MM1S) cell lines (Figure 2C). In all cell lines tested, 2-DOG, in contrast to 3-OMG, reduced cell growth by at least 50% over a 72-hour time period compared with cells treated with glucose. 2-DOG had an IC50 (50% inhibitory concentration) less than 1 mg/mL in most cell lines but was less effective in growth inhibition of some solid-tumor cell lines, including the small cell lung cancer cell lines H345 and H69 as well as the breast cancer cell lines MDA-MB-231 and HTB-30 (Figure 2D). Also, in the BCR-ABL-expressing cell lines K562, KU812, BV173, and MEG01, cell growth was not inhibited significantly by 3-OMG compared with glucose-treated cells. Glucose treatment itself reduced cell growth in all cell lines at concentrations greater than 10 mg/mL, and 3-OMG reached an IC50 less than 10 mg/mL only in the multiple myeloma cell line MM1S (data not shown). It is expected that 2-DOG will have similar effects on metabolically active hematopoietic cells, stimulated by growth factors. Indeed, preliminary data suggest that 2-DOG inhibits cell growth and viability, as well as reduces ROS levels, in cells with elevated intracellular ROS, such as interleukin-3-stimulated BaF3 cells (data not shown).
2-DOG induces apoptosis in BCR-ABL-transformed cells
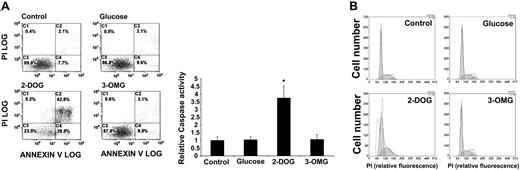
Apoptosis is a complex cellular function that is regulated in part through the availability of nutrients, and inhibition of the glucose pathway is therefore expected to induce an increase in apoptosis. We measured the change in Annexin V-positive staining of cells, an indication for increased exposure of phosphatidylserine to the outer cell membrane during apoptosis. Using BCR-ABL-transformed MO7e cells, we found that treatment with 2-DOG (72 hours, 4.5 mg/mL) led to an increase in Annexin V-positive cells compared with either 3-OMG- or glucose-treated cells (Figure 3A). In the control cells less than 10% of the total population showed signs of apoptosis; however, the number of apoptotic cells increased to more than 70% after 2-DOG treatment in a typical experiment. We next measured the activation status of caspase-3, a downstream effecter of the proapoptotic caspase-9. Similar to the previous data, we observed a consistent increase in caspase-3 activity (3.7-± 0.8-fold increase; n = 3; P < .02) in 2-DOG-treated cells compared with untreated, glucose-, or 3-OMG-treated MO7e.BCR-ABL cells (Figure 3A).
Induction of apoptosis 2-deoxyglucose. MO7e cells transformed by BCR-ABL were treated with glucose (4.5 mg/mL), 3-OMG (4.5mg/mL), and 2-DOG (4.5 mg/mL) or left untreated (control) as indicated (A-B). Annexin V-positive cell staining, induction of caspase-3 activity (n = 3) (A), and cell-cycle distribution indicated by shaded areas (B) were determined in a 72-hour culture. Error bars indicate standard error of the mean. *Significant differences (P < .05) were observed between treated and control cells (n = 3).
Induction of apoptosis 2-deoxyglucose. MO7e cells transformed by BCR-ABL were treated with glucose (4.5 mg/mL), 3-OMG (4.5mg/mL), and 2-DOG (4.5 mg/mL) or left untreated (control) as indicated (A-B). Annexin V-positive cell staining, induction of caspase-3 activity (n = 3) (A), and cell-cycle distribution indicated by shaded areas (B) were determined in a 72-hour culture. Error bars indicate standard error of the mean. *Significant differences (P < .05) were observed between treated and control cells (n = 3).
We also determined whether inhibition of glucose metabolism with 2-DOG would induce cell-cycle arrest. Cells were left untreated or treated with 2-DOG and compared with 3-OMG- and glucose-treated cells, and the different phases of cell-cycle distribution were determined (Figure 3B; Table 1). The percentage of cells in G2/M phase decreased from 6.3% to less than 1% in cells that were treated for 72 hours with 2-DOG (4.5 mg/mL), whereas the percentage of cells in S phase (increase from 34.9% to 37%) and G1 phase (increase from 58.8% to 62.6%) increased. This suggests that inhibition of the glucose pathway does not lead to a significant cellcycle arrest in the transformed cells. However, there was a significant increase of cells in sub-G1 phase, which was consistent with apoptotic cell death. However, 3-OMG and glucose had little effect on cell-cycle distributions. These data demonstrate that 2-DOG-induced apoptosis is likely to contribute to the reduced cell growth of 2-DOG-treated BCR-ABL-transformed cells. Interestingly, we found in additional experiments that 2-DOG reduces BCR-ABL-dependent tyrosine phosphorylation of cellular proteins, and this could be overcome in part by treatment with the ROS hydrogen peroxide (data not shown).
Tyrosine 177 in BCR-ABL regulates intracellular levels of ROS
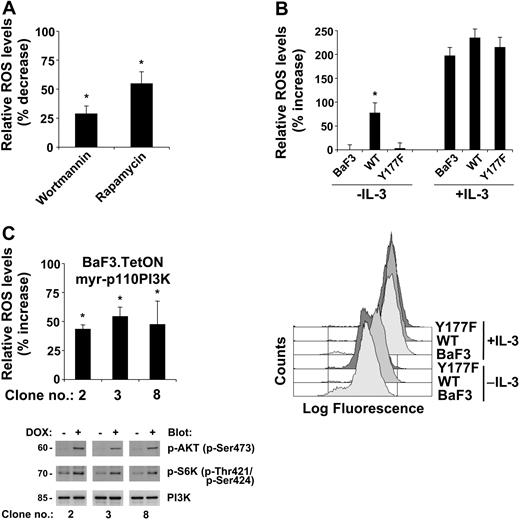
There are likely multiple proteins and pathways regulated through the effects of 2-DOG. Of special interest is the PI3K pathway since it was previously shown to be involved in the regulation of glucose uptake, sensing, and metabolism. To evaluate the potential role of the PI3K pathway in the regulation of ROS levels, we used wortmannin, an inhibitor of PI3K activity, and rapamycin, an inhibitor of mTOR activity, to treat a BCR-ABL-transformed cell line. There was a significant inhibition of ROS with either inhibitor, reducing the ROS levels by 29.2% with wortmannin and by 56.2% with rapamycin treatment compared with controls (Figure 4A). Neither treatment reduced intracellular ROS to levels found in growth factor-deprived untransformed cells (not shown). In addition, rapamycin was consistently more effective than wortmannin in reducing ROS, suggesting that there are additional pathways involved in the regulation of ROS that may also be involved the regulation of mTOR and are independent of PI3K.
Activation of the PI3K pathway by BCR-ABL is required for elevated levels of ROS. Intracellular ROS levels were measured by DCF-DA staining (A-C). (A) BCR-ABL-transformed BaF3 cells were treated for 4 hours with wortmannin (10 nM) or rapamycin (1 nM) and compared with control-treated cells (top). (B) Parental BaF3 cells and cells expressing either BCR-ABL wild type (WT) or BCR-ABL containing a Tyr177Phe substitution (Y177F) were used and left untreated or stimulated with IL-3 (top). Representative FACS plots of the data are presented in the bottom panel; the top 3 curves represent cells treated with IL-3; the bottom 3, untreated. (C) Three individual clones of starved BaF3.TetON.myr-p110PI3K cells were either left untreated or treated for 18 hours with doxycycline (1 μg/mL). The changes in relative ROS levels were determined (top), and p85 PI3K expression or phosphorylation on specific serine and threonine residues in AKT and S6K was detected as indicated (bottom). The error bars indicate the standard errors of the mean. *Significant differences (P < .05) were observed between treated and control cells (n = 3).
Activation of the PI3K pathway by BCR-ABL is required for elevated levels of ROS. Intracellular ROS levels were measured by DCF-DA staining (A-C). (A) BCR-ABL-transformed BaF3 cells were treated for 4 hours with wortmannin (10 nM) or rapamycin (1 nM) and compared with control-treated cells (top). (B) Parental BaF3 cells and cells expressing either BCR-ABL wild type (WT) or BCR-ABL containing a Tyr177Phe substitution (Y177F) were used and left untreated or stimulated with IL-3 (top). Representative FACS plots of the data are presented in the bottom panel; the top 3 curves represent cells treated with IL-3; the bottom 3, untreated. (C) Three individual clones of starved BaF3.TetON.myr-p110PI3K cells were either left untreated or treated for 18 hours with doxycycline (1 μg/mL). The changes in relative ROS levels were determined (top), and p85 PI3K expression or phosphorylation on specific serine and threonine residues in AKT and S6K was detected as indicated (bottom). The error bars indicate the standard errors of the mean. *Significant differences (P < .05) were observed between treated and control cells (n = 3).
We had previously shown that the autophosphorylation site Tyr177 of BCR-ABL regulates activation of PI3K. We therefore hypothesized that this site may also be involved in the regulation of ROS. We used the interleukin-3-dependent cell line BaF3 expressing either wild-type BCR-ABL or the Tyr177Phe mutation. Consistent with our previous data demonstrating that BCR-ABL can maintain high levels of ROS in transfected cell lines, we found a 77.2% higher level of ROS in the BCR-ABL-expressing cells compared with the parental growth factor-deprived cells. Most importantly, introduction of the Tyr177Phe mutation in BCR-ABL failed to increase intracellular levels of ROS (Figure 4B, top left panel), suggesting that pathways regulated through this site are important in the regulation of ROS. As a control, cells were treated with interleukin-3, and intracellular levels of ROS were analyzed. Cell lines were stimulated at an interleukin-3 concentration that causes a maximal proliferative and antiapoptotic response. It is likely and desired that these conditions lead to a saturation of IL-3 receptors and a maximum induction of ROS level. Stimulation of the parental and the BCR-ABL-transfected cell lines with interleukin-3 led to a comparable increase of intracellular ROS (Figure 4B, top right panel). The ability of interleukin-3 to stimulate ROS production to a similar extent in these different cells suggests that the increase in ROS in the absence of interleukin-3 was specific to BCR-ABL and not due to clonal selection of cell lines. It should be emphasized that conditions used in this control experiment are unlikely to occur often in a human body.
These results demonstrate that activation of the PI3K pathway is required for optimal induction of ROS in BCR-ABL-transformed cells. To further determine whether activation of PI3K is sufficient to increase cellular levels of ROS, we generated BaF3 cells with doxycycline-inducible myristoylated p110PI3K (myr-p110PI3K). Three subclones with doxycycline-inducible expression of myr-p110PI3K were characterized for altered levels of intracellular ROS in response to doxycycline. Doxycycline treatment by itself does not alter intracellular ROS levels (data not shown). Induction of Myr-p110PI3K expression led to a significant increase in ROS in all 3 cell lines tested with an increase of 43% to 54% compared with untreated cells (Figure 4C, top panel). In control experiments the functional activation of the PI3K pathway in these cell lines was determined by immunoblotting using phospho-specific antibodies against activation sites in AKT and p70S6K. As a control for equal loading, membranes were probed for actin (not shown) and p85PI3K. In all 3 subclones tested there was a significant induction of phosphorylation at Ser473 in AKT and Thr421/Ser424 in p70S6K (Figure 4C, bottom panels), demonstrating the activation of PI3K-dependent signaling pathways in cells expressing myr-p110PI3K. Overall, these results demonstrate that activation of the PI3K pathway is sufficient for the induction of elevated levels of ROS.
Discussion
The available evidence suggests that production of ROS contributes to signal transduction, viability, and possibly genomic instability associated with tyrosine kinase oncogenes, including BCR-ABL. The exact mechanisms whereby BCR-ABL regulates intracellular ROS have not yet been determined. These data presented here suggest that the origin of ROS associated with BCR-ABL transformation is linked to the mitochondrial electron transport chain and is under metabolic control in the cell. We found that the electron transport inhibitor rotenone and the Glut1 glucose transport inhibitor phloretin led to a reduction of ROS similar to that in cells treated with the ABL kinase inhibitor imatinib mesylate. Therefore, the generation of ROS depends mainly on the supply of energy in the form of glucose or pyruvate. The increase in intracellular ROS associated with BCR-ABL can be reduced by the glucose analog 2-DOG. Phosphorylation of 2-DOG to 2-DOG-6-phosphate reduces the amount of intracellular glucose-6-phosphate formation, which in turn has recently been suggested to be involved in the regulation of ROS levels.13 In cells transformed by the BCR-ABL or v-Abl tyrosine kinases, the uptake of glucose through the glucose transporter is tightly regulated by the oncoprotein itself.14-16 Activated ABL kinases are likely to regulate glucose uptake by increasing the affinity of glucose to Glut1 and regulating transporter activity, but there may be additional mechanisms.17-19 Targeting the glucose pathway with 2-DOG demonstrates potent anticancer activity of this drug in vitro by causing apoptosis, reduced cell growth, and cooperative effects with imatinib mesylate. 2-DOG can also reduce cell growth in cells transformed by a mechanism different from that of BCR-ABL, as we have shown in leukemia, lymphoma, and multiple myeloma cell lines. Identifying targets for other signal transduction inhibitors that are complementary to imatinib mesylate without adding toxicity to normal blood cells is of great interest. However, preliminary data suggest that 2-DOG inhibits cells growth and ROS levels not only in BCR-ABL-transformed cell lines but also to a certain extent in the parental cells. This is consistent with our observation that transformation by BCR-ABL and stimulation with hematopoietic growth factors up-regulate intracellular ROS levels in cell line models.2,4 The ROS pathway is chronically active only in BCR-ABL-transformed cells, suggesting that a combination of traditional therapy with improved inhibitors of glucose pathways may be beneficial for the treatment of CML.
Normal cells also produce ROS and contain a number of mechanisms to carefully regulate production and metabolism of these potentially toxic molecules. In addition to the direct induction of ROS through mitochondrial pathways, levels of ROS can be regulated indirectly though a variety of mechanisms. For example, thiols such as thioredoxin or glutathione reduce ROS, and it is possible that growth stimuli in hematopoietic cells affect the activity of one of these pathways. Superoxide dismutase generates H2O2 from
Of special interest for BCR-ABL transformation is the requirement for an active PI3K/mTOR pathway in the induction of elevated levels of intracellular ROS. This is consistent with previous findings, demonstrating a requirement of PI3K activation in the PDGF receptor-dependent production of hydrogen peroxide.21 Activation of the PI3K pathway has already been shown to be important for transformation by BCR-ABL.22 Inhibition of either PI3K or mTOR with specific inhibitors cooperates with imatinib mesylate in BCR-ABL-transformed cells, while having reduced or little effect on normal bone marrow cells.23,24 We have previously shown that cells expressing BCR-ABL with a Tyr177Phe substitution exhibit markedly reduced activation of the PI3K pathway.25 Evidence that the PI3K pathway is critical for the generation of ROS comes from the finding of reduced levels of ROS in the BCR-ABL Tyr177Phe mutant, which we have previously shown to be defective in its ability to activate PI3K. Confirmation that PI3K is likely to be important comes from the observation that expression of an activated allele of PI3K in nontransformed cells results in production of ROS by itself. BCR-ABL activates PI3K likely through recruitment of a scaffolding adapter Grb2/Gab2 complex to autophosphorylated Tyr177 in BCR.25 Gab2 and its associated proteins may therefore be key mediators of ROS signaling by BCR-ABL-transformed cells. Interestingly, Momose et al26 recently implicated Gab2 phosphorylation-dependent activation of PI3K in Fcγ and fMLP (formyl-methionyl-leucyl-phenylalanine)-stimulated cells in superoxide formation. It is therefore likely that Gab2 may also have a predominant role in the regulation of ROS in BCR-ABL-transformed cells. It will now be interesting not only to determine the requirement for Gab2/PI3K in the induction of ROS but also to look at its regulation of the mTOR pathway, which we have also identified as a key regulator of ROS. The activity of mTOR is regulated at least in part through the PI3K effecter AKT and can be specifically inhibited by rapamycin. mTOR has previously been implicated in nutrient sensing by activating S6kinase and inducing gene expression.27,28 The involvement of mTOR in the glucose-dependent regulation of ROS in BCR-ABL-transformed cells remains to be determined.
The predominant role of PI3K in the regulation of ROS may also have implications for the regulation of downstream effectors. It has previously been suggested that the fine balance between activity of tyrosine kinases and tyrosine phosphatases can be shifted toward increased tyrosine phosphorylation of cellular proteins through an increase in cellular ROS. A similar mechanism may also occur in the regulation of bioactive phosphatidylinositol-3′-phosphates. PI3K activity has been shown to be increased as a result of oxidative stress.29 Also, the phosphatydylinositol-3-phosphatase PTEN has recently been shown to be redox-regulated through inactivation by oxidation of Cys124 in the active site.30 PTEN is a tumor suppressor and a target of mutations in solid tumors, but it may not be mutated in hematologic malignancies.31 Leslie et al32 recently suggested that inactivation of PTEN by itself can lead to oxidative stress and activation of PI3K. Activation of the PI3K effector AKT by hydrogen peroxide in U87 glioma cells required expression of PTEN. Our results would favor a model in hematopoietic cells wherein activation of PI3K leads to an increase in ROS independent of PTEN. However, the exact role of PTEN in the direct regulation of ROS and PI3K in hematopoietic cells still needs to be determined. It is also possible that there are additional PI3K-independent pathways that contribute to the regulation of ROS in BCR-ABL-transformed cells.
In summary, these studies have characterized the dramatic effects of targeting the glucose pathway with 2-DOG on cell growth, viability, cellular tyrosine phosphorylation, and intracellular ROS. To verify the potential of the glucose pathway for targeted therapy it will be important to identify novel drugs with higher specificity. Since increased glucose metabolism is a common feature among metabolically active hematopoietic cells, it is likely that the efficacy of targeting this pathway will be increased in vivo by combination with standard therapy, such as imatinib mesylate treatment. Our results illustrate the potential of targeting pathways involved in the regulation of ROS for therapeutic use in leukemias associated with activated forms of ABL as well as in other cancers.
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-03-0849.
Supported in part by National Institutes of Health (NIH) (grant DK66996), a Leukemia and Lymphoma Society Specialized Centers of Research (SCOR) grant (J.D.G.), and an American Cancer Society Research Scholar grant (M.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.