Abstract
Gene therapy is a potential route for the delivery of secreted therapeutic proteins, but pharmacologic control of expression will generally be required for optimal safety and efficacy. Previous attempts to achieve regulated expression in largeanimal models have been thwarted by transient expression or immune responses to regulatory proteins. We evaluated the ability of the dimerizer-regulated gene expression system to achieve controlled, long-term production of erythropoietin (Epo) following intramuscular administration of adeno-associated virus (AAV) vectors to 16 primates. All animals showed dose-responsive and completely reversible elevation of Epo and hematocrit in response to the dimerizer rapamycin, or analogs with reduced immunosuppressive activity, administered intravenously or orally. Animals that received optimized dual vectors showed persistent regulated expression for the duration of the study, with no apparent immune response to Epo or the regulatory proteins. Similar results were obtained with single vectors incorporating both the Epo and regulatory genes, including those packaged into serotype 1 AAV vectors to allow use of lower viral doses. For the longest-studied animal, regulated expression has persisted for more than 6 years and 26 induction cycles. These data indicate that one-time or infrequent gene transfer followed by dimerizer regulation is a promising approach for delivery of therapeutic proteins.
Introduction
Therapeutic proteins are conventionally delivered by repeated parenteral administration. In principle, the use of gene therapy to achieve stable protein expression in vivo has several potential advantages, including eliminating frequent injections and obviating the need to manufacture recombinant protein.1 Indeed, adeno-associated virus (AAV) vectors have proved capable of stable transduction of a variety of target tissues, leading to persistent expression of secreted proteins in mice,2,3 dogs,4,5 and some primates.6,7 Intramuscular or intrahepatic administration of AAV vectors encoding coagulation Factor IX can correct hemophilia B in dogs,4,5 and has been evaluated clinically.8
The relatively benign safety profile of Factor IX makes it a good candidate protein for delivery by gene therapy. However, many proteins have more narrow therapeutic windows, and over-production could result in toxicity. An example is erythropoietin (Epo), a hormone widely used to treat anemia.9 Constitutive expression of Epo and elevated hematocrit has been achieved using AAV vectors administered intramuscularly to mice6,10-12 or primates.6,13,14 However, excessive levels of Epo induce a potentially fatal polycythemia,6,13 requiring the use of careful vector titration and/or therapeutic phlebotomy. To deliver such proteins using gene therapy, it will probably be essential to equip the transgene with a regulatory system, ideally one that allows expression to be controlled pharmacologically by an orally administered drug. Such a system would allow protein levels to be titrated into the therapeutic window, to be altered as the disease evolves, and to be terminated if required.1
Several regulatory systems have been designed in which the gene of interest is controlled by an engineered transcription factor inducible by drugs such as tetracycline (Tet), mifepristone, or ecdysone.1 We have developed a system in which the DNA-binding and activation domains of a transcription factor are expressed separately as fusion proteins that can be reversibly crosslinked by addition of a bivalent “dimerizer” drug, such as the natural product rapamycin or its analogs.15,16 Addition of the drug dimerizes the fusion proteins and activates transcription of the target gene. The system is characterized by dose-dependent, reversible induction, with negligible background in the absence of inducer, in a variety of vector systems in vitro15-18 and in vivo.6,12,15,19-25 Importantly, the system is built exclusively from human proteins to minimize the potential for immunogenicity in a clinical setting.
In mice, controlled Epo expression following AAV-mediated gene transfer to muscle has been achieved with both the Tet-26,27 and rapamycin-regulated6,12 systems. However, this has proved difficult to translate to the large animal setting. We previously described rapamycin-regulated production of Epo in a rhesus macaque, but inducibility only persisted through 2 induction cycles (∼ 3 months).6 Tet-inducible expression has been achieved in 2 cynomolgus macaques for approximately one year, but at least 5 others developed an immune response against the bacterially derived Tet-regulated transcription factor, eliminating transduced myofibers and abolishing expression.7,28 These results suggested that the apparent promise of regulated protein delivery using AAV vectors might not extend to primates and potential clinical applications. In this study, we describe the design and validation of next-generation rapamycin-regulated AAV vectors that reproducibly allow multiyear inducible Epo expression in primates, with 100% long-term persistence and with no apparent immune response. These data establish the viability and clinical potential of regulated gene therapy.
Materials and methods
Plasmid construction
The following vectors were described previously: AAV-CMV-Epo,6,22 AAV-CMV-TF1,20 and AAV-CMV-TF1Nc.22 AAV-RSV-Epo was generated by replacing the cytomegalovirus (CMV) enhancer of AAV-CMV-Epo with the Rous sarcoma virus (RSV) enhancer (pREP8; Invitrogen, Carlsbad, CA). AAV-Z12I-rhEpo-3 is identical to AAV-Z12I-rhEpo-2,6 except that it contains a 5′ chimeric intron (pCI; Promega, Madison, WI). To construct AAV-CMV-TF-rhEpo2.3, a transcription factor expression cassette was derived from AAV-CMV-TF1Nc by removing the intron and replacing the 250–base pair (bp) human growth hormone (hGH) 3′ untranslated region (UTR) with a minimal 120-bp hGH UTR generated by polymerase chain reaction (PCR) with primers 5′-CCACTCCAGTGCCCACCAGC-3′ and 5′-TTGCTCCAAACCACCCCCC-3′. An Epo target gene cassette was derived from AAV-Z12I-rhEpo-2 by removing 4 copies of the ZFHD1 binding site and replacing the 240-bp SV40 UTR with a minimal 53-bp rabbit β-globin UTR consisting of the following sequence: 5′-GCCTAATAAAGAGCTCAGATGCATGCATCAGAGTGTGTTGGTTTTTTGTGTGT-3′. The Epo target cassette was then inserted at the 3′ end of the transcription factor vector cassette (head to tail).
Epo transgene
The rhesus Epo transgene used in this study6 was cloned from the genomic DNA of a rhesus monkey by standard methods, and found to match the endogenous Epo gene sequence in both a persistently inducible (94B091) and a nonpersistent (EWP) monkey. This transgene encodes leucine, isoleucine, and methionine at amino acids 17, 33, and 81, respectively, whereas the published rhesus Epo sequence29 encodes valine, valine, and isoleucine at these positions. The endogenous Epo genes cloned from 2 cynomolgus monkeys were also found to encode an identical amino acid sequence to this rhesus Epo transgene.
AAV-vector generation and production
AAV vectors were generated by triple plasmid transfection of 293 cells or by an Ad/AAV hybrid system, purified by cesium gradient sedimentation or column chromatography and assayed for infectivity, endotoxin contamination, and purity.30 AAV1-pseudotyped vector was generated by triple transfection to package vectors carrying AAV2 inverted terminal repeats (ITRs) with capsid from AAV1, followed by cesium gradient sedimentation.31 Viral genome titer (genome copies) was determined by either slot blot hybridization or by TaqMan real-time PCR. The single vector AAV-CMV-TF-rhEpo2.3 (4938 nucleotide [nt]) was efficiently packaged; average titers of 1.5 × 1012 were obtained for the AAV2-packaged vector, and 25.0 × 1012 for the AAV1-pseudotyped vector, compared with 10.9 × 1012 and 9.5 × 1012 for AAV2-packaged AAV-CMV-TF1Nc and AAV-Z12I-rhEpo-2, respectively.
Animal work
Rhesus monkeys were treated and cared for at the University of Pennsylvania. The study protocol was approved by the institutional animal care and use committee, and use of the vectors in the protocol was approved by the Environment Health and Radiation Safety Office and the Institutional Biosafety Committee. Blood samples were taken via venipuncture of the saphenous vein. Therapeutic phlebotomy was performed for any animal with a hematocrit over 65%. The FFT monkey was administered 166 mg/kg sodium 4-phenylbutyrate orally 3 times a day on days 291 to 338.
Vector and drug administration
Viruses were generally resuspended in 4 mL to 10 mL total phosphatebuffered saline (PBS) and an equal number of 1-mL injections were administered to the vastus lateralis of each leg at doses indicated in Table 1. The injection volumes for 00E022, 97E036, and 97E081 were 3 mL, 1.6 mL, and 0.5 mL, respectively. For intravenous administration, rapamycin and analogs were initially dissolved in N, N dimethylacetamide (DMA) to a concentration of 50 mg/mL and then diluted in propylene glycol to generate a 2-mg/mL stock solution. Just before use, the stock solution was diluted to an appropriate concentration in diluent (1.2% Tween 80, 27% polyethylene glycol 400 [PEG-400]) and injected in a dose volume of 0.5 mL/kg. For oral administration, an appropriate concentration of an AP22594 dispersion in water was administered in a dose volume of 2 mL/kg to animals fasted overnight. AP22594 was synthesized as described.32
Determination of Epo levels
Levels of rhesus Epo in serum were measured in duplicate using the Quantikine Epo enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN), which was calibrated against a human Epo standard. To determine the relative sensitivity of the assay for rhesus Epo, we generated purified recombinant human and rhesus Epo, each fused to the same epitope tag. Comparison of equal concentrations of both proteins revealed the ELISA to be approximately 4-fold less sensitive for rhesus versus human Epo (n = 4; data not shown). For consistency with other studies, we report here uncorrected values as measured by the ELISA.
Mouse skin graft rejection assay
Assays were carried out as previously described,33 using Balb/c mice as skin graft donors and C57bl/6 mice as recipients. Beginning on the day of grafting (day 1), groups of mice (n = 10) received daily subcutaneous administrations of rapamycin or AP22594 in vehicle (10% DMA, 90% [9:1 PEG-400/Tween 80]), or vehicle alone, in a dose volume of 4 mL/kg. The day of complete graft rejection was scored.
Results
Long-term constitutive Epo expression in monkeys
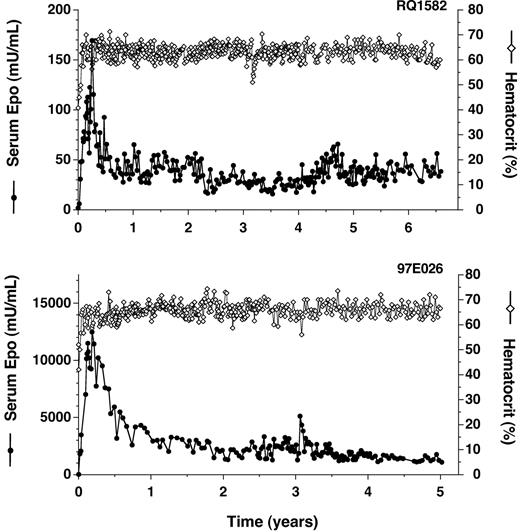
AAV-mediated delivery to rhesus monkey skeletal muscle was first studied with a vector that expresses rhesus Epo constitutively from a cytomegalovirus enhancer (AAV-CMV-Epo). Following a single intramuscular injection of AAV-CMV-Epo to a rhesus macaque (4.8 × 1012 genome copies [GCs]/kg), serum Epo levels have been maintained at supraphysiologic levels for more than 6 years, with Epo levels peaking 3 months after vector administration and stabilizing at a 4-fold lower level by 6 months (Figure 1A; a preliminary description of this animal was presented previously6,34 ). Essentially all of the serum Epo detected is vectorderived since endogenous Epo levels in rhesus monkeys are measured as less than 3 mU/mL (see “Materials and methods”). As a result of the sustained over-production of Epo, hematocrit levels rose from a baseline of 40% and have been maintained at more than 60% for longer than 6 years (Figure 1; this and all subsequently studied animals were phlebotomized for safety each time hematocrit rose above 65%). Similar results have been obtained in a second rhesus macaque injected with AAV-CMV-Epo (3.9 years; data not shown), and dramatically higher levels of Epo obtained in 2 rhesus macaques injected with a 4-fold higher dose of vector incorporating the Rous sarcoma virus (RSV) enhancer in place of CMV (Figure 1B and data not shown). These data confirm the promise of intramuscular AAV delivery for long-term protein expression, but also illustrate the problem of polycythemia caused by high Epo expression.
Long-term constitutive expression of Epo in AAV-transduced primates. (A) Transduction with AAV-CMV-Epo (primate RQ1582). (B) Transduction with AAV-RSV-Epo (primate 97E026). Times following administration of vector are indicated. Serum Epo levels (•) and hematocrit levels (⋄) were measured.
Long-term constitutive expression of Epo in AAV-transduced primates. (A) Transduction with AAV-CMV-Epo (primate RQ1582). (B) Transduction with AAV-RSV-Epo (primate 97E026). Times following administration of vector are indicated. Serum Epo levels (•) and hematocrit levels (⋄) were measured.
Long-term control of Epo expression in a subset of monkeys using first-generation vectors
To achieve regulated expression, our initial approach was to deliver the components of the system in 2 separate vectors. AAV-CMV-TF1 (Figure 2) expresses the regulated transcription factors constitutively from the CMV enhancer. AAV-Z12I-rhEpo-3 (Figure 2) contains the rhesus Epo gene downstream of a minimal promoter containing binding sites for the regulated transcription factors. An alternative target gene vector, AAV-Z12I-rhEpo-2, lacks an intron but is otherwise identical to AAV-Z12I-rhEpo-3. We coadministered vectors intramuscularly to the animals and administered single doses of rapamycin intravenously to study the kinetics and reproducibility of Epo induction.
AAV vectors used in this study. Components of the vectors have been described previously.6,21 The inducible Epo reporter vector AAV-Z12I-rhEpo-3 (A) consists of 12 binding sites for the ZFHD1 DNA binding domain, a minimal interleukin-2 promoter (Min. IL2), a chimeric intron, the rhesus Epo (rhEpo) coding sequence, and an SV40 late gene 3′ untranslated region (UTR). The transcription factor construct AAV-CMV-TF1 (B) contains a human cytomegalovirus (CMV) enhancer driving expression of a bicistronic gene with the following components: an activation domain fusion (FRAP*, the FRB fragment of human FRAP [mTOR] in which threonine 2098 is mutated to leucine, fused to an activation domain derived from the p65 subunit of human nuclear factor κB [NF-κB]), the internal ribosome entry sequence (IRES) derived from encephalomyocarditis virus, the DNA-binding domain fusion (ZFHD1 and 3 copies of human FKBP12), and the final intron and 3′ UTR of the rabbit β-globin (RBG) gene. The FRAP*-p65 and ZFHD1-3xFKBP fusion proteins both contain an amino-terminal epitope tag from influenza virus hemagglutin (HA tag) and a nuclear localization signal (NLS) from SV40 large T antigen. AAV-CMV-TF1Nc (C) is identical to AAV-CMV-TF1 except that the HA tags are eliminated, each SV40 NLS is replaced by an NLS from human c-myc, a chimeric intron is inserted downstream of the transcription start site, and the 3′ UTR is derived from the human growth hormone (hGH) gene. In AAV-CMV-TF-rhEpo2.3 (D), elements of the above vectors were combined as indicated in “Materials and methods.” ITR indicates inverted terminal repeat sequences of AAV2.
AAV vectors used in this study. Components of the vectors have been described previously.6,21 The inducible Epo reporter vector AAV-Z12I-rhEpo-3 (A) consists of 12 binding sites for the ZFHD1 DNA binding domain, a minimal interleukin-2 promoter (Min. IL2), a chimeric intron, the rhesus Epo (rhEpo) coding sequence, and an SV40 late gene 3′ untranslated region (UTR). The transcription factor construct AAV-CMV-TF1 (B) contains a human cytomegalovirus (CMV) enhancer driving expression of a bicistronic gene with the following components: an activation domain fusion (FRAP*, the FRB fragment of human FRAP [mTOR] in which threonine 2098 is mutated to leucine, fused to an activation domain derived from the p65 subunit of human nuclear factor κB [NF-κB]), the internal ribosome entry sequence (IRES) derived from encephalomyocarditis virus, the DNA-binding domain fusion (ZFHD1 and 3 copies of human FKBP12), and the final intron and 3′ UTR of the rabbit β-globin (RBG) gene. The FRAP*-p65 and ZFHD1-3xFKBP fusion proteins both contain an amino-terminal epitope tag from influenza virus hemagglutin (HA tag) and a nuclear localization signal (NLS) from SV40 large T antigen. AAV-CMV-TF1Nc (C) is identical to AAV-CMV-TF1 except that the HA tags are eliminated, each SV40 NLS is replaced by an NLS from human c-myc, a chimeric intron is inserted downstream of the transcription start site, and the 3′ UTR is derived from the human growth hormone (hGH) gene. In AAV-CMV-TF-rhEpo2.3 (D), elements of the above vectors were combined as indicated in “Materials and methods.” ITR indicates inverted terminal repeat sequences of AAV2.
We have previously reported the ability to regulate Epo for several months following coadministration of AAV-CMV-TF1 and AAV-Z12I-rhEpo-3 to the muscle of a rhesus monkey.6 However, administration of rapamycin on day 90 or beyond failed to provoke a significant induction (95C002; Table 1). When the same vectors were administered to 2 additional monkeys, similar outcomes were observed (EWP and FJX; Table 1).
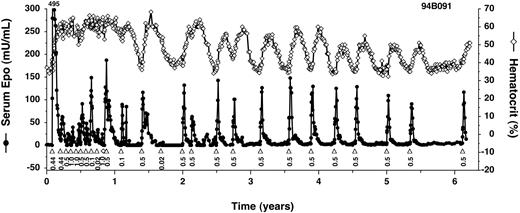
In contrast, 3 rhesus monkeys that were coinjected with the slightly modified, intronless target vector, AAV-Z12I-rhEpo-2, have all remained inducible for the duration of study (GXF, FFT, and 94B091; Table 1). In the longest studied monkey, 94B091, Epo expression has been controlled by the administration of rapamycin for more than 6 years and 26 induction cycles, to date (Figure 3). Induced Epo levels have been remarkably consistent, with peak levels of between 50 mU/mL and 150 mU/mL being achieved after nearly every administration of 0.1 mg/kg rapamycin. A lower dose of rapamycin, 0.02 mg/kg, provoked a smaller increase in Epo levels. Importantly, in the absence of induction, Epo expression has always fallen to undetectable levels, followed by a return of hematocrit to baseline with kinetics that are consistent with the 2-week half-life of red blood cells.35 These studies demonstrate that long-term and tightly regulated expression of Epo in primates is achievable.
Long-term regulated expression of Epo using first-generation AAV vectors. Primate 94B091 was cotransduced with AAV-CMV-TF1 and AAV-Z12I-rhEpo-2 once at the beginning of the study (Table 1 shows the doses), and subsequently administered rapamycin (▵) or on one occasion rapamycin analog AP1861 (⋄) intravenously at the indicated doses (mg/kg). Epo levels (•) peaked at 495 mU/mL after the first induction. Hematocrit levels (⋄ with solid line) are also given.
Long-term regulated expression of Epo using first-generation AAV vectors. Primate 94B091 was cotransduced with AAV-CMV-TF1 and AAV-Z12I-rhEpo-2 once at the beginning of the study (Table 1 shows the doses), and subsequently administered rapamycin (▵) or on one occasion rapamycin analog AP1861 (⋄) intravenously at the indicated doses (mg/kg). Epo levels (•) peaked at 495 mU/mL after the first induction. Hematocrit levels (⋄ with solid line) are also given.
A consistent feature of these studies was the higher levels of Epo obtained in the first few months after transduction compared with later times. The timing of these “hyperresponsive” and “plateau” phases matches the peak and plateau we observed with constitutive Epo expression (Figure 1), suggesting that these phases reflect changes in the expression levels of the regulated transcription factor proteins. In vitro analyses have shown that target gene inducibility correlates with transcription factor expression levels, and that inducibility can be lost if levels fall below a critical threshold (data not shown), potentially explaining the loss of expression in primates 95C002, EWP, and FJX. Alternatively, the loss of inducibility could be due to an immune response against residual nonhuman components of the transcription factors encoded by AAV-CMV-TF1 (Figure 2) and/or a detrimental effect of the intron in the target vector AAV-Z12I-rhEpo-3.
Long-term control of Epo expression in monkeys using second-generation vectors
To obtain reproducible inducible expression, we addressed these potential problems by constructing a second transcription factor vector with nonhuman sequences removed and with additional changes in noncoding regions (AAV-CMV-TF1Nc; Figure 2). In vitro and in vivo studies in mice revealed that these changes also increased transcription factor expression levels and vector potency (data not shown). In addition, subsequent studies used only the AAV-Z12I-rhEpo-2 target gene vector.
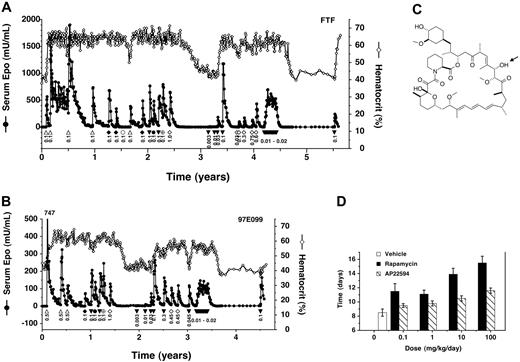
AAV-CMV-TF1Nc and AAV-Z12I-rhEpo-2 were coadministered to a total of 8 rhesus monkeys at various doses (Table 1). In all 8 monkeys, the ability to control Epo and hematocrit by administration of rapamycin, or rapamycin analogs (see Figure 4), has been maintained for the duration of study (0.5 years to 5.5 years). Data from 2 of the longest studied monkeys are shown (Figure 4A-B). In the first-studied monkey, FTF, much higher Epo levels were obtained after administering the vectors at doses comparable to those given previously (compare Figure 3 and Figure 4A), presumably due to the increased potency of the new transcription factor vector. Therefore, monkeys dosed subsequently (eg, 97E099; Figure 4B) received approximately 10-fold-lower vector doses (Table 1). In all 8 animals, Epo levels consistently returned to baseline after each induction, and on each occasion when induction was withheld for sufficient time, hematocrit also returned to baseline levels. All 8 animals exhibited the previously observed hyperresponsive and plateau phases. These data indicate that the second-generation vectors solve the problem of variable persistence and consistently confer reliable, long-term inducible expression.
Long-term regulated expression of Epo using second-generation AAV vectors and rapamycin or rapamycin analogs. (A) High vector doses. Primate FTF was cotransduced with AAV-CMV-TF1Nc and AAV-Z12I-rhEpo-2 once at the beginning of the study and subsequently administered rapamycin (▵) or rapamycin analogs intravenously (AP22594, ▾ ; AP22660, ♦; AP21370, •; AP23054, ⊕), or AP22594 orally (⋄), at the indicated doses (mg/kg). Beginning at year 4.2, AP22594 was dosed intravenously weekly at 0.02 mg/kg 4 times, 0.01 mg/kg 2 times, and 0.015 mg/kg 7 times in consecutive weeks. Serum Epo levels (• with solid line) and hematocrit levels (⋄ with solid line) are also given. (B) Lower vector doses. Primate 97E099 was transduced similarly to FTF but using a 17-fold-lower total dose of vector (Table 1). Weekly dosing was performed as described for primate FTF (A), but beginning at year 3.2. Epo levels peaked at 747 mU/mL after the first induction as indicated. (C) Chemical structure of rapamycin analog AP22594. The arrow indicates the position of epimerization with respect to rapamycin (C28). (D) Reduced immunosuppressive activity of AP22594 in a mouse model of immune rejection. C57bl/6 mice were grafted with skin patches from Balb/c mice, and animals subsequently received daily subcutaneous administrations of rapamycin (▪) or AP22594 (▧) at the indicated dose, or vehicle (□). The time for complete graft rejection is plotted (mean ± SD).
Long-term regulated expression of Epo using second-generation AAV vectors and rapamycin or rapamycin analogs. (A) High vector doses. Primate FTF was cotransduced with AAV-CMV-TF1Nc and AAV-Z12I-rhEpo-2 once at the beginning of the study and subsequently administered rapamycin (▵) or rapamycin analogs intravenously (AP22594, ▾ ; AP22660, ♦; AP21370, •; AP23054, ⊕), or AP22594 orally (⋄), at the indicated doses (mg/kg). Beginning at year 4.2, AP22594 was dosed intravenously weekly at 0.02 mg/kg 4 times, 0.01 mg/kg 2 times, and 0.015 mg/kg 7 times in consecutive weeks. Serum Epo levels (• with solid line) and hematocrit levels (⋄ with solid line) are also given. (B) Lower vector doses. Primate 97E099 was transduced similarly to FTF but using a 17-fold-lower total dose of vector (Table 1). Weekly dosing was performed as described for primate FTF (A), but beginning at year 3.2. Epo levels peaked at 747 mU/mL after the first induction as indicated. (C) Chemical structure of rapamycin analog AP22594. The arrow indicates the position of epimerization with respect to rapamycin (C28). (D) Reduced immunosuppressive activity of AP22594 in a mouse model of immune rejection. C57bl/6 mice were grafted with skin patches from Balb/c mice, and animals subsequently received daily subcutaneous administrations of rapamycin (▪) or AP22594 (▧) at the indicated dose, or vehicle (□). The time for complete graft rejection is plotted (mean ± SD).
Dose-responsive regulation using analogs of rapamycin
A potential limitation of rapamycin as an inducing drug is its immunosuppressive activity. Daily administration of rapamycin is used clinically to prevent transplant rejection,36 although immunosuppressive activity is dramatically diminished when the drug is dosed on intermittent schedules, such as weekly,36,37 as would be the case in most gene therapy applications. To further reduce the potential for immunosuppression, we synthesized and tested several analogs of rapamycin that have substantially reduced immunosuppressive activity in vivo due to a shorter half-life and diminished affinity for endogenous FKBP-12 and/or mammalian target of rapamycin/FKBP12-rapamycin–associated protein (mTOR/FRAP) in T cells (data not shown). Several analogs induced Epo expression potently in primates (Figure 4A-B), indicating that their reduced binding affinity is still sufficient to effectively crosslink the multivalent transcription factor fusion proteins.16 One such analog, AP22594 (28-epi-rapamycin32 ; Figure 4C), was selected for further study.
AP22594 is approximately 100-fold less immunosuppressive than rapamycin when administered daily in a mouse skin allograft rejection assay (Figure 4D). However, the 2 drugs induce Epo expression with equal potency in primates (Figure 4A-B). AP22594-induced expression was dose dependent, with a maximal response obtained with an intravenous dose of 0.1 mg/kg to 0.3 mg/kg (Figure 4A, years ∼3-3.5 and Figure 4B, years ∼2-2.5). Epo expression could also be induced by orally administered AP22594 (Figure 4A-B); like rapamycin,38 AP22594 exhibited approximately 10% oral bioavailability. For example, administration of a 0.45-mg dose orally gave a similar response to 0.045 mg/kg administered intravenously (Figure 4B; compare open diamonds and closed triangles).
We were also able to use more frequent (weekly) administration of lower AP22594 doses to generate sustained elevations of Epo as opposed to individual peaks (Figure 4A, years ∼4-4.5 and Figure 4B, years ∼3-3.5). This dosing regime is likely one that would be used clinically as it delivers Epo with kinetics that approximate those of the natural protein.
Tightly regulated expression of Epo in monkeys using a single AAV vector
Vector manufacture and delivery would be greatly simplified if the regulatory system and target gene could be incorporated into a single AAV vector. Since the packaging capacity of AAV vectors is limited to approximately 5 kb, this would require eliminating or minimizing noncoding components of the existing vectors. We prepared candidate single vectors by joining multiple minimal transcription factor and target gene cassettes in a variety of configurations, and screened them by transient transfection and by injection into mouse muscle (data not shown). Several vectors functioned comparably to the 2-vector system, with no basal expression and potent induction. AAV-CMV-TF-rhEpo2.3, in which transcription factor and target gene cassettes were assembled in a head-to-tail manner (Figure 2), was chosen for characterization in primates.
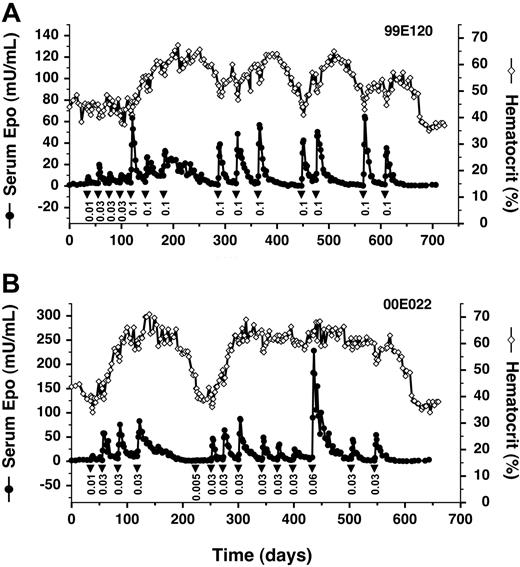
We found that AAV-CMV-TF-rhEpo2.3 retains all the desirable properties of the 2-vector system when injected intramuscularly into a rhesus monkey, with AP22594 dose-dependent production of Epo for over 20 months (Figure 5A). Remarkably, despite the colocalization of the strong CMV enhancer and the Epo target promoter on the same vector, there is no evidence of Epo production in the absence of AP22594. In this study, we also sought to avoid the potentially dangerous over-production of Epo during the early hyperresponsive phase by starting with low drug doses and then escalating. This strategy was successful in achieving steady inductions of Epo at more physiologically appropriate levels (Figure 5A).
Long-term regulated expression of Epo using a single AAV vector. (A) AAV2 single vector. Primate 99E120 was transduced with AAV-TF-rhEpo-2.3 once on day 1, and subsequently administered AP22594 (▾) intravenously at the indicated doses (mg/kg). Serum Epo levels (• with solid line) and hematocrit levels (⋄ with solid line) are also given. (B) AAV1-pseudotyped vector. Primate 00E022 was transduced with an AAV1-pseudotyped AAV-TF-rhEpo-2.3 vector once on day 1, at a 10-fold-lower dose than that administered to 99E120 (Table 1).
Long-term regulated expression of Epo using a single AAV vector. (A) AAV2 single vector. Primate 99E120 was transduced with AAV-TF-rhEpo-2.3 once on day 1, and subsequently administered AP22594 (▾) intravenously at the indicated doses (mg/kg). Serum Epo levels (• with solid line) and hematocrit levels (⋄ with solid line) are also given. (B) AAV1-pseudotyped vector. Primate 00E022 was transduced with an AAV1-pseudotyped AAV-TF-rhEpo-2.3 vector once on day 1, at a 10-fold-lower dose than that administered to 99E120 (Table 1).
Improved potency of an AAV1 pseudotyped single vector
In common with most previous work, the studies cited to this point all used AAV serotype 2 (AAV2). However, serotype 1 (AAV1) has been shown to be a much more efficient vector for gene delivery to muscle in mice39,40 and larger animals.28,41 We packaged AAV-CMV-TF-rhEpo2.3 as an AAV1-pseudotyped vector and administered it to a rhesus monkey at a 10-fold-lower dose compared with the previous AAV2 vector (Figure 5B). Despite this lower dose, we obtained 5- to 10-fold-higher levels of Epo in response to a nonsaturating dose of AP22594 (0.03 mg/kg; compare Figure 5A-B). Inducibility persisted for more than 18 months. These data indicate that AAV1-pseudotyped vectors confer enhanced transduction of primate muscle, and allow persistent regulated expression of Epo to be achieved with a vector dose of 1011 GC/kg, more than 100-fold lower than that used at the start of this study (Table 1).
Discussion
In this work, we demonstrated that a single intramuscular injection of AAV vectors into rhesus macaques can sustain either constitutive (Figure 1) or regulated gene expression (Figures 3, 4, 5) for at least 6 years. To our knowledge, these studies, which are still ongoing, represent the longest observed expression following AAV administration. The drug-regulated expression we obtained was dose dependent and fully reversible at the levels both of Epo and hematocrit in all animals. These results indicate that a one-time or infrequent intramuscular administration of AAV vector, followed by regulation with an orally active rapamycin analog, is a clinically feasible and promising approach for the delivery of secreted therapeutic proteins.
The most likely explanation for the success of the optimized vectors is their higher transcription factor potency, since this is consistent with prior observations that inducibility can be lost if the potency or expression levels of these factors fall below a critical threshold, and with the observation that expression levels from AAV vectors injected into primate muscle drop off in the initial months after transduction (Figure 1). Although we did observe some low-titer transient antibody responses to the HA tag and human FKBP and FRAP portions of the first-generation transcription factors, their presence did not correlate with the loss of expression and their relevance is unclear (data not shown). The long-term inducibility we observed in 13 primates with, cumulatively, more than 41 years of regulated expression, implies that there was no functional immune response to the transcription factors in these animals.
Induced expression exhibited an initial hyperresponsive phase followed by a plateau phase characterized by more modest and highly reproducible responses to drug. In some cases we observed very prolonged kinetics in the initial hyperresponsive phase (Figure 4A), although this was only seen when induced levels of Epo were exceptionally high: more than 2000 mU/mL (corrected units; see “Materials and methods”) compared with a normal physiologic range of 8 mU/mL to 24 mU/mL.42 In our later studies we were able to avoid excessive inductions of Epo by administering low doses of AP22594 and then escalating the dose (Figure 5). In general, incorporating tight regulation into gene therapy applications allows variations in expression associated with the vector or target tissue (Figure 1) to be addressed by adjustments in the dose of inducing drug.
Although infrequent dosing of rapamycin is not likely to lead to immunosuppression,36,37 for many applications it will be desirable to further reduce the potential for this side effect by using a rapamycin analog with reduced immunosuppressive activity. We showed here that the analog AP22594 induces gene expression in primates equivalently to rapamycin, despite exhibiting approximately 100-fold-lower immunosuppressive activity in a mouse model of immune rejection. The comparative immunosuppressive activities could also, in principle, be determined in primates; for example, using baboons receiving kidney grafts or cynomolgus monkeys receiving heart grafts.36,43
An emerging issue in the use of gene therapy to deliver therapeutic proteins is the induction of autoimmune responses against the expressed protein. Two groups have recently observed autoimmune anemia following administration to cynomolgus monkeys of constitutive44 or Tet-regulated45 AAV vectors expressing (cynomolgus) Epo. Expression of Epo apparently broke tolerance to this self-antigen, leading to a potent humoral anti-Epo response and severe anemia, requiring that the animals be killed. By contrast, we have observed no anemia in the 16 rhesus macaques that received regulated Epo AAV vectors in this study (Table 1), or 5 additional rhesus macaques that received slightly modified transcription factor vectors (data not shown), or the 4 rhesus macaques that received constitutive Epo vectors (Figure 1), despite the expression of extremely high protein levels in some cases (Figures 1B, 4A). The explanation for these dramatically different results is unclear. The effects do not appear to be completely species specific, since autoimmunity has been observed in rhesus monkeys following administration of constitutive AAV Epo vectors to the lung.44 Moreover, we have administered AAV1-CMV-TF-rhEpo2.3 intramuscularly to 5 cynomolgus monkeys and achieved persistent regulated expression for 6 months to date in all animals, again with no anemia (data not shown; the amino acid sequences of rhesus and cynomolgus Epo are identical; see “Materials and methods”). Overall, our data in a total of 21 rhesus and 5 cynomolgus monkeys demonstrate that safe and persistent regulated expression of Epo is reproducibly achievable in primates using the dimerizer-inducible system. It is possible that this regulation system confers specific benefits that minimize the chances of autoimmunity, such as tight regulation and minimal immunogenicity of the human-derived transcription factor components. There may be benefit in delaying expression of the transgene after the initial injection of vector—a time of potential immune activation due to the inflammation associated with vector injection.
In summary, we have developed and validated in primates a general platform for long-term regulated delivery of proteins using a gene therapy approach: one-time intramuscular delivery of a modest dose of a single AAV1-pseudotyped vector, followed by oral administration of a rapamycin analog such as AP22594 to control protein production. Our data support the further exploration of the clinical potential of this system to deliver a range of secreted therapeutic proteins.
Prepublished online as Blood First Edition Paper, October 26, 2004; DOI 10.1182/blood-2004-06-2501.
Supported in part by grants from the Juvenile Diabetes Research Foundation (JDRF) (4-1999-840) and the National Institutes of Health (NIDDK P30 DK47757).
V.M.R., L.R., and T.C. have declared a financial interest in ARIAD Pharmaceuticals, a company whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lori Berk, Steven Boule, Steven Notari, Karen Russian, Hao Tang, Xiurong Wang, and Scott Wardwell for assistance, and David Dalgarno for bioanalytical and formulation contributions.

![Figure 2. AAV vectors used in this study. Components of the vectors have been described previously.6,21 The inducible Epo reporter vector AAV-Z12I-rhEpo-3 (A) consists of 12 binding sites for the ZFHD1 DNA binding domain, a minimal interleukin-2 promoter (Min. IL2), a chimeric intron, the rhesus Epo (rhEpo) coding sequence, and an SV40 late gene 3′ untranslated region (UTR). The transcription factor construct AAV-CMV-TF1 (B) contains a human cytomegalovirus (CMV) enhancer driving expression of a bicistronic gene with the following components: an activation domain fusion (FRAP*, the FRB fragment of human FRAP [mTOR] in which threonine 2098 is mutated to leucine, fused to an activation domain derived from the p65 subunit of human nuclear factor κB [NF-κB]), the internal ribosome entry sequence (IRES) derived from encephalomyocarditis virus, the DNA-binding domain fusion (ZFHD1 and 3 copies of human FKBP12), and the final intron and 3′ UTR of the rabbit β-globin (RBG) gene. The FRAP*-p65 and ZFHD1-3xFKBP fusion proteins both contain an amino-terminal epitope tag from influenza virus hemagglutin (HA tag) and a nuclear localization signal (NLS) from SV40 large T antigen. AAV-CMV-TF1Nc (C) is identical to AAV-CMV-TF1 except that the HA tags are eliminated, each SV40 NLS is replaced by an NLS from human c-myc, a chimeric intron is inserted downstream of the transcription start site, and the 3′ UTR is derived from the human growth hormone (hGH) gene. In AAV-CMV-TF-rhEpo2.3 (D), elements of the above vectors were combined as indicated in “Materials and methods.” ITR indicates inverted terminal repeat sequences of AAV2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-06-2501/6/m_zh80040573920002.jpeg?Expires=1768154389&Signature=ITjp2gDRgcXbAqvmpIhnomVLhc8svlkU~uIIf75Dq7A60THwj-RuGaIclH0mwHlJHwW-EFDhD68ifJTqErBa1VxxpwqmxzxsFcnVdOBGfP5chIxtQOLyf1M~Ov~cNHUSAnGrWArIkG3ItHSdpN5NbfHeBM0FNYxKdXFlsyNzJJnD-HzrW4zsNuaidf-zAa8L6Y9t6-W7SvI8qSvHsU9Vb9LmWlwKfMd2TUVdM-t6S5X6rxJsAY562SRtcxoa7HZEmdyaVjTwGthRlXHkUAcvsSbJqzgPwIuiCjs-RVjNH9hN5ecALooBnC1RCTsgBLjr33FV9FSzfppRchBx-34wJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)