Abstract
FOXP1 (Forkhead box-P1) is a winged-helix transcription factor that is differentially expressed in resting and activated B cells. FOXP1 expression has been demonstrated in a subset of diffuse large B-cell lymphomas (DLBCLs) and is more common in the nongerminal center (non-GC), activated B-cell type; however, its prognostic significance is uncertain. We analyzed presentation lymph nodes from 126 patients with nodal DLBCL, previously classified according to GC and BCL2 status, for FOXP1 protein expression using standard immunocytochemistry. Uniform high FOXP1 expression was demonstrated in 23 of 126 patients with DLBCL. This high level of expression was almost exclusively confined to patients who lacked the GC phenotype, who expressed MUM-1 and BCL2 in the absence of t(14; 18), and who were identified as a subgroup of patients with particularly poor outcomes in a group with already poor prognoses. The data presented suggest that high FOXP1 expression is an independent prognostic factor in DLBCL.
Introduction
FOXP1 (Forkhead box protein P1) is a member of the FOXP subfamily (FOXP1-4) of transcription factors, characterized by a common DNA-binding, winged-helix or forkhead domain, together with N-terminal zinc finger and leucine zipper domains. Spontaneous mutations in FOXP2 have been linked to speech disorders,1 whereas FOXP3 mutations cause X-linked autoimmunity-allergic dysregulation.2 Despite these findings, there is little information on how FOXP transcription factors mediate signaling and affect gene regulation. At least 4 FOXP1 isoforms have been characterized3 and have been shown to be expressed in the adult lung4 and brain5 and to be implicated in the development of the central nervous system.6 Unlike other FOX proteins, FOXP members require homodimerization and heterodimerization to bind DNA.7 FOXP1 has been shown to be expressed in normal activated B cells using genomic-scale expression profiling8 and in mantle zone and some germinal center (GC) B cells using immunohistochemistry.9 However, the physiologic role of FOXP1 in lymphocytes is unclear. Preliminary data have shown FOXP1 to be strongly overexpressed in a subset of diffuse large cell lymphomas (DLBCLs),9,10 suggesting a possible role in the pathogenesis of this group of tumors.
DLBCLs can be classified as a germinal center (GC) type, an activated B-cell (post-GC) type,11 and an undefined type 312 according to patterns of gene expression. This classification scheme has prognostic significance and can be replicated using immunohistochemical detection of CD10, BCL6,13 and MUM-114,15 to define GC status. Correlation of a microarray classification of DLBCL with immunohistochemistry showed that FOXP1 is predominantly expressed in non-GC DLBCL10,15 but that its prognostic significance is ambiguous. One study found no effect on outcome,15 but another found FOXP1 expression to be associated with inferior survival.16
This study examines FOXP1 protein expression in DLBCL and its relationship to immunophenotype. In addition, it demonstrates a correlation between strong nuclear positivity and poor prognosis in a subset of patients with BCL2-positive, t(14;18)-negative, non-GC DLBCL.
Study design
Presentation lymph nodes from 126 patients with previously untreated nodal DLBCL were examined. Mediastinal B-cell lymphoma, Burkitt lymphoma, DLBCL with evidence of underlying follicular lymphoma, anaplastic variants, and primary extranodal disease (stage IE or IIE) were excluded to ensure maximum uniformity of the patient cohort. All patients received anthracycline-containing combination chemotherapy with curative intent. This study had local ethics committee approval obtained from the Leeds Teaching Hospitals' NHS trust institutional review board. Informed consent was provided according to the Declaration of Helsinki, and treatment was not modified as a consequence of the study.
Patients had been previously classified according to GC and BCL2 status, using CD10 and BCL6 coexpression to define a GC phenotype and a 50% cutoff to define BCL2 positivity.13 Patients were defined as positive for MUM-1 when more than 30% of tumor cells expressed the nuclear protein.14 Paraffin sections measuring 3 μm were stained for FOXP1 protein using heat-mediated antigen retrieval and standard immunocytochemistry. The primary antibody was the mouse monoclonal antibody JC12 (isotype immunoglobulin G2a [IgG2a]). Reactive tonsil was used as a positive control.
Two independent assessors (S.L.B. and A.S.J.) classified patients with DLBCL into 3 groups according to FOXP1 expression, as follows: negative; weak expression in a variable proportion of tumor cells; uniform high expression in all tumor cells.
Statistical testing with χ2 analysis was used to examine relationships between variables. Overall survival (OS) time was the time from diagnosis until death or last follow-up. Survival curves were estimated by the Kaplan-Meier method, using log rank to analyze the differences between groups.
Results and discussion
Overall, the percentage of cells expressing FOXP1 ranged from 0% to 100%. In a significant subset of patients, however, uniform high FOXP1 expression was noted (n = 23; 18%). This level of expression was strikingly different from that observed in patients with normal B cells and other DLBCLs, in which FOXP1 expression was variable (n = 51) or absent (n = 52) (Figure 1). Classification using FOXP1 expression was highly reproducible, with 100% concordance between observers. The proportion of patients with high FOXP1 expression was lower than the percentages previously reported (61%15 and 40%16 ). However, in these studies, an arbitrary 30% cutoff was used to define positivity. Distinction between patients with high uniform expression was not considered, and some patients with variable/weak expression were included as positive. Twenty-one (91%) of 23 patients with high FOXP1 expression were non-GC (Pearson χ2, 10.335; P = .006), confirming previous findings.10,15 In addition, MUM-1 (IFR4) and BCL2 were positive in 21 (91%) of 23 patients with high FOXP1 (Pearson χ2, 23.720 and 18.093, respectively; P < .0001), and 11 (85%) of 13 patients with available results lacked a t(14;18) (Pearson χ2, 7.742; P = .021). The fact that uniform high expression of FOXP1 was confined to a specific subgroup of patients with non-GC DLBCL and BCL2 expression in the absence of a t(14;18) supported the stratification of patients in this way. Furthermore, patients with variable and negative expression showed equal distribution of all variables studied (data not shown), suggesting that these 2 groups represented a normal range of FOXP1 expression for GC and post-GC B cells.
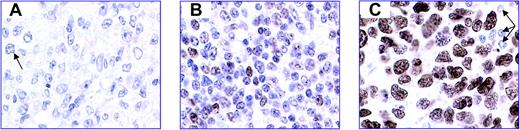
Representative images of FOXP1 immunostaining demonstrate the 3 groups of DLBCLs classified according to FOXP1 expression. Standard streptavidin ABC immunocytochemistry with heat-mediated antigen retrieval was used, with DAB as the chromogen. FOXP1 is visualized in brown. The blue nuclear counterstain is hematoxylin. (A) FOXP1 negative. Occasional cells have very weak expression of FOXP1 (arrow). (B) FOXP1 variable; weak positive. Approximately 50% of the cells express FOXP1 with variable intensity. (C) FOXP1 uniform high expression. One hundred percent of tumor cells show strong, uniform expression of FOXP1. Small lymphocytes are negative (arrows). All images were captured at 60 × magnification using an Olympus Bx50 microscope (Olympus, Tokyo, Japan), a Plan Apo 60×/1.40 oil objective lens (Tokyo, Japan), a Leica DFC-320 digital camera (Leica, Solms, Germany), and associated Leica IM50 software.
Representative images of FOXP1 immunostaining demonstrate the 3 groups of DLBCLs classified according to FOXP1 expression. Standard streptavidin ABC immunocytochemistry with heat-mediated antigen retrieval was used, with DAB as the chromogen. FOXP1 is visualized in brown. The blue nuclear counterstain is hematoxylin. (A) FOXP1 negative. Occasional cells have very weak expression of FOXP1 (arrow). (B) FOXP1 variable; weak positive. Approximately 50% of the cells express FOXP1 with variable intensity. (C) FOXP1 uniform high expression. One hundred percent of tumor cells show strong, uniform expression of FOXP1. Small lymphocytes are negative (arrows). All images were captured at 60 × magnification using an Olympus Bx50 microscope (Olympus, Tokyo, Japan), a Plan Apo 60×/1.40 oil objective lens (Tokyo, Japan), a Leica DFC-320 digital camera (Leica, Solms, Germany), and associated Leica IM50 software.
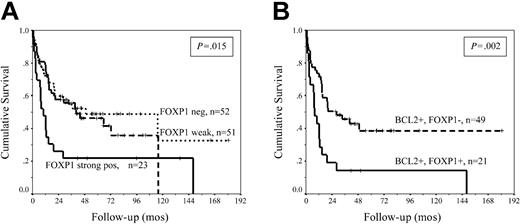
Kaplan-Meier analysis showed a significantly inferior median OS of 10 months in patients with high FOXP1 expression (n = 23), in contrast to patients with negative and variable expression whose median OS times were almost identical at 40 months and 49 months, respectively (P = .015) (Figure 2A; Table 1). This apparent discrepancy from previously published data15 may be attributed to different criteria in defining antigen expression. In our analysis, the poor prognostic effect was confined to patients with uniform high expression and was lost when a 30% cutoff was used. The outcome for patients with DLBCL of a non-GC phenotype and BCL2 expression is poor,13 but even within this poor prognostic subgroup, strong FOXP1 expression had an additive effect and reduced the median OS from 28 months to 8 months (Figure 2B; Table 1). There was no association between FOXP1 expression and the International Prognostic Index (IPI) score or any of its components (data not shown), suggesting that FOXP1 is an independent prognostic factor in DLBCL. Furthermore, strong FOXP1 expression retained its prognostic value in patients with low and high IPI score. In patients with a low IPI score (0-2) and variable/weak FOXP1, median OS was not reached (2-year survival, 76%), compared with the 15-month OS (2-year survival, 41%) in those with low IPI and strong FOXP1 expression (P = .04). Similarly, in the high IPI group (3-5), median OS was reduced from 10.3 to 2.3 months (P = .017) in patients in whom FOXP1 was strongly expressed. The prognostic effect of FOXP1 and the correlation with other biologic parameters was only seen in patients with high expression, suggesting that stratification according to FOXP1 defines a specific subgroup of patients with DLBCL. The high, uniform expression of the protein demonstrated in these patients also suggested that the FOXP1 gene may be deregulated and may play a key role in the pathogenesis and clinical behavior of DLBCL. The potential mechanism of deregulation is unknown, but translocation events upstream of other FOX gene family members have been reported as causing deregulated gene expression resulting in various pathologic conditions.17,18 Given that hypermutation of multiple loci, including immunoglobulin genes, BCL6, BCL2, and c-MYC,19 is frequently demonstrated in DLBCL, it is conceivable that mutational activation of FOXP1 may be an alternative mechanism of deregulation, but this requires further study.
Survival analysis of DLBCL patients stratified using FOXP1 expression. (A) Kaplan-Meier analysis of OS of 126 patients DLBCL stratified according to FOXP1 positivity. Patients with high FOXP1 expression had significantly inferior median OS compared with patients with negative and variable weak expression. (B) OS of 70 patients with DLCBL of a non-GC phenotype and BCL2 expression, stratified according to FOXP1 expression. High FOXP1 expression has an additive adverse prognostic effect and identifies a group of patients with very poor outcome, even within this already poor prognostic subgroup.
Survival analysis of DLBCL patients stratified using FOXP1 expression. (A) Kaplan-Meier analysis of OS of 126 patients DLBCL stratified according to FOXP1 positivity. Patients with high FOXP1 expression had significantly inferior median OS compared with patients with negative and variable weak expression. (B) OS of 70 patients with DLCBL of a non-GC phenotype and BCL2 expression, stratified according to FOXP1 expression. High FOXP1 expression has an additive adverse prognostic effect and identifies a group of patients with very poor outcome, even within this already poor prognostic subgroup.
In summary, uniform high expression of FOXP1 occurs in a subgroup of patients with non-GC DLBCL who express BCL2 in the absence of a t(14;18). Significantly, this appears to be an independent adverse prognostic factor in a group of patients with already poor prognoses.
Prepublished online as Blood First Edition Paper, July 6, 2004; DOI 10.1182/blood-2004-03-1209.
Supported by a grant from the United Kingdom Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.