Abstract
We performed a prospective, randomized, open, blinded end point (PROBE) study to assess the efficiency of transfusing high doses of platelets in patients with thrombocytopenia, either acute leukemia (AL) or those undergoing autologous hematopoietic stem cell transplantation (AT). Patients were randomly assigned to receive transfusions with a target dose of 0.5 × 1011/10 kg (arm A) or 1 × 1011/10 kg (arm B). A total of 101 patients were included, of whom 96 were given at least one transfusion. The median time between the first transfusion and when the platelet count reached at least 20 × 109/L increased from 63 hours to 95 hours in the arm B group (P = .001), and the median number of transfusions was lower in this group (2; P = .037). The total number of transfused platelets did not differ between groups (14.9 × 1011 for arm A versus 18.5 × 1011 for arm B; P = .156). In such patients, a prophylactic strategy of high doses of platelets could improve platelet transfusion efficiency.
Introduction
In hematologic patients with thrombocytopenia, platelet transfusions remain vital in supportive care. If the dose of 0.5 × 1011/10 kg1 remains the standard,2-5 the optimal dose for prophylactic treatment is debatable. Results of 2 studies comparing different doses of platelets showed an increased platelet count and time between 2 transfusions according to the dose.6,7 Both were crossover studies; however, transfusion efficiency may be associated with the number of previous transfusions and dose. To assess the overall efficiency of transfusing a high dose of platelets, we performed a prospective randomized study to compare the effect of a single dose (0.5 × 1011/10 kg) versus a double dose (1 × 1011/10 kg) of platelets on repeat transfusion in hematologic patients with thrombocytopenia.
Study design
Patients
Patients who had not undergone transfusion who had acute leukemia (AL; AML3 excluded) undergoing first-line treatment or autologous hematopoietic stem cell transplantation (AT) without criteria impairing platelet efficiency were enrolled.
Setting and ethical approval
The study protocol was approved by the institutional review board of Brest, France, and written informed consent of patients was obtained. Four regional blood banks in France (Etablissement Français du Sang [EFS]) and hematology departments from university hospitals in Angers, Besançon, Brest, and Tours, France, participated.
Trial design
The design was multicenter, randomized, parallel group. Physicians and patients were not blinded to the randomization arm. The main outcome parameter was platelet count and the laboratory was blinded to the dose of platelets received, thus defining the study as a prospective, randomized, open, blinded end point (PROBE) study.8 Randomization was based on the center and type of pathology.
Platelet transfusion protocol
Blood cells were counted daily between 7:00 and 8:00 AM, and transfusions were given when the platelet count was less than 20 × 109/L. To avoid interference with criteria known to impair recovery,9-13 patients were given leukocyte-depleted single-donor apheresis platelet concentrates (APCs) less than 72 hours old, and ABO identical or donor platelets were compatible with patients' isoagglutinins. Patients were randomly assigned to receive doses in arm A (single dose; target 0.5 × 1011/10 kg) or arm B (double dose; target 1 × 1011/10 kg) and were followed from the first platelet transfusion until they were discharged and had a stable platelet count more than 25 × 109/L or died.
Outcome measures
We measured the time between the first transfusion and the daily platelet count reaching 20 × 109/L, which allowed for calculating the risk of retransfusion and the theoretical time between the first and second transfusion. Secondary outcome measures were (1) the corrected count increment (CCI), which was calculated as (posttransfusion count - pretransfusion count) × body surface area (m2)/platelet dose (× 1011) for the first transfusion; (2) number of transfusions; and (3) number of transfused platelets. Bleeding complications were assessed daily according to World Health Organization (WHO) criteria (0 = none; 1 = petechial; 2 = mild blood loss; 3 = gross blood loss; 4 = debilitating blood loss).
Sample size
Hypothesizing an increase of 75% in the median delay between 2 transfusions, the hazard ratio was thus hypothesized at 0.57, leading to a required number of events (retransfusion) of 100 (α 5% and β 20%).
Statistical analysis
Analyses were conducted according to a prespecified plan based on the principle of intention to treat. Patients had to undergo transfusion at least once during the study; patients never having a transfusion were excluded. Analysis was global and by 2 subgroups (AL or AT groups). The primary outcome was analyzed by means of a log-rank test. For the CCI, comparison was by means of the Student t test. The number of transfusions and transfused platelets were analyzed in the framework of a generalized linear model with negative binomial distribution14 and a linear model, respectively. We adjusted for weight at baseline, and patients who died were excluded because of lack of transfusion history. Analyses involved use of SAS (version 8.1; SAS Institute, Cary, NC).
Results and discussion
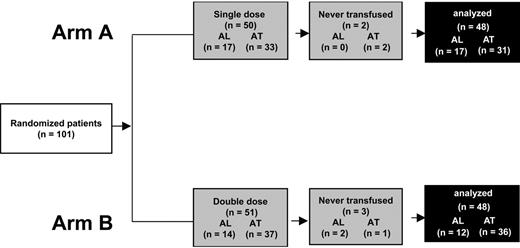
Between May 1999 and October 2001, 101 patients were randomly assigned to receive transfusions in arm A (50 patients, 17 with AL and 33 AT) and arm B (51 patients, 14 with AL and 37 AT). The groups were balanced according to age, weight, and baseline hematology findings (Table 1). Three patients with AL died during the study period, with the death not related to bleeding or transfusion. We analyzed the results for 96 patients (Figure 1). The mean number of transfused platelets was 0.57 ± 0.15/10 kg for the arm A group and 0.96 ± 0.18/10 kg for the arm B group.
Procedure for including patients in trial of transfusing platelets in patients with hematologic thrombocytopenia. Randomized patients had no clinical or biologic features that would impair platelet transfusion efficiency. For the final analysis, 5 patients never receiving a transfusion after randomization were excluded. (AL indicates acute leukemia undergoing first-line treatment; AT, undergoing autologous hematopoietic stem cell transplantation.
Procedure for including patients in trial of transfusing platelets in patients with hematologic thrombocytopenia. Randomized patients had no clinical or biologic features that would impair platelet transfusion efficiency. For the final analysis, 5 patients never receiving a transfusion after randomization were excluded. (AL indicates acute leukemia undergoing first-line treatment; AT, undergoing autologous hematopoietic stem cell transplantation.
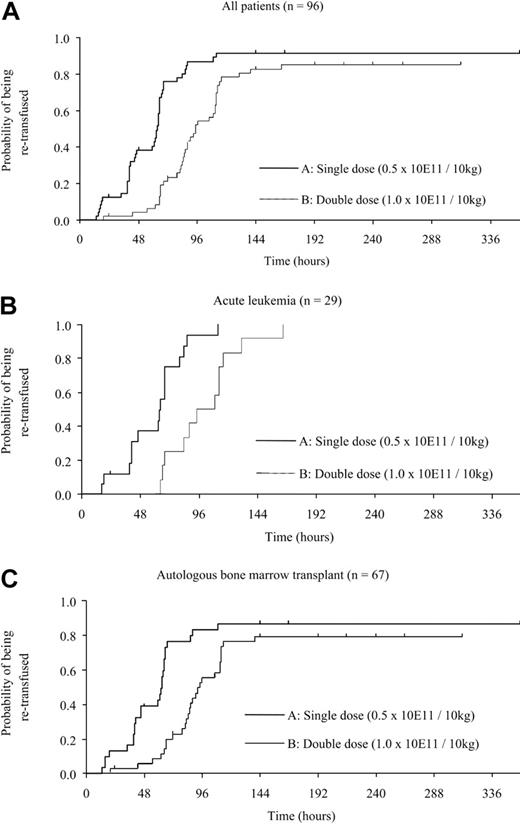
The median theoretical time between the first and the second transfusion was 63 hours (95% confidence interval [CI], 46-65) for the arm A group and 95 hours for the arm B group (95% CI, 85-112; P = .001; Figure 2), the difference remaining significant whatever the pathology (AL or AT) or number of subsequent transfusions (data not shown). The mean posttransfusion CCI was not statistically different between patients who were given a single or double dose (Table 2). Arm B patients underwent fewer transfusions with a median of 2 (range, 1-13) versus a median of 3 (range, 1-12) for the arm A group (P = .037). This benefit was absent in the AT (median 2 for the arm B versus 2 for the arm A group; P = .064) and AL (median 5 versus 5; P = .584) subgroups. The adjusted means of total number of transfused platelets were not significantly different between groups (14.9 × 1011 arm A versus 18.5 × 1011 arm B group; P = .156) and within subgroups: AL, 21.9 × 1011 (arm A) versus 26.7 × 1011 (arm B; P = .356); AT, 11.9 × 1011 (arm A) versus 15.3 × 1011 (arm B; P = .172).
Probability of retransfusion after the first platelet transfusion. For patients with thrombocytopenia undergoing single or double doses of platelet transfusion the probability of retransfusion in arm A (single dose) and arm B group (double dose): panel A the probability of retransfusion decreased significantly in the arm B group (P = .001). The probability of retransfusion in patients with AL (panel B; 17 in arm A group; 12 in arm B) and AT (panel C; 31 in arm A group; 36 in arm B) was lower in the arm B than in the arm A group (P = .001 for AL, P = .003 for AT). Comparison involved use of the log-rank test.
Probability of retransfusion after the first platelet transfusion. For patients with thrombocytopenia undergoing single or double doses of platelet transfusion the probability of retransfusion in arm A (single dose) and arm B group (double dose): panel A the probability of retransfusion decreased significantly in the arm B group (P = .001). The probability of retransfusion in patients with AL (panel B; 17 in arm A group; 12 in arm B) and AT (panel C; 31 in arm A group; 36 in arm B) was lower in the arm B than in the arm A group (P = .001 for AL, P = .003 for AT). Comparison involved use of the log-rank test.
During the study period, hemorrhaging was seen in 14 patients (5 in arm A and 9 arm B; P = .247). WHO grade 2 and 3 hemorrhages were seen equally in the arm A (2 patients) and arm B (3 patients) groups. The 3 patients with recurrent bleeding were given single-dose transfusions.
In a retrospective study, Andreu15 found good response to platelet transfusion with high doses. In 1998,6 the first prospective comparison demonstrated good platelet count increment and increased transfusion time with high doses. In 1999,7 the first randomized study to involve 2 different doses (3.1 × 1011 and 4.9 × 1011) found that platelet count and transfusion interval increased with increased number of transfused platelets. Finally, in a study involving platelets collected from apheresis donors receiving thrombopoietin, increasing the dose of platelets led to an increased transfusion interval and platelet count.16 These studies did not adjust platelet dosage to body weight, which led to underestimated results. Here, for the first time, our randomized prospective study of a double dose of platelets and the period of platelet transfusion demonstrates increased transfusion interval and decreased number of transfusion episodes with a double dose of platelets.
The absence of decreased number of transfusions in patients with AL could be due to the small number of patients with AL in our study or the known decrease in platelet transfusion efficiency over time. Our results on the number of transfusions could be underestimated because of a real increase of 68% and not 100% in platelet dose between patients given single and double doses. Although the number of transfusion events was small, recurrent bleeding occurred only in patients given a single dose, which suggests a better control of hemorrhage with high doses.
Thus, for prophylaxis, high doses of platelets can reduce the number of transfusions in hematologic patients with thrombocytopenia, without a significant increase in amount of transfused platelets.
Prepublished online as Blood First Edition Paper, September 14, 2004; DOI 10.1182/blood-2004-05-1841.
Supported by a grant from Etablissement Français du Sang (E.F.S.; FORTS no. 99004250837).
We thank C. Monpouet for her excellent data management and G. Andreu for helpful comments.