Abstract
Congenital thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS) is associated with an inherited von Willebrand factor-cleaving protease (ADAMTS13 [a disintegrin and metalloprotease with thrombospondin type I domains 13]) deficiency. In this study, we identified novel mutations in the ADAMTS13 gene in a patient with TTP. The patient was a 51-year-old Japanese male who exhibited TTP symptoms at frequent intervals. The ADAMTS13 activity during acute episodes was less than 3% that of normal. The enzyme activities of the patient's father and mother were both 46%, and both parents were asymptomatic. Genetic analysis revealed that the patient was a compound heterozygote for 2 mutations. One mutation was a missense mutation in the metalloprotease domain (A250V, exon 7), and the other was a guanine to adenine substitution at the 5′ end of intron 3 (intron 3 G→A). In vitro expression studies revealed that the A250V mutation markedly reduced ADAMTS13 activity and the intron 3 G→A mutation caused abnormal mRNA synthesis. (Blood. 2004;104: 2081-2083)
Introduction
ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type I domains 13) is a metalloprotease that specifically cleaves the multimeric von Willebrand factor (VWF).1-5 VWF is a large glycoprotein essential for high-shear stress-associated platelet adhesion and aggregation.6 VWF is synthesized in vascular endothelial cells7-9 and released into the plasma as unusually large multimeric forms (UL-VWFM).10 In the presence of excess amounts of UL-VWFM, the more effective platelet interaction with VWF multimers is thought to foster thrombus formation in the microcirculation.11
Severely deficient ADAMTS13 activity (less than 5% of that in normal plasma) caused either by mutations of the ADAMTS13 gene2,12-15 or by inhibitory antibodies against ADAMTS1316-18 is linked to thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS). In this article, we report 2 novel ADAMTS13 mutations in a Japanese patient with TTP who had reduced plasma ADAMTS13 activity. In vitro studies indicated that the 2 mutations affected the enzymatic activity and mRNA splicing, which would account for the patient's phenotype.
Study design
Patient
All experiments were performed with the permission of the ethics committees of both the sample-collecting hospital (Yamada Red Cross Hospital, Watarai-gun, Japan) and the gene-analyzing institute (Toyobo, Osaka, Japan and TAKARA BIO, Otsu, Japan). The patient was a 51-year-old Japanese male. He had a past history of thrombocytopenia of unknown etiology, but now it is impossible to identify whether he had such episodes during his early childhood. He developed convulsive seizures after surgery for hemorrhoids in March 2000. He was diagnosed with TTP because of progressing renal failure and decreased platelet counts. Fresh-frozen plasma (FFP) infusion was effective. After recovery, he was observed in an outpatient clinic with no maintenance FFP infusion. In March 2001, TTP symptoms again developed without any triggering episodes. He was treated with FFP for several consecutive days. After recovery, however, the patient stopped visiting the hospital. In August 2001, he was hospitalized because of the recurrence of TTP. No apparent triggering infection or trauma was documented for this episode. Laboratory data on admission were as follows: red blood cells (RBCs), 2.12 × 1012/L (212 × 104/μL); hemoglobin (Hb), 68 g/L (6.8 g/dL), white blood cells (WBCs), 3.6 × 109/L (3600/μL); platelets (PLTs), 70 × 109/L (7.0 × 104/μL); reticulocytes, 130 × 109/L (13 × 104/μL); lactate dehydrogenase (LDH), 414 U/L; total bilirubin (T-bilirubin), 37.62 μmol/L (2.2 mg/dL); blood urea nitrogen (BUN), 44 mg/dL; and creatinine, 335.92 μmol/L (3.8 mg/dL). FFP infusion was again effective, and the patient regularly received 4 units of FFP infusion every week at the outpatient clinic, by which hemolysis and thrombocytopenia were well controlled. In January 2002, he was hospitalized because of abdominal pain with gallstone disease. No apparent biliary tract infection was documented at this point, but cholecystectomy was performed to control pain. After surgery, thrombotic microangiopathy with severe thrombocytopenia developed, which was refractory to FFP infusion. Gastrointestinal bleeding occurred, and the patient died of renal failure in July 2002. The plasma ADAMTS13 activity of this patient during an acute episode was less than 3% of normal, and ADAMTS13 inhibitor was not detected. The ADAMTS13 activities of his parents were both 46%, and both were asymptomatic.
ADAMTS13 gene analysis
Human genomic DNA was isolated from peripheral leukocytes. The 29 exons and exon-intron boundary sites of the ADAMTS13 gene were analyzed using an ABI3100 sequencer (Applied Biosystems, Foster City, CA). The frequencies of newly discovered mutations were investigated using genomic DNA from the general Japanese population without a history of TTP/HUS.
Expression of recombinant ADAMTS13
ADAMTS13 cDNA (GenBank accession no. NM139025) was polymerase chain reaction (PCR) amplified from a human fetal liver cDNA library (BioChain Institute, Hayward, CA) and cloned into pcDNA3.1/myc-His (Invitrogen, Carlsbad, CA). Construction of mutants was performed using PCR-based mutagenesis. Each expression vector was transfected into HEK293 cells using FuGENE6 (Roche Molecular Biochemicals, Indianapolis, IN). After a 24-hour incubation, the medium was changed to serum-free OPTI-MEM I (Invitrogen), and the cells were cultured for 48 hours. The media were analyzed for protein expression with Western blot using anti-ADAMTS13 monoclonal antibodies.19 Wild-type (WT) and A250V mutant culture media were concentrated appropriately so that the 2 culture media had an equal concentration of ADAMTS13 protein. Assays of ADAMTS13 activity were performed using the method of Furlan et al16,20 with a slight modification as described previously.21
Minigene expression system
The exon 3, intron 3, and exon 4 of ADAMTS13 genomic DNA was cloned into pcDNA3.1 (Invitrogen). The vector was transfected into HEK293 cells. After 48 hours of incubation, we extracted total RNA and performed reverse transcription (RT)-PCR.
Results and discussion
Genetic analysis of the ADAMTS13 gene
We analyzed the ADAMTS13 gene in the patient with TTP and his mother. Two novel mutations, A250V and intron 3 G→A, were identified. A250V localizes in the metalloprotease domain, caused by a missense mutation in exon 7. Intron 3 G→A was a guanine to adenine substitution at the 5′ end of intron 3 in the ADAMTS13 gene. The patient was heterozygous for both mutations. His mother had the heterozygous intron 3 G→A mutation but did not have the A250V mutation. Genomic DNA of his father was not available. These results suggested that the patient was a compound heterozygote for A250V and intron 3 G→A. The frequencies of these mutations were investigated in the general Japanese population. Although 66 individuals were analyzed for A250V and 772 individuals were analyzed for intron 3 G→A, we found no individuals with these mutations.
Effect of A250V mutation on ADAMTS13 activity
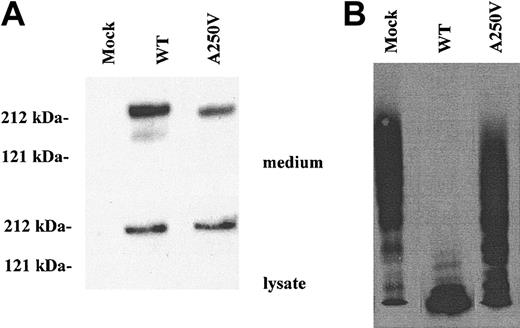
To determine the effect of the A250V mutation on ADAMTS13 activity, WT and A250V were expressed in HEK293 cells. Lysate of A250V-transfected cells contained basically the same amount of ADAMTS13 as WT (Figure 1A). On the other hand, in culture media, the amount of A250V antigen was less than that of WT, indicating that the mutation affected secretion or stability of the protein. After normalization of protein concentration for WT and A250V, enzymatic activities were measured. A250V had markedly reduced activity as compared with WT (Figure 1B). A250 localizes in the Zn2+ binding domain, which is highly conserved in ADAMTS families. In particular, the M249 residue is essential for Zn2+ binding.22,23 The A250V mutation might influence the structure of the Zn2+ binding domain, resulting in reduced protease activity.
Effect of the A250V mutation on ADAMTS13 activity. The wild-type (WT) and A250V mutant ADAMTS13 were expressed in HEK293 cells. (A) The culture media and cell lysate were analyzed by Western blot. The molecular sizes as judged by protein standards are indicated at the left. (B) After normalization of ADAMTS13 protein concentration, the culture media were analyzed for the enzymatic activity by measuring the degradation of VWF multimers. The A250V mutant had markedly reduced activity compared with wild type.
Effect of the A250V mutation on ADAMTS13 activity. The wild-type (WT) and A250V mutant ADAMTS13 were expressed in HEK293 cells. (A) The culture media and cell lysate were analyzed by Western blot. The molecular sizes as judged by protein standards are indicated at the left. (B) After normalization of ADAMTS13 protein concentration, the culture media were analyzed for the enzymatic activity by measuring the degradation of VWF multimers. The A250V mutant had markedly reduced activity compared with wild type.
Effect of intron 3 G→A mutation on RNA splicing
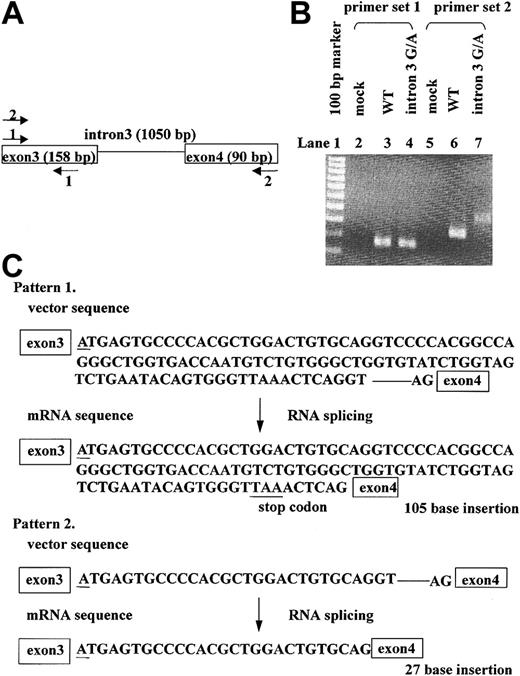
The intron 3 G→A mutation was found at the 5′ border of intron 3 in the ADAMTS13 gene. The guanine at this position is highly conserved in general exon-intron boundary sites, and most RNA splicing occurs due to recognition of the GT-AG motif at both ends of the intron. It was expected that this mutation would affect RNA splicing. We constructed the minigene expression system because mRNA of the patient was not available. The transcription products were analyzed by RT-PCR using 2 primer sets (Figure 2A). The WT-expressing cells produced normally spliced RNA, whereas the intron 3 G→A mutant produced splicing variants (Figure 2B). To further analyze the aberrantly spliced RNA products, RT-PCR products of intron 3 G→A mutant were subcloned into pDrive cloning vector (Qiagen, Valencia, CA), and 49 colonies were picked for sequence analysis. As a result, 2 splicing variants were detected (Figure 2C). One variant in 38 of 49 clones had a 105-nucleotide insertion containing an in-frame stop codon between exon 3 and exon 4, which would result in the synthesis of a truncated form that stops at the metalloprotease domain. The remaining 11 clones had a 27-bp insertion, which would lead to a 9-amino acid polypeptide-inserted phenotype.
The effect of the intron 3 G→A mutation on RNA splicing. To evaluate the effect of the intron 3 G→A mutation on RNA splicing, we transfected minigene expression vectors into HEK293 cells. Total RNA was purified, which was subjected to RT-PCR analysis. (A) Primer sets for the PCR analysis are shown. Set 1 amplified exon 3 (158 bp), whereas set 2 amplified exon 3 and exon 4 (248 bp). (B) Results of RT-PCR. Primer set 2 yielded the 248-bp band from WT-transfected cells (lane 6), and primer set 2 yielded different products from intron 3 G→A mutant-transfected cells (lane 7). (C) Sequence analysis of the transcript from intron 3 G→A mutant-transfected cells. Underlines indicate the position of the G→A mutation. Forty-nine clones were analyzed: 38 clones had a 105-bp insert, and 11 clones had a 27-bp insert. Each variant was spliced with recognition at the GT-AG motif.
The effect of the intron 3 G→A mutation on RNA splicing. To evaluate the effect of the intron 3 G→A mutation on RNA splicing, we transfected minigene expression vectors into HEK293 cells. Total RNA was purified, which was subjected to RT-PCR analysis. (A) Primer sets for the PCR analysis are shown. Set 1 amplified exon 3 (158 bp), whereas set 2 amplified exon 3 and exon 4 (248 bp). (B) Results of RT-PCR. Primer set 2 yielded the 248-bp band from WT-transfected cells (lane 6), and primer set 2 yielded different products from intron 3 G→A mutant-transfected cells (lane 7). (C) Sequence analysis of the transcript from intron 3 G→A mutant-transfected cells. Underlines indicate the position of the G→A mutation. Forty-nine clones were analyzed: 38 clones had a 105-bp insert, and 11 clones had a 27-bp insert. Each variant was spliced with recognition at the GT-AG motif.
These results indicate that A250V and intron 3 G→A mutations might be the cause of the decreased plasma ADAMTS13 activity in this patient, the former abolishing ADAMTS13 enzymatic activity and the latter impairing normal mRNA synthesis.
Prepublished online as Blood First Edition Paper, May 4, 2004; DOI 10.1182/blood-2004-02-0715.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.