Comment on Knuepfer et al, page 4078
Malaria parasites export virulence proteins through intermediate protein complexes and membranous clefts in the host erythrocyte cytoplasm. GFP fusion proteins carrying the necessary export motifs provide new ways to study this phenomenon.
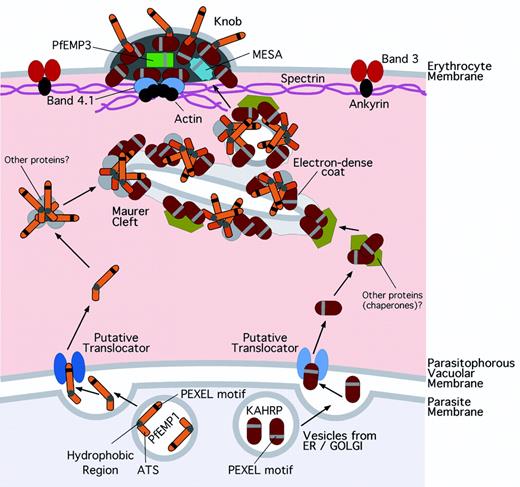
Plasmodium falciparum erythrocyte membrane protein-1 (PfEMP1) comprises a family of antigenically diverse adhesins responsible for the sequestration of parasitized erythrocytes in such critical tissues as the brain, lung, and placenta in malaria. Intraerythrocytic parasites are surrounded by a parasitophorous vacuolar membrane (PVM) that fences off the parasitophorous vacuole (PV) in which the parasite resides. Because human erythrocytes lack the cellular machinery of protein secretion, the mechanism by which newly synthesized PfEMP1 is transferred to the surface of the host cell has been as baffling as it is important.
Knuepfer and colleagues have used chimeric fusions of P falciparum protein sequences and green fluorescent protein (GFP) to identify regions of PfEMP1 that provide the necessary information for its transport. The authors also report that exported PfEMP1-GFP chimeras are present in the erythrocyte in 2 states: in a smoothly distributed, low-intensity pool throughout the cytoplasm and in brightly fluorescent populations concentrated at membranous structures (Maurer clefts) beneath the host cell membrane. Exchange of the PfEMP1-GFP molecules between these 2 states, or movement of PfEMP1-GFP between separate clefts, must be slow and inefficient, as fluorescence remained extinguished for at least several minutes from clefts that had been photobleached. In contrast, photobleaching of regions of the smoothly distributed cytoplasmic pool was followed by recovery of fluorescence within seconds. This recovery time indicates that the molecules are present as large, mobile complexes of proteins in the host cell and is consistent with previous studies showing pools of readily extracted PfEMP1 molecules in the parasitized erythrocyte cytoplasm (see figure).1,2
Maurer clefts are thought to derive from the PVM and to play an important role in protein sorting and trafficking. As the parasite matures, some PfEMP1 molecules from the cytoplasmic pool embed in the electron dense coats of Maurer clefts so that only their acidic terminal segment (ATS) regions are exposed to the erythrocyte cytoplasm.1 They may become associated at this time with proteins necessary for their placement in the knobby protrusions at the erythrocyte surface, notably including a knob-associated histidine-rich protein (KAHRP) from a separate cytoplasmic pool of protein complexes.3 How the PfEMP1-knob protein complexes transfer from Maurer clefts to the erythrocyte membrane is unclear, but electron microscopy results suggest that budding events from the clefts are involved in the process.1 Association with knob components is necessary for the transfer of PfEMP1 to the erythrocyte surface since P falciparum mutants that lack these components (KAHRP or other proteins such as PfEMP34 ) fail to properly display PfEMP1 in knobs.
Model of PfEMP1 trafficking to the surface of P falciparum–parasitized erythrocytes. PfEMP1 and KAHRP carry protein export element (PEXEL) motifs that enable them to traverse the PVM, perhaps in association with specific translocases or helper proteins. In knobs, KAHRP binds the ATS of PfEMP1 and ties it to actin-spectrin-band 4.1 in the cytoskeleton.5
Model of PfEMP1 trafficking to the surface of P falciparum–parasitized erythrocytes. PfEMP1 and KAHRP carry protein export element (PEXEL) motifs that enable them to traverse the PVM, perhaps in association with specific translocases or helper proteins. In knobs, KAHRP binds the ATS of PfEMP1 and ties it to actin-spectrin-band 4.1 in the cytoskeleton.5
Export of PfEMP1 to Maurer clefts through an intermediate cytoplasmic pool of vesicle-free protein complexes may seem peculiar, but this is perhaps the parasite's shrewd solution to survival in a host erythrocyte that lacks protein secretory ability. Knuepfer and colleagues have opened new doors to investigations of this unusual process and show how to express and direct engineered proteins of interest to the parasitized erythrocyte surface. Further study of PfEMP1 export is clearly important from the standpoint of basic biology and as a possible point of departure for new therapeutic ideas in today's world of multiple-drug resistant malaria. ▪