Abstract
The chemokine stromal-derived factor-1α (SDF-1α) is an essential regulator of hematopoiesis, lymphocyte homing, pre-B-cell growth, and angiogenesis. As SDF-1α is constitutively expressed in many tissues, chemokine function is mostly regulated by proteolytic degradation. Human serum cleaves the 68-amino acid chemokine, SDF-1α, at both termini. The enzyme or enzymes responsible for the removal of the carboxy-terminal lysine from SDF-1α, leading to significant reduction in biologic activity, have not been identified. Using a new biochemical assay for measuring the carboxy-terminal cleavage activity, we purified from serum and plasma a peptidase that specifically removes the carboxy-terminal lysine from SDF-1α and identified it as carboxypeptidase N (CPN, also known as kininase I, arginine carboxypeptidase, and anaphylotoxin inactivator). We demonstrate that SDF-1α in serum and plasma lacks the carboxy terminal lysine, and depletion of CPN from serum and plasma significantly reduces the SDF-1α carboxypeptidase activity. Purified CPN effectively and specifically removes the carboxy-terminal lysine from SDF-1α and significantly reduces the chemokine's biologic activity as a pre-B-cell growth factor and chemoattractant. Thus, in addition to its role as a regulator of the biologic activity of kinins and anaphylatoxins, CPN is an important regulator of the biologic activity of SDF-1α by reducing the chemokine-specific activity. (Blood. 2005;105:4561-4568)
Introduction
The chemokine stromal-derived factor-1 (SDF-1) and its unique receptor, CXCR4, are key regulators of the hematopoietic, nervous, and cardiovascular systems during embryogenesis.1-3 Knock-out mice for SDF-1 or CXCR4 die perinatally with a similar phenotype, including defects in the formation of the cerebellum, the ventricular septum of the heart, the vasculature of the gastrointestinal system, and the lympho-hematopoietic system.4-7 After birth, SDF-1 and CXCR4 continue to play key roles in regulating physiologic hematopoiesis and angiogenesis.8-15 Studies in vitro have shown that SDF-1 functions as a growth factor for immature B lymphocytes and promotes chemotaxis in CXCR4-expressing cells, including tumor cells.1,3,9,16-18 Since CXCR4 can function as a coreceptor for T-cell tropic HIV-1, SDF-1 and CXCR4 may also affect the course of HIV-1 infection.19,20
SDF-1α is a highly conserved chemokine in evolution, only a single conservative substitution (I to V) distinguishes the human chemokine from the mouse chemokine.21,22 Two forms of SDF-1 are generated, α and β, which are identical in amino acid sequence except for the presence of 4 additional amino acids at the carboxy terminus of SDF-1β.21,22 A third form of SDF-1 was identified in rat, SDF-1γ, which is predicted to code for a protein identical to SDF-1β, except for the insertion of 30 additional amino acids at the carboxy terminus.23 Structure-function studies have identified the amino terminal region of SDF-1 as critical to CXCR4 receptor activation.22,24 SDF-1 lacking the amino terminal lysine alone did not trigger CXCR4 signaling while retaining substantial receptor binding activity, whereas SDF-1 lacking the first 8 N-terminal amino acids displayed substantially reduced CXCR4 binding.22
Recently, we found that full-length SDF-1α (residues 1-68) undergoes rapid processing when exposed to human serum, first at the carboxy terminus to produce SDF-1α (residues 1-67) and subsequently at the N terminus to produce SDF-1α (residues 3-67).25 By contrast, full-length SDF-1β (residues 1-72) is processed only at the N terminus by removal of 2 amino acids to produce SDF-1β (residues 3-72).25 Functionally, SDF-1α lacking the carboxy terminal lysine displays a significantly reduced ability to stimulate pre-B-cell proliferation and chemotaxis compared with the full-length molecule.25 Since SDF-1β is not cleaved at the carboxy terminus, carboxy terminal processing distinguishes SDF-1α from SDF-1β, and provides a mechanism for fine regulation of SDF-1 functional activity.
Serum processing of SDF-1α and β at the N terminus, which functionally inactivates the molecules, has been attributed to a number of different enzymes. Dipeptidyl peptidase/CD26 is catalytically active in serum and can remove the first 2 amino acids,25,26 cathepsin G can remove the first 5 amino acids of SDF-1,27 neutrophil elastase removes the first 3 amino acids,28 and matrix metalloproteinases can remove the first 4 amino acids.29 However, the enzyme responsible for C-terminal SDF-1α cleavage by serum has not been previously identified. Here, we identify carboxypeptidase N (CPN) as an enzyme responsible for the lysine removal from SDF-1α carboxy terminus.
Materials and methods
Reagents and samples
Recombinant SDF-1α (1-68) was from R&D Systems (Minneapolis, MN). An 11 amino acid SDF-1 C-terminal peptide, IQEYLEKALNK, was obtained from Sigma-Genosys (Woodlands, TX). Also, a scrambled version of the SDF-1 peptide (LAKELYEQIK), the C-terminal 11 amino acids of SDF-1β (LEKALNKRFKM), an SDF-1 peptide containing a C-terminal arginine (IQEYLEKALNR), and a peptide with a C-terminal glutamic acid (IQEYLEKALNE) were prepared. Antigen-affinity purified goat anti-human SDF-1α and β (BAF-310), and biotin-labeled mouse immunoglobulin G 1 (IgG1) monoclonal anti-human/mouse SDF-1 antibodies (MAB 310) were from R&D Systems; rabbit anti-human SDF-1 antigen affinity-purified antibodies were from PeproTech (Rocky Hill, NJ). Anti-CPN immune serum30 was a kind gift of Dr E. G. Erdös (UIC College of Medicine, Chicago, IL). Purified CPN was a kind gift of Dr Mariko Nagashima, Berlex Biosciences (Richmond, CA). D-Phe-Pro-Arg chloromethylketone (PPACK) and heparin were from Calbiochem (La Jolla, CA). Serum and plasma samples were obtained with institutional approval from blood donors. Purified thrombin-activatable fibrinolysis inhibitor (TAFI) protein and anti-TAFI antibody, which recognizes the active (TAFIa) and inactive (TAFI) form of TAFI, was obtained from Haematologic Technologies (Essex Junction, VT).
SDF-1 Western analysis for cleavage of carboxy-terminal lysine
SDF-1α (3 ng-25 ng) with no additives or with addition of serum samples (1%-10%) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose as previously described.25 Immunoblotting was performed as described25 using rabbit anti-human SDF-1 antigen affinity-purified antibody (PeproTech), which does not recognize SDF-1α lacking the carboxy-terminal lysine,31 followed by membrane reblotting with antigen affinity-purified goat anti-human SDF-1α and β (BAF-310; R&D Systems), which recognizes SDF-1α with or without the carboxy-terminal lysine.25,31 Bound antibodies were detected by addition of peroxidase-linked donkey anti-rabbit or anti-goat IgG antibody (Amersham Pharmacia Biotech) followed by a chemiluminescence detection system (ECL kit; Amersham Pharmacia Biotech).
SDF-1 affinity purification from serum or plasma
Biotin-labeled mouse monoclonal anti-SDF-1 antibody (BAF 310; R&D Systems, Piscataway, NJ) was immobilized on beads coated with NeutrAvidin TM Biotin-Binding Protein (Pierce Biotechnology, Rockford, IL). Aliquots (1 mL) of serum or plasma were precleared 3 times by 30-minute incubations with Protein-G Sepharose beads (packed 50 μL; Amersham Pharmacia Biotech) followed by removal of precipitate, and incubated by rotating for 30 minutes with 200 μL beads coated with anti-SDF-1 antibody. After 5 washes with 0.5 mL binding buffer (Pierce Biotechnology), bound protein was eluted with 125 μL elution buffer (Pierce Biotechnology) and neutralized with 6.5 μL 1 M Tris buffer, pH 9.5. Eluted samples were analyzed by Western blot.
CPN immunoprecipitation from plasma and testing for residual carboxypeptidase activity
Aliquots (50 μL) of normal plasma and serum precleared with Protein-G Sepharose beads (3 times incubation followed by removal of precipitate) were incubated overnight at 4°C with 3 μL phosphate-buffered saline (PBS), rabbit anti-CPN immune serum (a gift of Dr E. G. Erdös), or control rabbit serum. Following immunoprecipitation, samples were tested for their ability to cleave recombinant SDF-1α (diluted 1:10, 10 minutes) or the SDF-1α peptide.
MALDI-TOF-MS analysis
Analysis of SDF-1α was performed on a Voyager-DE STR matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF-MS) operating in linear mode using delayed extraction under standard conditions. α-Cyano-4-hydroxycinnamic acid was used as matrix at a concentration of 10 mg/mL in 60% acetonitrile with 0.1% trifluoroacetic acid (TFA). Matrix and sample were mixed in a volume ratio of 5:1. A 1-μL aliquot of the mixture was applied to the sample plate and allowed to air dry. Spectra were acquired with 200 shots per spectrum. MALDI data processing was performed using DataExplorer software (PerSeptive Biosystems, Framingham, MA) for noise removal, noise filter, and Gaussian smooth.
Determination of SDF-1 carboxypeptidase activity by RP-HPLC/MS analysis
The SDF-1α peptide (IQEYLEKALNK) was separated using an Agilent Technologies (Wilmington, DE) 1100 reversed phase-high-performance liquid chromatography (RP-HPLC)/MSD (mass selective detector) system with a Thermo BetaBasic-18 column (50 mm × 4.6 mm, 5 μM) from Western Analytical Products (Murrieta, CA). Peptides were eluted with a 0.1% formic acid/water (solvent A) and acetonitrile/0.1% formic acid (solvent B) gradient at a flow of 0.8 mL per minute over 35 minutes. The column was equilibrated with 5% solvent B then increased linearly to 25% over 20 minutes. Peptides were detected at 205 nm using a diode array detector and by electrospray mass spectrometry detector set under positive polarity scan mode.
Purification of SDF-1 carboxypeptidase activity
Protein concentrations were determined using the bicinchoninic acid (BCA) assay from Pierce Biotechnology. Human serum (100 mL) from Sigma (St Louis, MO) was filtered to remove particulate matter. Protease inhibitors (0.2 mM phenylmethylsulfonyl fluoride [PMSF], 4 μg/mL aprotonin) were added and 16.6 g solid ammonium sulfate was added to bring the serum to 30% ammonium sulfate saturation. The solution was stirred for 1 hour at 4°C and the precipitate removed by centrifugation at 5000g. The remaining 30% supernatant was brought to 40% saturation with 5.7g solid ammonium sulfate. The solution was stirred for 1 hour at 4°C and the precipitate collected by centrifugation at 5000g. The pellet was resuspended in 100 mL cold 50 mM phosphate buffer, pH 7.5. Each fraction (100 μg protein) was tested for peptide cleavage activity. The material precipitated with 40% ammonium sulfate was fractionated by anion exchange chromatography using Vivapure Maxi Q columns from Vivascience (Carlsbad, CA). The protein solution (475 mg) was diluted 1:6 with loading buffer (25 mM Tris/HCl buffer, pH 8.0) and loaded onto Maxi Q columns (45 mL total per column) by centrifugation. The columns were washed twice by centrifugation with 5 mL loading buffer and eluted twice each by centrifugation with 0.20 M, 0.25 M, and 0.30 M NaCl in 50 mM Na phosphate buffer, pH 7.5. Fractions containing the highest specific activity were concentrated using a 10 000 MW cutoff Centricon concentration device and further fractionated on a lysine Sepharose column. The concentrated material was dialyzed (2 hours) against 1 L cold 50 mM phosphate buffer, pH 7.5, and applied (6 mL containing 8.3 mg total protein) to a 3 mL lysine Sepharose 4B column (Amersham Biosciences) equilibrated with 4 bed volumes of 50 mM phosphate binding buffer, pH 7.5. The eluate was reapplied 3 times and the column was washed with 4 bed volumes of 0.1 M NaCl followed by elution of tightly bound protein with 100 μM SDF-1 peptide in 0.1 M NaCl. The SDF-1 peptide eluted fractions were pooled, dialyzed free of peptide against 50 mM phosphate buffer, pH 7.5, and concentrated.
CPN immunoblotting
Protein samples (1 μg) and purified standards (CPN 0.3 μg and TAFI 0.2 μg) were denatured by heating at 95°C for 2 minutes in 1× Invitrogen sample buffer, loaded onto a precast 4% to 12% Bis-Tris gel (Invitrogen, Carlsbad, CA), run using 1X MES buffer (Invitrogen), and transferred onto a nitrocellulose membrane (Invitrogen). After blocking in 5% milk, membranes were incubated with polyclonal rabbit anti-CPN serum (a kind gift from Dr E. G. Erdös) or a mouse monoclonal antibody to TAFI followed by a secondary alkaline phosphatase-labeled goat antirabbit or antimouse antibody, respectively. Blots were developed using Western Blue stabilized substrate for alkaline phosphatase from Promega (Madison, WI).
Cells, cell proliferation, and chemotaxis
The murine DW34 pre-B-cell line (a gift from Dr P. Kincade, Oklahoma Medical Research Foundation, Oklahoma City) was propagated in culture with RPMI 1640 medium (Invitrogen, Grand Island, NY) supplemented with 50 μM 2-mercaptoethanol, 10% fetal bovine serum (Biofluids, Rockville, MD), and 10 ng/mL interleukin 7 (IL-7; a gift Dr C. Mackall, National Cancer Institute, Bethesda, MD). DW34 cell proliferation was as described.25
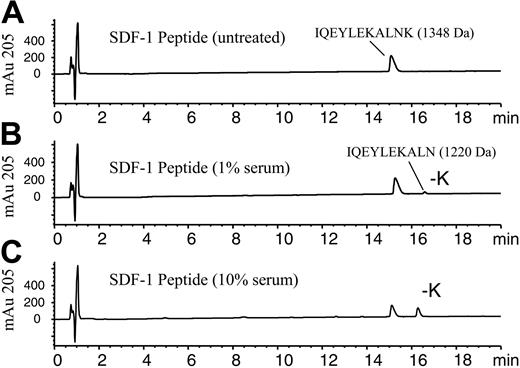
SDF-1α peptide cleavage assay using RP-HPLC/MSD analysis. The 11-amino acid C-terminal peptide of SDF-1α corresponding to amino acids 58-68, IQEYLEKALNK, was incubated (100 μM) in PBS in the absence (A) or presence of (B) 1% human serum or (C) 10% human serum and incubated for 10 minutes at 37°C. Samples were stopped by acidification with TFA and analyzed by RP-HPLC/MSD. Representative elution profiles for the intact peptide and the -K peptide as measured at 205 nm are shown along with their respective molecular masses determined by electrospray mass spectrometry.
SDF-1α peptide cleavage assay using RP-HPLC/MSD analysis. The 11-amino acid C-terminal peptide of SDF-1α corresponding to amino acids 58-68, IQEYLEKALNK, was incubated (100 μM) in PBS in the absence (A) or presence of (B) 1% human serum or (C) 10% human serum and incubated for 10 minutes at 37°C. Samples were stopped by acidification with TFA and analyzed by RP-HPLC/MSD. Representative elution profiles for the intact peptide and the -K peptide as measured at 205 nm are shown along with their respective molecular masses determined by electrospray mass spectrometry.
The human Burkitt lymphoma cell line BL41 was propagated in culture with RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Biofluids). Primary human umbilical vein endothelial cells (HUVECs), derived as previously described,32 were propagated up to passage 5 in culture medium consisting of M199 (Invitrogen) supplemented with 5% human AB serum (Sigma), 20% newborn calf serum (Invitrogen), 1.6 mM l-glutamine (Invitrogen), 25 ng/mL porcine heparin (Sigma), 50 ng/mL ascorbate (Fisher Scientific, Fair Lawn, NJ), and 15 μg/mL endothelial cell growth supplement (Sigma). Transendothelial migration assays were performed as described.18
Results
Purification of carboxypeptidase activity toward SDF-1α
To identify the carboxypeptidase that cleaves the C-terminal lysine from full-length SDF-1α (1-68), we purified the activity from human serum. To assess the activity, a peptide-based assay was developed, which used an SDF-1α peptide consisting of the 11 C-terminal amino acids (58-68) of SDF-1α (IQEYLEKALNK). This SDF-1α peptide contains a C-terminal lysine. Addition of 1% or 10% serum to this peptide resulted in a decrease in the amount of intact peptide and the appearance of a later-eluting peptide (Figure 1A-C). RP-HPLC/MSD analysis indicated the new peptide had a mass of 1220 Da and was 128 Da less than the original peptide of 1348 Da, consistent with the loss of the C-terminal lysine. After a 10-minute incubation with 10% serum, more than 40% of the peptide was hydrolyzed at the C-terminus (Figure 1C). Similar results were also obtained with human plasma (data not shown). By contrast, the C-terminal lysine of a scrambled version of the SDF-1 peptide (NLAKELYEQK) was not hydrolyzed by serum nor was the SDF-1 peptide containing a C-terminal glutamic acid (data not shown). However, an SDF-1 peptide in which the C-terminal amino acid was changed from lysine to arginine was hydrolyzed by serum with the elimination of the arginine (data not shown), reflecting a preference for basic amino acids at the C-terminus. This data indicated some amino acid sequence specificity required for this activity. A peptide consisting of the 11 C-terminal amino acids of SDF-1β, LEKALNKRFKM, was unaffected by treatment with 10% serum under the same conditions (data not shown). This preliminary characterization of the enzymatic activity suggested that the serum enzyme or enzymes responsible for SDF-1α peptide cleavage is/are likely a carboxypeptidase with specificity for basic amino acids at the SDF-1α carboxy-terminus and the adjacent amino acid sequence.
We used the RP-HPLC/MSD peptide-based assay to identify fractions containing SDF-1α peptidase activity, and to purify the enzyme responsible for carboxypeptidase activity toward SDF-1α. The serum was precipitated with 30% ammonium sulfate to remove much of the inactive protein and the remaining activity in the supernatant was precipitated with 40% ammonium sulfate (Table 1). The 40% ammonium sulfate fraction was further fractionated using anion exchange chromatography. The majority of the activity bound to the anion exchange column. The column was step eluted with NaCl and the activity eluted at 300 mM NaCl (Table 1). The active fractions from anion exchange were dialyzed against 50 mM phosphate buffer and then applied to a lysine Sepharose column. The majority of the active material was retained on the column and could be eluted with 150 mM salt (100 mM NaCl in 50 mM Na phosphate, pH 7.5). Additional activity could be eluted with 1 mM SDF-1α C-terminal peptide in 150 mM NaCl buffer (Table 1). The eluted fractions were pooled and this fraction represented a 175-fold purification of the SDF-1α peptidase activity.
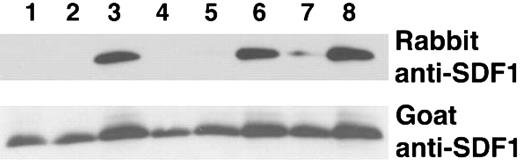
We verified that the lysine Sepharose fraction enriched with SDF-1 peptide cleavage activity was able to cleave the intact recombinant SDF-1α protein. To distinguish between full-length SDF-1α (1-68) from SDF-1α lacking the carboxy-terminal lysine, we used a rabbit polyclonal antibody that recognizes only full-length SDF-1α (1-68) but not SDF-1α that lacks the carboxy-terminal lysine (1-67), combined with goat anti-SDF-1α antibodies that recognize both SDF-1α species (1-68 and 1-67).31 As demonstrated by immunoblotting, incubation of SDF-1α with 100 μg (10% serum) or 10 μg (1% serum) of total serum protein completely cleaved full-length SDF-1α whereas 1 μg (0.1% serum) was ineffective (Figure 2, lanes 1-3). The lysine Sepharose fraction was at least 100-fold more active as demonstrated by the complete cleavage of SDF-1α by 1 μg and 0.1 μg of the purified material but not by 0.01 μg (Figure 2, lanes 4-6). However, even 1 μg of the protein fraction that did not bind to the lysine Sepharose column failed to completely cleave the SDF-1 protein; some activity remained in this unbound fraction as indicated by the decrease in band intensity as compared with the control (Figure 2, lane 7).
SDF-1α cleavage by crude and lysine Sepharose-purified serum fractions. Recombinant SDF-1α (25 ng in 18 μL PBS) was incubated at room temperature for 10 minutes with serum, plasma or serum components, and subsequently immunoblotted with rabbit and goat anti-SDF-1α antibodies (10%). Lanes 1-3: SDF-1α incubated with 10% serum (100 μg), 1% serum (10 μg), or 0.1% (1 μg) serum, respectively. Lanes 4-6: SDF-1α incubated with 1 μg, 0.1 μg, or 0.01 μg lysine Sepharose fraction from purification of stored serum. Lane 7: SDF-1α incubated with 1 μg of the flow-through fraction from lysine Sepharose column. Lane 8: SDF-1α incubated with PBS.
SDF-1α cleavage by crude and lysine Sepharose-purified serum fractions. Recombinant SDF-1α (25 ng in 18 μL PBS) was incubated at room temperature for 10 minutes with serum, plasma or serum components, and subsequently immunoblotted with rabbit and goat anti-SDF-1α antibodies (10%). Lanes 1-3: SDF-1α incubated with 10% serum (100 μg), 1% serum (10 μg), or 0.1% (1 μg) serum, respectively. Lanes 4-6: SDF-1α incubated with 1 μg, 0.1 μg, or 0.01 μg lysine Sepharose fraction from purification of stored serum. Lane 7: SDF-1α incubated with 1 μg of the flow-through fraction from lysine Sepharose column. Lane 8: SDF-1α incubated with PBS.
Identification of carboxypeptidase N in the purified fractions
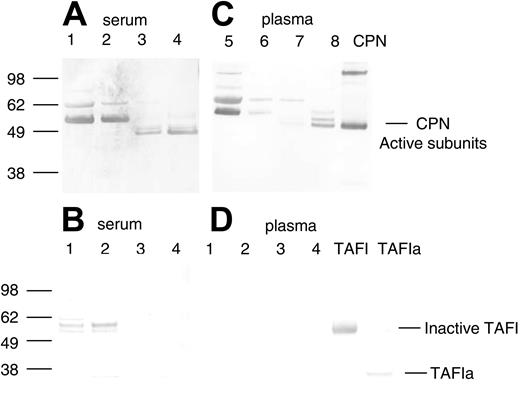
Two serum-derived enzymes have previously been described as capable of selective cleavage of basic amino acid residues (lysine and arginine) from the carboxy terminus of a number of different polypeptides, carboxypeptidase N (CPN, also known as kininase I, arginine carboxypeptidase, and anaphylatoxin inactivator) and TAFI (also known as carboxypeptidase R, carboxypeptidase U, and carboxypeptidase B). To determine if the purification of SDF-1α peptidase activity copurified with either of these 2 previously reported serum-derived carboxypeptidases, we used specific antibodies against CPN and TAFI in Western blotting. A rabbit immune serum (a gift of Dr E. G. Erdös) against the purified active subunit of CPN (a kind gift of Dr Mariko Nagashima, Berlex Biosciences)33 recognized bands corresponding in size to the CPN active subunits (48 kDa and 55 kDa subunits) in the purified fractions from serum containing high SDF-1α peptidase activity (Figure 3A, lanes 3 and 4). Bands corresponding to the CPN active subunits were not detected in unfractionated human serum (Figure 3A, lane 1) or in the 40% ammonium sulfate fraction (Figure 3A, lane 2). Nonspecific bands were also recognized by the polyclonal CPN antibody in the crude fractions (Figure 3, lanes 1 and 2), but these were no longer present in the more purified fractions, and thus may reflect either nonspecific reactivity toward serum proteins or inactive/aggregated forms of the enzyme (Figure 3A). In contrast to the results with the CPN antibody, a monoclonal antibody that recognizes inactive (TAFI) and active (TAFIa) forms of TAFI did not recognize this protein in the purified fractions containing carboxypeptidase activity (Figure 3B, lanes 3 and 4). Bands corresponding to inactive TAFI were present in the unfractionated and ammonium sulfate fractions but were not present in the purified fractions. These results provide evidence that CPN present in human serum is responsible, at least in part, for SDF-1α C-terminal peptidase activity.
Detection of CPN and TAFIa in crude and purified serum and plasma fractions containing SDF-1α peptidase activity. Protein samples from the purification of SDF-1α peptidase activity from human serum or plasma as described in Tables 1 and 2 were electrophoresed, transferred to nitrocellulose, and immunoblotted in panels A and C with rabbit polyclonal antibody against the active subunits of CPN, and in panels B and D with a mouse monoclonal antibody against TAFI. (A-B) Lane 1: human serum (1.0 μg); lane 2: 40% ammonium sulphate pellet (1.0 μg); lane 3: anion exchange fraction eluted with 0.25 M NaCl (1.0 μg); lane 4: pooled lysine Sepharose fraction eluted with 150 mM NaCl and 150 mM NaCl containing 100 μM SDF-1 peptide (1.0 μg). (C-D) Lane 1: human plasma (0.25 μg); lane 2: 50% ammonium sulphate pellet (0.25 μg); lane 3: anion exchange fraction eluted with 0.25 M NaCl (0.25 μg); lane 4: pooled lysine Sepharose fraction eluted with 150 mM NaCl and 150 mM NaCl containing 100 μM SDF-1 peptide (0.25 μg). Bands were visualized using Promega stabilized alkaline phosphatase substrate following incubation with secondary antirabbit or antimouse antibodies linked to alkaline phosphatase. The locations of the active subunits of purified CPN (0.3 μg) and the active (TAFIa) (0.01 μg) and inactive subunits of TAFI (0.01 μg) are indicated.
Detection of CPN and TAFIa in crude and purified serum and plasma fractions containing SDF-1α peptidase activity. Protein samples from the purification of SDF-1α peptidase activity from human serum or plasma as described in Tables 1 and 2 were electrophoresed, transferred to nitrocellulose, and immunoblotted in panels A and C with rabbit polyclonal antibody against the active subunits of CPN, and in panels B and D with a mouse monoclonal antibody against TAFI. (A-B) Lane 1: human serum (1.0 μg); lane 2: 40% ammonium sulphate pellet (1.0 μg); lane 3: anion exchange fraction eluted with 0.25 M NaCl (1.0 μg); lane 4: pooled lysine Sepharose fraction eluted with 150 mM NaCl and 150 mM NaCl containing 100 μM SDF-1 peptide (1.0 μg). (C-D) Lane 1: human plasma (0.25 μg); lane 2: 50% ammonium sulphate pellet (0.25 μg); lane 3: anion exchange fraction eluted with 0.25 M NaCl (0.25 μg); lane 4: pooled lysine Sepharose fraction eluted with 150 mM NaCl and 150 mM NaCl containing 100 μM SDF-1 peptide (0.25 μg). Bands were visualized using Promega stabilized alkaline phosphatase substrate following incubation with secondary antirabbit or antimouse antibodies linked to alkaline phosphatase. The locations of the active subunits of purified CPN (0.3 μg) and the active (TAFIa) (0.01 μg) and inactive subunits of TAFI (0.01 μg) are indicated.
Comparative analysis of SDF-1α cleavage by human serum and plasma. Recombinant SDF-1α (1 μgin18 μL PBS) was incubated for 10 minutes at room temperature with PBS (2 μL; lane 1); normal human serum (2 μL; lane 2); normal human plasma collected in heparin (2 μL; lane 3); and normal human plasma collected in heparin and the thrombin inhibitor PPACK (2 μL; lane 4). Serum and plasma samples were from the same individual. At the end of incubation, 1 μL of the mixture was used for immunoblotting and the remainder was used for MALDI. (A) Western blot analysis of SDF-1α cleavage using rabbit and goat anti-SDF-1 antibodies. (B) MALDI analysis of SDF-1α cleavage by serum or plasma collected in heparin (hep). The experiment was carried out 4 times and a representative set of data is shown with similar results from each.
Comparative analysis of SDF-1α cleavage by human serum and plasma. Recombinant SDF-1α (1 μgin18 μL PBS) was incubated for 10 minutes at room temperature with PBS (2 μL; lane 1); normal human serum (2 μL; lane 2); normal human plasma collected in heparin (2 μL; lane 3); and normal human plasma collected in heparin and the thrombin inhibitor PPACK (2 μL; lane 4). Serum and plasma samples were from the same individual. At the end of incubation, 1 μL of the mixture was used for immunoblotting and the remainder was used for MALDI. (A) Western blot analysis of SDF-1α cleavage using rabbit and goat anti-SDF-1 antibodies. (B) MALDI analysis of SDF-1α cleavage by serum or plasma collected in heparin (hep). The experiment was carried out 4 times and a representative set of data is shown with similar results from each.
Comparison of serum and plasma carboxypeptidase activity toward SDF-1α
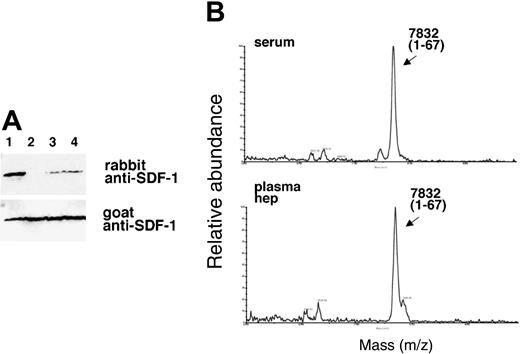
Like serum, plasma is known to contain active CPN.34 By contrast, TAFI is an inactive plasma enzyme that is activated during clotting by thrombin-catalyzed proteolysis to an active but unstable carboxypeptidase enzyme designated TAFIa. If TAFIa were responsible for the SDF-1α cleavage activity we would not expect to detect this activity in plasma. Therefore, we first compared the ability of serum and plasma to remove the carboxy-terminal lysine from SDF-1α. When recombinant SDF-1α (25 ng) was incubated with either fresh serum or plasma collected with heparin alone or with PPACK (used to block TAFI activation) from the same individual, each displayed SDF-1α carboxypeptidase activity as judged by the absence of an SDF-1α-related band or presence of only a faint band following serum or plasma treatment (Figure 4A). The decreased recognition of SDF-1α by the rabbit antibody could not be attributed to differences in loading because bands of equal intensity were detected upon membrane reprobing with goat anti-SDF-1 antibodies that recognize both forms of SDF-1α (Figure 4A). Analysis of the samples by MALDI (Figure 4B), confirmed25 that SDF-1α displays a relative molecular mass of 7832 Da following incubation with serum or plasma, corresponding to the loss of the carboxy-terminal lysine from full-length SDF-1α (calculated mass of 7959 Da average). The immunoblotting and MALDI results demonstrate that serum and plasma display lysine carboxypeptidase activity for SDF-1α.
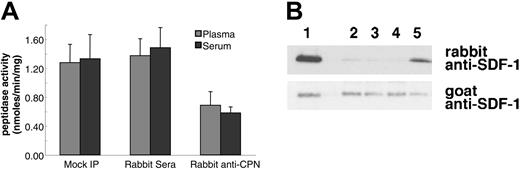
To confirm that the SDF-1α carboxypeptidase activity detected in serum and plasma is attributable to CPN, we depleted serum and plasma of endogenous CPN by immunoprecipitation with rabbit anti-CPN antibodies against the active subunit (a gift of Dr E. G. Erdös). Immunoprecipitation of CPN from human plasma and serum resulted in a 47% and 57% reduction in SDF-1 peptide cleavage activity compared with control antibodies, respectively (Figure 5A). In addition, plasma immunoprecipitated with rabbit anti-CPN antiserum displayed a markedly reduced ability to cleave SDF-1α protein (Figure 5B, lane 5) as compared with untreated plasma (Figure 5B, lane 2), plasma mock precipitated (Figure 5B, lane 3), or plasma precipitated with control rabbit serum (Figure 5B, lane 4). However, immunoprecipitation with sheep polyclonal antibodies to TAFI had no effect on the peptide cleavage activity (data not shown).
Immunoprecipitation of CPN from human plasma reduces SDF-1α and SDF-1α C-terminal peptide cleavage activity. Plasma or serum was either mock immunoprecipitated (no antibody added) or immunoprecipitated with control rabbit serum or with rabbit anti-CPN antibodies. After removal of the immunoprecipitate with protein-G, supernatant was tested for enzymatic activity. (A) SDF-1 carboxypeptidase activity in serum or plasma following immunoprecipitation. Peptidase activity was calculated for untreated 10% plasma or serum (Mock IP); 10% plasma or serum immunoprecipitated with control rabbit serum; and 10% plasma or serum immunoprecipitated with rabbit anti-CPN antibodies. Peptide cleavage was evaluated by RP-HPLC. Shown is the mean plus or minus the standard deviation from 3 independent experiments. (B) SDF-1α cleavage evaluated by immunoblotting. SDF-1α incubated for 10 minutes at room temperature with 10% of the following: lane 1: PBS alone; lane 2: plasma (10%); lane 3: 10% plasma, mock immunoprecipitated; lane 4: 10% plasma immunoprecipitated with control rabbit IgG; lane 5: 10% plasma immunoprecipitated with rabbit anti-CPN antibodies.
Immunoprecipitation of CPN from human plasma reduces SDF-1α and SDF-1α C-terminal peptide cleavage activity. Plasma or serum was either mock immunoprecipitated (no antibody added) or immunoprecipitated with control rabbit serum or with rabbit anti-CPN antibodies. After removal of the immunoprecipitate with protein-G, supernatant was tested for enzymatic activity. (A) SDF-1 carboxypeptidase activity in serum or plasma following immunoprecipitation. Peptidase activity was calculated for untreated 10% plasma or serum (Mock IP); 10% plasma or serum immunoprecipitated with control rabbit serum; and 10% plasma or serum immunoprecipitated with rabbit anti-CPN antibodies. Peptide cleavage was evaluated by RP-HPLC. Shown is the mean plus or minus the standard deviation from 3 independent experiments. (B) SDF-1α cleavage evaluated by immunoblotting. SDF-1α incubated for 10 minutes at room temperature with 10% of the following: lane 1: PBS alone; lane 2: plasma (10%); lane 3: 10% plasma, mock immunoprecipitated; lane 4: 10% plasma immunoprecipitated with control rabbit IgG; lane 5: 10% plasma immunoprecipitated with rabbit anti-CPN antibodies.
Analysis of SDF-1α purified from human serum and plasma
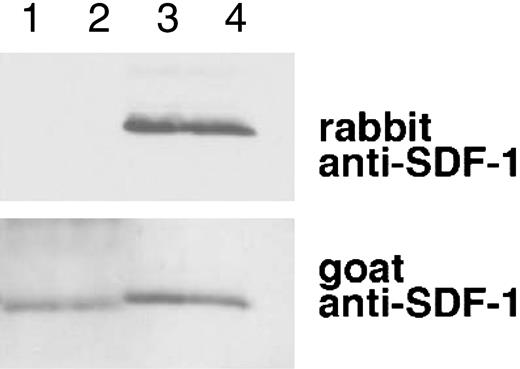
SDF-1α is present in normal human serum at concentrations of 15.4 ng/mL ± 3.0 ng/mL as measured by enzyme-linked immunosorbent assay (ELISA), but serum SDF-1α lacks the carboxy-terminal lysine, as assessed by immunoblotting after immunoprecipitation.31 We examined whether SDF-1α in plasma also lacks the carboxy-terminal lysine as one might expect due to the presence of CPN activity in plasma. To this end, we affinity purified SDF-1α from plasma collected in heparin and 5 μM PPACK (an inhibitor of TAFIa activation) and from serum collected from the same individual. As shown in Figure 6, SDF-1α purified from plasma was indistinguishable from SDF-1α purified from serum. In particular, serum- and plasma-derived SDF-1α were not visualized by rabbit anti-SDF-1α antibodies (as expected from SDF-1α lacking the carboxy-terminal lysine), but was recognized by goat anti-SDF-1α antibodies. In addition, SDF-1α purified from serum or plasma displayed a slightly reduced size compared with the recombinant SDF-1α control. The pattern of antibody recognition and the size reduction were indistinguishable in SDF-1α purified from serum or plasma, providing further evidence that CPN present in plasma can cleave circulating SDF-1 at the carboxy terminus.
Characterization of SDF-1α present in the circulation. SDF-1 was affinity purified from aliquots (1 mL) of human serum and plasma (collected in heparin and PPACK) from the same individual using biotin-labeled goat anti-SDF-1 antibodies (BAF 310) immobilized onto beads. Aliquots of the purified material (20 μL) were immunoblotted with rabbit anti-SDF-1α antibodies that do not recognize SDF-1α missing the C-terminal lysine (aa 1-67), and reblotted with goat anti-SDF-1 antibodies that recognize SDF-1 lacking the C-terminal lysine. Lane 1: SDF-1α purified from serum; lane 2: SDF-1α purified from plasma; lane 3: recombinant SDF-1α, 3 ng; lane 4: recombinant SDF-1α, 1.5 ng. The experiment was carried out 3 times with similar results and a representative set of this data is shown.
Characterization of SDF-1α present in the circulation. SDF-1 was affinity purified from aliquots (1 mL) of human serum and plasma (collected in heparin and PPACK) from the same individual using biotin-labeled goat anti-SDF-1 antibodies (BAF 310) immobilized onto beads. Aliquots of the purified material (20 μL) were immunoblotted with rabbit anti-SDF-1α antibodies that do not recognize SDF-1α missing the C-terminal lysine (aa 1-67), and reblotted with goat anti-SDF-1 antibodies that recognize SDF-1 lacking the C-terminal lysine. Lane 1: SDF-1α purified from serum; lane 2: SDF-1α purified from plasma; lane 3: recombinant SDF-1α, 3 ng; lane 4: recombinant SDF-1α, 1.5 ng. The experiment was carried out 3 times with similar results and a representative set of this data is shown.
CPN purification from plasma and characterization of SDF-1α cleavage
To further confirm that CPN is responsible for the cleavage of SDF-1α in the circulation and to separate it from enzymes derived from clotting, we purified the activity from plasma. The activity from plasma was purified using the same steps as that used for serum. Very similar results were obtained for plasma when compared with those seen with serum (Table 2). The active fractions purified from plasma contained CPN as determined by Western blotting (Figure 3C, lanes 7 and 8). CPN was enriched during the purification process whereas TAFIa was not detected in any of the fractions (Figure 3D, lanes 1-4).
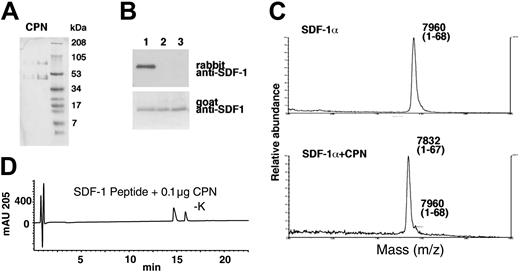
Finally, purified CPN (a gift of Dr M. Nagashima, Berlex Biosciences) (Figure 7A) was tested for SDF-1α cleavage. By immunoblotting, we documented that recombinant SDF-1α treated for 30 minutes at room temperature with purified CPN (Figure 7B, lane 2: 10 μg/mL; lane 3: 50 μg/mL) was no longer recognized by the rabbit anti-SDF-1α antibodies, but continued to be recognized by goat anti-SDF-1α antibodies, providing evidence for the occurrence of carboxy-terminal cleavage (Figure 7B). As determined by MALDI, SDF-1α treated for 30 minutes with purified CPN displayed a mass of 7832 Da (average) corresponding to SDF-1α that has lost a lysine residue (Figure 7C). Purified CPN also hydrolyzed the C-terminal amino acid from the 11 amino acid SDF-1 peptide (Figure 7D), but was ineffective against the scrambled SDF-1 peptide (data not shown). These results confirm that CPN is capable of processing SDF-1α at the C-terminus by enzymatic removal of the carboxy-terminal lysine.
Characterization of the biologic activity of SDF-1α cleaved by CPN
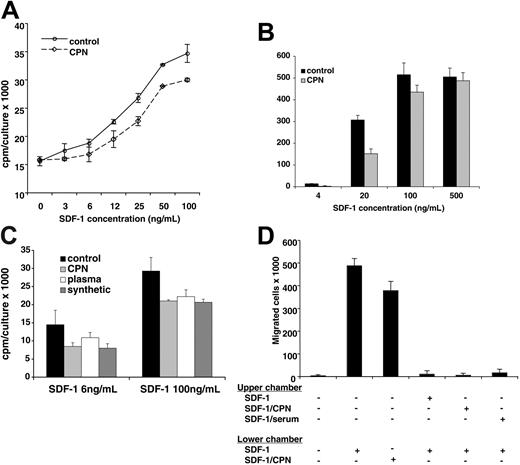
We have previously demonstrated that SDF-1α lacking the carboxy-terminal lysine, generated synthetically or by exposure to human serum, displays a reduced biologic activity compared with full-length SDF-1α.25 In addition, it was previously reported that full-length SDF1β is approximately 2-fold more potent than SDF-1α lacking the carboxy-terminal lysine.22 We now examined the biologic activity of SDF-1α treated with CPN in comparison to the untreated chemokine. Full-length SDF-1α (R&D Systems; 5 μg in 100 μL PBS) was incubated for 10 minutes at room temperature with or without purified CPN (Berlex Biosciences; final dilution 1:50). We confirmed by MALDI that SDF-1α had lost the carboxy-terminal lysine after treatment with CPN, whereas the control SDF-1α was full length. Using the pre-B-cell line DW34, which responds to SDF-1 with increased proliferation, we measured SDF-1 activity.25 As shown in Figure 8A, control SDF-1α and CPN-treated SDF-1α induced a dose-dependent rise in DW34 cell proliferation. However, control (full-length, 1-68) SDF-1α was approximately 2-fold more active than CPN-treated SDF-1α (1-67) over a wide range of concentrations: levels of proliferation induced by 3 ng/mL, 6 ng/mL, and 12 ng/mL full-length SDF-1α were achieved by 6 ng/mL, 12 ng/mL, and 25 ng/mL CPN-treated SDF-1α, respectively (P < .05 all determinations). In control experiments (not shown), the appropriately diluted CPN enzyme had minimal effects on DW34 cell proliferation. We also compared the chemotactic activity of CPN-treated SDF-1α (1-67) with that of control SDF-1α (1-68) in trans-endothelial migration assays (Figure 8B). The human Burkitt lymphoma cell line BL41, which expresses surface CXCR4 at high levels,18 displayed a brisk chemotactic response to control and CPN-treated SDF-1α, but at the suboptimal SDF-1 concentration of 20 ng/mL the chemotactic response to the full-length (control) SDF-1α was significantly greater (P < .05) than the response to CPN-treated SDF-1α. This suggests that CPN-treated SDF-1α displays a reduced chemotactic activity that is revealed at nonsaturating concentrations.
Characterization of SDF-1α and SDF-1 peptide cleavage by purified CPN. (A) SDS-PAGE analysis of purified CPN. Lane 1: 1 μg and lane 2: 4 μg (stained with Coomassie). (B) SDF-1α cleavage by CPN evaluated by Western blotting with specific antibodies. Lane 1: SDF-1α was incubated for 10 minutes at room temperature with PBS alone; lane 2: purified CPN at 10 μg/mL; lane 3: 50 μg/mL. (C) SDF-1α cleavage by CPN evaluated by MALDI. SDF-1α was incubated for 10 minutes at room temperature in PBS or with PBS containing purified CPN at 10 μg/mL. (D) RP-HPLC tracing for SDF-1 peptide treated for 10 minutes with 0.1 μg purified CPN.
Characterization of SDF-1α and SDF-1 peptide cleavage by purified CPN. (A) SDS-PAGE analysis of purified CPN. Lane 1: 1 μg and lane 2: 4 μg (stained with Coomassie). (B) SDF-1α cleavage by CPN evaluated by Western blotting with specific antibodies. Lane 1: SDF-1α was incubated for 10 minutes at room temperature with PBS alone; lane 2: purified CPN at 10 μg/mL; lane 3: 50 μg/mL. (C) SDF-1α cleavage by CPN evaluated by MALDI. SDF-1α was incubated for 10 minutes at room temperature in PBS or with PBS containing purified CPN at 10 μg/mL. (D) RP-HPLC tracing for SDF-1 peptide treated for 10 minutes with 0.1 μg purified CPN.
Stimulation of cell proliferation and chemotaxis by SDF-1α cleaved by CPN. (A) DW34 pre-B-cell proliferation in response to various concentrations (3 ng/mL-100 ng/mL) of SDF-1α, which was incubated for 10 minutes at room temperature with or without purified CPN (1:50 dilution) prior to testing. The results reflect the mean counts per minute (cpm) per culture (± SEM) of triplicate determinations; representative experiment of 4 performed. (B) BL41 cell migration across endothelial cell monolayers in the presence of SDF-1α (4 ng/mL-500 ng/mL) in the lower chamber of the transwell. SDF-1α was incubated for 10 minutes at room temperature with or without purified CPN (1:50 dilution) prior to testing. The results reflect the mean (± SEM) number of cells that have transmigrated to the lower chamber over a 4-hour incubation from 3 replicate wells per condition. Representative experiment of 5 performed. (C) DW34 pre-B-cell proliferation in response to full-length SDF-1α (4 ng/mL and 100 ng/mL) or SDF-1α lacking the carboxy-terminal lysine due to treatment with purified CPN (1:50 dilution, 10 minutes at room temperature), treatment with normal human plasma (1:10 dilution, 10 minutes at room temperature) or synthetic (SDF-1α 1-67). The results reflect the mean counts per minute (cpm) per culture (± SEM) of triplicate determinations; representative experiment of 3 performed. (D) BL41 cell migration across endothelial cell monolayers in the presence or absence of full-length SDF-1α (100 ng/mL) and CPN-treated SDF-1α (100 ng/mL) in the lower chamber of the transwell. In the presence of full-length SDF-1α (100 ng/mL) in the lower chamber of the transwell, BL41 cell transmigration was evaluated with full-length SDF-1α (100 ng/mL), SDF-1α (100 ng/mL) treated with purified CPN (1:50 dilution, 10 minutes at room temperature) or SDF-1α (100 ng/mL) treated with normal human serum (1:10 dilution, 10 minutes at room temperature) in the upper chamber of the transwell. The results reflect the mean (± SEM) number of cells that have transmigrated to the lower chamber over a 4-hour incubation from 5 replicate wells per condition. Representative experiment of 3 performed.
Stimulation of cell proliferation and chemotaxis by SDF-1α cleaved by CPN. (A) DW34 pre-B-cell proliferation in response to various concentrations (3 ng/mL-100 ng/mL) of SDF-1α, which was incubated for 10 minutes at room temperature with or without purified CPN (1:50 dilution) prior to testing. The results reflect the mean counts per minute (cpm) per culture (± SEM) of triplicate determinations; representative experiment of 4 performed. (B) BL41 cell migration across endothelial cell monolayers in the presence of SDF-1α (4 ng/mL-500 ng/mL) in the lower chamber of the transwell. SDF-1α was incubated for 10 minutes at room temperature with or without purified CPN (1:50 dilution) prior to testing. The results reflect the mean (± SEM) number of cells that have transmigrated to the lower chamber over a 4-hour incubation from 3 replicate wells per condition. Representative experiment of 5 performed. (C) DW34 pre-B-cell proliferation in response to full-length SDF-1α (4 ng/mL and 100 ng/mL) or SDF-1α lacking the carboxy-terminal lysine due to treatment with purified CPN (1:50 dilution, 10 minutes at room temperature), treatment with normal human plasma (1:10 dilution, 10 minutes at room temperature) or synthetic (SDF-1α 1-67). The results reflect the mean counts per minute (cpm) per culture (± SEM) of triplicate determinations; representative experiment of 3 performed. (D) BL41 cell migration across endothelial cell monolayers in the presence or absence of full-length SDF-1α (100 ng/mL) and CPN-treated SDF-1α (100 ng/mL) in the lower chamber of the transwell. In the presence of full-length SDF-1α (100 ng/mL) in the lower chamber of the transwell, BL41 cell transmigration was evaluated with full-length SDF-1α (100 ng/mL), SDF-1α (100 ng/mL) treated with purified CPN (1:50 dilution, 10 minutes at room temperature) or SDF-1α (100 ng/mL) treated with normal human serum (1:10 dilution, 10 minutes at room temperature) in the upper chamber of the transwell. The results reflect the mean (± SEM) number of cells that have transmigrated to the lower chamber over a 4-hour incubation from 5 replicate wells per condition. Representative experiment of 3 performed.
In additional functional experiments, we tested 3 forms of SDF-1α lacking the carboxy-terminal lysine obtained by CPN treatment, plasma treatment (10% plasma, 10-minute incubation at room temperature), or through synthesis (Upstate Biotechnology, Lake Placid, NY). As shown in Figure 8C, all forms of SDF-1α lacking the carboxy-terminal lysine displayed a similarly reduced (P < .05 all determinations) ability to promote DW34 cell proliferation compared with the full-length molecule. We also tested SDF-1 lacking the carboxy-terminal lysine in competitive trans-endothelial migration assays (Figure 8D). BL41 cells trans-migrated in response to full-length SDF-1α (100 ng/mL) and CPN-treated SDF-1α (100 ng/mL) placed in the lower chamber of the transwell. This response was significantly (P < .05) reduced by addition of full-length SDF-1α (100 ng/mL) to the upper chamber of the transwell. A similarly reduced migration to full-length SDF-1α (100 ng/mL) was observed when SDF-1α lacking the carboxy-terminal lysine, derived either from CPN or 10% serum treatment, was added to the upper chamber (Figure 8C). Together, these results provide evidence that CPN cleavage of full-length SDF-1α generates a biologically active chemokine with a reduced biologic activity compared with the full-length molecule.
Discussion
We identified CPN as an important blood enzyme that removes the C-terminal lysine from SDF-1α. CPN is a 280 kDa tetrameric glycoprotein that is synthesized in the liver and secreted into the bloodstream.33,35 It comprises two 83-kDa regulatory subunits (CPN2) and two approximately 50-kDa catalytic subunits (CPN1), which form a stable tetramer.35-38 CPN was originally identified in plasma as the enzyme responsible for inactivating bradykinin and kallidin II by removal of their C-terminal arginine.39 When cleaved by CPN, bradykinin and kallidin II change their receptor specificity, from B2 to B1, which effectively reduces their activity.40,41 Both receptors are 7-transmembrane G-protein-coupled receptors, but whereas the B1 receptor is normally present at low levels and is induced during chronic inflammation, the B2 receptor is constitutively expressed, and contributes to the acute phase of an inflammatory response, enhancing vascular permeability, bronchoconstriction, and hypotension.35,42 CPN also removes the C-terminal arginines from anaphylatoxin C3a, C4a, and C5a,35,43 and this reduces their biologic activities 10- to 100-fold.35 Another identified substrate for CPN is creatine kinase MM (CK-MM), an intracellular enzyme that catalyses the transfer of a phosphate group from adenosine triphosphate (ATP) to creatinine.35,44 CPN cleaves the 2 carboxy-terminal lysines from CK-MM to generate CK-MM1 and CK-MM2 detected in the circulation.45 Increased levels of intact CK-MM compared with its cleaved forms CK-MM1 and CK-MM2 correlate with tissue damage following myocardial infarction.45 Interestingly, removal of the C-terminal lysines from CK-MM has no apparent detrimental effect on its biologic activity.35
The chemokine SDF-1α can be added to the list of biologically important proteins affected by CPN enzymatic activity. Although CPN may not be the only circulating enzyme capable of SDF-1α cleavage at the C-terminus, it is unlikely that TAFIa is responsible for the physiologic cleavage activity of SDF-1α in vivo. TAFIa is not active in anticoagulated plasma. Endogenous SDF-1α from plasma or serum lacks the C-terminal lysine, suggesting that the enzyme responsible for C-terminal processing of SDF-1 is active in plasma. Unlike TAFIa, CPN is active in plasma and serum.33,35
We previously established that removal of the C-terminal lysine from SDF-1α reduces the activity of the chemokine by approximately 50% as a chemoattractant and pre-B-cell growth factor.25 We found that CPN removes the carboxy-terminal lysine from full-length SDF-1α and reduces its biologic activity as a chemoattractant and a pre-B-cell growth factor by approximately 50%. The reduced biologic activity of SDF-1 by CPN cleavage provides another tool for fine regulation of chemokine gradients that critically regulate cell migration within tissues and from the blood stream to tissues.8,18,46-48 SDF-1α lacking the C-terminal lysine can activate the CXCR4 receptor, but displays a reduced affinity for heparin/heparin sulfate glycosaminoglycans abundant on the cell surface and in the extracellular matrix, and enhances the local concentrations of SDF-1α available for activation of CXCR4.22,24,25,49 Endothelial cell surface full-length SDF-1α, which is mostly bound to heparin sulfate, can mediate lymphocyte attachment and transmigration under conditions of physiologic blood flow.18,50 SDF-1β is more effective than SDF-1α (1-67) at inhibiting HIV-1 replication.22 While the N-terminal sequence of SDF-1 is required for receptor binding, the C-terminal sequence of SDF-1 may affect the binding affinity to the CXCR4 receptor.51 The absence of the C-terminal lysine in SDF-1 (1-67) may explain its reduced ability to inhibit HIV-1 replication, as we have already shown that SDF-1 (1-67) has reduced binding affinity to heparin/heparin sulfate glycosaminoglycans as compared with SDF-1 (1-68).25
In addition to being cleaved at the C-terminus by CPN, SDF-1α is cleaved at the N-terminus by a number of different enzymes to generate an inactive chemokine that can no longer activate the CXCR4 receptor, including CD26/dipeptidyl peptidase,22,26 MMPs,29 neutrophil elastase,28 and cathepsin G.27 The C-terminal loss of lysine is very rapid as compared with a much slower removal of the N-terminal amino acids lysine and proline.25 This stepwise reduction in SDF-1α activity is consistent with the important role played by degradation in the control of SDF-1α biologic activity. Unlike other chemokines whose expression is induced by specific signals, SDF-1α is constitutively expressed.1,2 Thus, degradation is critical to regulation of SDF-1α activity in vivo.
There is no individual known to have a complete deficiency of CPN, likely a reflection of the important biologic functions of this enzyme. A patient with markedly reduced levels of circulating CPN had recurrent angioedema associated with increased C3a and histamine levels.52-55 The patient also had an abnormal cutaneous polymorphonuclear cell infiltration,52 which could be related to SDF-1 dysfunction due to the chemokine's established role as a key regulator of neutrophil mobilization from the bone marrow to the peripheral circulation and chemotaxis.5,12,13,52 The results presented here derive from in vitro experiments and their impact to the situation in vivo is speculative. Nonetheless, CPN is identified here as an enzyme capable of regulating SDF-1α biologic activity, a chemokine that controls hematopoiesis, lymphocyte homing, B-lineage cell growth, and angiogenesis.
Prepublished online as Blood First Edition Paper, February 17, 2005; DOI 10.1182/blood-2004-12-4618.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Drs. E. G. Erdös, M. Nagashima, J. Farber, and C. Kimmel Williams for their help in various aspects of this work.