Comment on Bayry et al, page 2441
DCs are highly specialized antigen-presenting cells that play a central role in initiating T-cell responses. DC–T-cell interactions lead to up-regulation of costimulatory molecules important for an effective T-cell activation.
Common variable immunodeficiency (CVID) is a heterogeneous immunodeficiency with characteristic recurrent bacterial infections due to hypogammaglobulinemia and incapacity to generate memory B cells.1 Patients further suffer from gastrointestinal infections, autoimmune disease, and various B-cell neoplasm, such as non-Hodgkin lymphoma. While no genetic cause has been found, CVID may be connected to immunoglobulin A (IgA) deficiency as members of the same family can have either disease, suggesting a genetic link. In addition to dysfunctional immunoglobulin production, patient's T-cell activation is impaired, resulting in low production of interleukin 2 (IL-2) and the T-helper 2 (Th2)–type cytokines IL-4, IL-5, and IL-10 and decreased T-cell proliferation. Levels of peripheral blood natural killer (NK) cells are also reduced, which in turn may affect tumor surveillance.
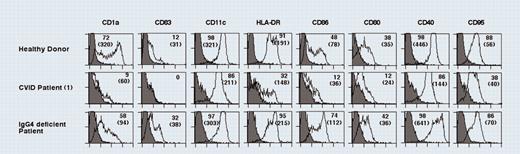
In the present issue of Blood, Bayry and colleagues make an interesting observation in that dendritic cells (DCs) from CVID patients have severely perturbed maturation. Although the total number of DCs appears normal as determined by CD11c expression, they express only nominal levels of CD1a, a hallmark of Langerhans cells, and completely fail to up-regulate CD83, typically expressed on fully mature DCs.2 Moreover, patient'sDC show only low levels of the accessory molecules CD80, CD86, and major histocompatibility complex (MHC) class II compared with healthy controls. In turn, this result in impaired T-cell proliferation and CVID-derived DCs produces virtually no IL-12 (p70) in response to CD40 ligand stimulation. Since DCs play a central role in T-cell activation, a failure of DCs to mature into fully stimulatory cells may provide a more general explanation for the various symptoms of CVID patients.
In an effort to find a molecular marker for CVID, a subgroup of patients has been found that lack inducible costimulatory molecule (ICOS), a costimulatory molecule expressed on T cells only after activation and present in particularly high levels on IL-10 –producing T cells.3 Interaction of ICOS with ICOS-L on B cells is a necessary requirement for their immunoglobulin isotype switching and differentiation into memory B cells. Failure of DCs from CVID patients to activate T cells would hence impair B-cell differentiation. Moreover, IL-10 specifically promotes B-cell isotype switch to the IgA subclass, and IgA in turn is typical of mucosal responses resulting in clearance of bacteria. Thus, lack of IL-10 –producing, ICOS-positive T cells may contribute to the recurrent intestinal bacterial infections, such as inflammatory bowel disease, of these patients.
In contrast to bacterial infections, CVID patients cope relatively well with viruses.1 This suggests that the plasmacytoid DC subset, which primarily responds to viral pathogens by the production of type I interferons, is unaffected. However, some reports exist on a correlation between CVID and an increased incidence of hepatitis C and herpes zoster infection.1 It would be interesting to determine whether the differences in susceptibility to virus are due to selective depletion or inactivation of one of the patient's DC subsets.FIG1
Dendritic cells from CVID patients display impaired differentiation. See the complete figure in the article beginning on page 2441.
Dendritic cells from CVID patients display impaired differentiation. See the complete figure in the article beginning on page 2441.
Although the present study does not reveal the reason for impaired DC maturation (genetic, developmental), the data suggests DCs as a common denominator for the multiple symptoms displayed in CVID. DC maturation stage could perhaps be used as a complementary marker in the clinical characterization of this disease. ▪