Abstract
Familial hemophagocytic lymphohistiocytosis (FHL) is an inherited, fatal disorder of infancy. We report here a 17-day-old female infant who presented with high fever, hepatosplenomegaly, hypertriglyceridemia, hypofibrinogenemia, thrombocytopenia, and liver failure. Leukocytosis was detected with circulating “atypical” lymphoid cells. Flow cytometric studies revealed expanded subpopulations of CD8+ T cells with unusual immunophenotypic features, including a subset that lacked CD5 expression. A liver biopsy showed hemophagocytic lymphohistiocytosis with exuberant infiltrates of CD8+ T cells that lacked perforin. Mutational studies revealed a 666C→A (H222Q) missense mutation in the perforin gene. T-cell receptor studies on flow-sorted T-cell subpopulations revealed no evidence of monoclonality. Analysis of T-cell receptor excision circle levels indicated long proliferative history in the aberrant CD8+ T-cell subsets. This case provides an instructive example of uncontrolled reactive proliferation of CD8+ T cells in FHL, resulting in atypical morphology and unusual immunophenotypic features that might suggest malignancy in other clinical settings.
Introduction
Familial hemophagocytic lymphohistiocytosis (FHL), an inherited form of hemophagocytic lymphohistiocytosis (HLH), is a disorder of infancy that usually presents with acute illness characterized by fever, hepatosplenomegaly, cytopenias, hyperlipidemia, coagulopathy, and hemophagocytosis.1,2 Most patients with FHL have deficient natural killer (NK) and cytotoxic T-cell function associated with T-cell activation, impaired apoptosis, increased cytokine secretion, and activation of mononuclear phagocytes.1-3 An inherited mutation of the perforin gene underlies the pathophysiology of FHL in a subset of patients,4,5 and other patients might bear mutations in different cytotoxicity-associated genes.6 Deficient expression of perforin is associated with increased activation and expansion of cytotoxic T cells.7-9
The morphologic and immunophenotypic features of these T cells have not been characterized. Here, we report the case of a 17-day-old girl with liver failure who was suspected to have leukemia because of the presence of circulating atypical lymphoid cells. A liver biopsy revealed HLH associated with perforin deficiency. The case provides an instructive example of the morphologic, immunophenotypic, and molecular attributes of T cells that have undergone dysregulated activation and proliferation in the setting of FHL.
Study design
Patient presentation, clinical course, and laboratory studies
A 17-day-old female infant presented with high fever, hepatosplenomegaly, hematuria, and progressive liver failure. An older sibling had died following a similar presentation. There was no evidence of bacterial or viral infection, including cytomegalovirus (CMV) and Epstein-Barr virus (EBV). There was mild anemia (hemoglobin, 11.7 g/dL), thrombocytopenia (21 × 109/L), and a leukocytosis (26.9 × 109/L) composed of atypical lymphoid cells (54% of leukocyte differential). Blood was submitted for flow cytometric studies to evaluate for leukemia. The patient had hypertriglyceridemia (153 mg/dL) and hypofibrinogenemia (< 70 mg/dL). Her prothrombin time and partial thromboplastin time were both more than 114 seconds. Liver biopsy findings were consistent with FHL (described in “Results and discussion”). The patient suffered from postbiopsy blood loss and died.
Flow cytometric evaluation
Blood leukocytes were processed and analyzed as described previously.10 The following markers were evaluated: CD1a, CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD11b, CD14, CD15, CD16, CD19, CD20, CD22, CD25, CD33, CD34, CD36, CD38, CD45, CD45RA, CD45RO, CD56, CD57, CD61, CD64, CD79a, T-cell receptor (TCR)–αβ, TCR-γδ, kappa light chain, lambda light chain, myeloperoxidase, and terminal deoxynucleotidyl transferase (TdT).
Flow sorting
Leukocytes were stained for CD5, CD8, and CD4 and sorted into the indicated subsets with the use of the BD FACSVantage SE sorter (Becton Dickinson, San Jose, CA).11 After sorting, purity was more than 95% for each subpopulation.
TCR-γ gene rearrangement studies
DNA was extracted from blood leukocytes or flow-sorted T cells and used for TCR-γ polymerase chain reaction (PCR).12 The PCR product (2.0 μL) was added to 1.0 μL ROX500 molecular markers and 24 μL deionized formamide, denatured at 95° C for 5 minutes, and immediately cooled on ice. Analysis on an ABI310 Genetic Analyzer using GeneScan software (Applied Biosystems, Foster City, CA) followed.
Perforin gene mutational analysis
Sequencing of the perforin (PRF1) gene was performed for mutational analysis.4
T-cell receptor excision circle (TREC) levels
Photomicrography
Photomicrographs were obtained using an Olympus BX50 microscope equipped with an Olympus PM-C35DX camera (Olympus America, Melville, NY). Liver sections and Wright-stained blood smear micrographs were viewed with 50×/0.90 and 100×/1.30 oil objectives, respectively (Olympus, Tokyo, Japan). Kodachrome slides (35 mm; Eastman Kodak, Rochester, NY) were scanned in using Adobe Photoshop software (Adobe, San Jose, CA).
Results and discussion
Morphology and flow cytometry
The peripheral blood contained numerous medium-sized to large lymphoid cells that showed abundant basophilic cytoplasm with intense peripheral basophilia, oval to irregular nuclear contours, slightly dispersed chromatin, and occasional nucleoli (Figure 1A).
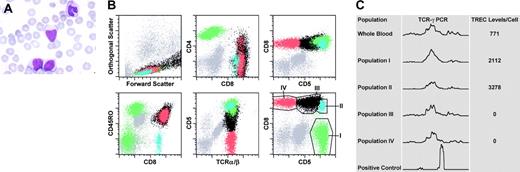
Blood findings: morphologic, flow cytometric, and molecular studies. (A) The morphologic appearance of the “atypical” lymphoid cells is shown in the peripheral blood of the patient (magnification × 1000). (B) Six representative dot plots from the flow cytometric studies are shown. The population in green (population I) represents CD4+ T lymphocytes. The 3 subpopulations of CD8+ T lymphocytes (populations II, III, and IV) are represented in blue, black, and red, respectively. The regions drawn around populations in the sixth dot plot approximate the electronic gates used for flow-sorting the corresponding subsets. (C) Shown are the results from the TCR-γ PCR reactions performed on genomic DNA extracted from either bulk blood leukocytes or sorted populations of T cells (as indicated). The bottom row indicates a positive control PCR reaction (monoclonal TCR). TREC levels (per cell) from the corresponding populations are also indicated in the same panel to the right of each tracing.
Blood findings: morphologic, flow cytometric, and molecular studies. (A) The morphologic appearance of the “atypical” lymphoid cells is shown in the peripheral blood of the patient (magnification × 1000). (B) Six representative dot plots from the flow cytometric studies are shown. The population in green (population I) represents CD4+ T lymphocytes. The 3 subpopulations of CD8+ T lymphocytes (populations II, III, and IV) are represented in blue, black, and red, respectively. The regions drawn around populations in the sixth dot plot approximate the electronic gates used for flow-sorting the corresponding subsets. (C) Shown are the results from the TCR-γ PCR reactions performed on genomic DNA extracted from either bulk blood leukocytes or sorted populations of T cells (as indicated). The bottom row indicates a positive control PCR reaction (monoclonal TCR). TREC levels (per cell) from the corresponding populations are also indicated in the same panel to the right of each tracing.
Flow cytometry (Figure 1B) revealed that 78% of events were mature, surface CD3+ T lymphocytes with a predominance of CD8+ T cells (CD4/CD8 ratio = 0.35:1). CD4+ T cells were composed of a CD45RO– subset (presumably naive) and an expanded CD45RO+ subset (memory/effector). CD8+ T cells were composed of 3 major subpopulations (blue, black, and red in Figure 1B) with a CD2+, CD7+, CD45bright+, CD25predominantly–, CD56predominantly–, TCR-αβ+, and TdT– immunophenotype. TCR-γδ+ T cells were also present and were distinct from these populations.
The smallest subpopulation (∼ 2.5% of total events; blue) was composed of small cells (forward light scatter characteristics) and exhibited a CD4–, HLA-DR–, CD45RO–, CD45RAbright+, CD5bright+ phenotype and most likely represented normal, naive CD8+ T cells. The other 2 subpopulations showed partial dim CD4 and slightly bright CD8 expression and were predominantly CD57–, uniformly CD45RO+, and CD45RAdim+to–, bearing some similarity to a previously described case.15 The population indicated in black (∼ 43% of total events) showed variable cell size with dim CD5, variable HLA-DR, and bright CD38 expression. The population indicated in red (∼ 12% of total events) was composed of small- to medium-sized HLA-DR+ cells that showed complete lack of CD5 expression. Thus, the CD8+ T cells consisted of a small proportion of naive T cells and 2 unusual expanded subpopulations with an “effector/memory phenotype” and underexpression of CD5.
Liver biopsy
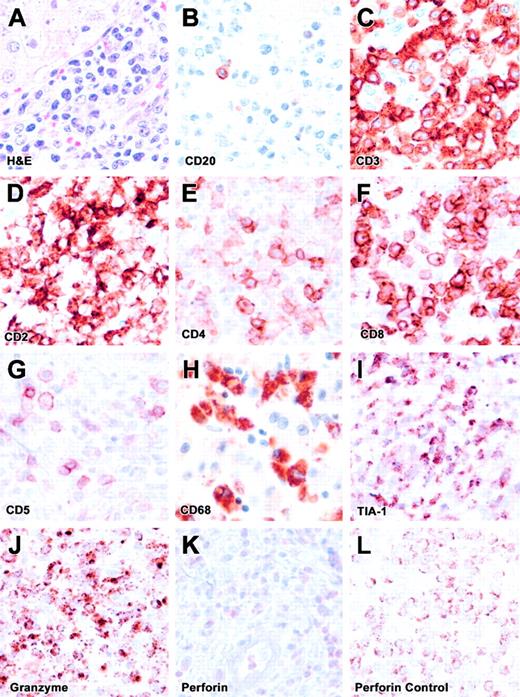
The liver biopsy showed an exuberant lymphocytic infiltrate within portal triads, with occasional foci within lobules. The infiltrates were composed predominantly of CD3+ T cells (Figure 2), including mainly CD8+ and a few CD4+ cells with a high degree of pleomorphism, enlarged nuclei, eosinophilic nucleoli, and abundant mitoses. Scattered macrophages were observed within sinusoids with occasional phagocytosis of neutrophils. Kupffer cell hyperplasia was noted on CD4 and CD68 staining. The infiltrating cells showed positive staining for granzyme B and T-cell intracellular antigen-1 (TIA-1). However, they were negative for perforin. Scattered cells showed weak diffuse cytoplasmic staining, which was distinct from the perforin staining pattern seen in positive controls.7 These findings are consistent with a diagnosis of FHL arising from a perforin mutation.7 Of interest, CD5 staining revealed few positive cells, likely corresponding to the CD4+ T cells. Thus, the infiltrating CD8+ T cells in the liver corresponded to the CD5dim+ or CD5– subpopulations identified in blood.
Liver biopsy. (A) The H&E-stained liver section is shown. (B-K) These panels represent immunohistochemical staining for CD20, CD3, CD2, CD4, CD8, CD5, CD68, TIA-1, granzyme B, and perforin. (L) Positive perforin staining is shown on a control section (spleen). All images are at × 500 magnification. The infiltrate is composed predominantly of CD8+ T cells with lack of perforin expression (FHL) and lack of CD5 expression, similar to flow cytometric findings.
Liver biopsy. (A) The H&E-stained liver section is shown. (B-K) These panels represent immunohistochemical staining for CD20, CD3, CD2, CD4, CD8, CD5, CD68, TIA-1, granzyme B, and perforin. (L) Positive perforin staining is shown on a control section (spleen). All images are at × 500 magnification. The infiltrate is composed predominantly of CD8+ T cells with lack of perforin expression (FHL) and lack of CD5 expression, similar to flow cytometric findings.
Clonality, proliferation history, and mutational analysis
To evaluate for potential monoclonality in the T cells, we flow-sorted 4 subpopulations (Figure 1B) and performed PCR reactions for TCR-γ rearrangement. All sorted populations and bulk blood leukocytes showed oligoclonal or polyclonal electrophoretograms (Figure 1C). Thus, despite the aberrant immunophenotype and atypical morphology, there was no evidence of a monoclonal or neoplastic lymphoproliferative disorder. This finding suggested a dysregulated reactive proliferation of CD8+ T cells in the setting of perforin deficiency, leading to expansions of phenotypically unusual subsets.
To test this hypothesis, we assessed proliferative history by measuring TRECs, which are episomal DNA fragments (excised from the TCR genes during thymic development) that are mitotically diluted during proliferation.13,14 As predicted, the highest TREC levels were observed in naive CD8+ T cells (population II). Importantly, there were no detectable TRECs in the 2 aberrant subpopulations (III and IV), confirming their prolonged proliferative history (Figure 1C).
To clarify the underlying genotype, we performed mutational analysis of the perforin gene4 and discovered a homozygous missense point mutation in exon 3 (666C→A [H222Q]). This point mutation was recently described in a study of North American families with HLH.16
This case is an example of FHL associated with perforin deficiency and dysregulated proliferation of CD8+ T cells, leading to atypical morphology and unusual immunophenotype. In other clinical settings (eg, in an adult patient), these findings would be suspicious for a clonal or malignant T-lineage proliferation. However, there was no evidence of monoclonality in any subpopulation, indicating that this was a reactive proliferation, probably driven by antigenic stimulation of CD8+ T cells with impaired cytotoxic and apoptotic functions.3,9,17
This case study provides several valuable clinical lessons. First, it depicts the wide spectrum of morphologic and immunophenotypic variability in reactive (nonmalignant) T cells. Second, it provides a detailed description of the morphology and immunophenotype of FHL-associated, dysregulated CD8+ T cells. In future studies, it might be worthwhile to test whether this phenotype is predictive of impaired cytotoxicity in the setting of HLH, thereby making it an indicator of focused diagnostic tests. Finally, this case re-emphasizes the need for a multimodal approach to the diagnosis of hematolymphoid disorders, in which data from morphology, immunophenotyping, and molecular or genetic tests contribute to the diagnostic process.
Prepublished online as Blood First Edition Paper, June 17, 2004; DOI 10.1182/blood-2004-04-1431.
Supported by US Public Health Service (USPHS) National Institutes of Health (NIH) grant (AI49990 to N.J.K.) and the University of Texas (UT) Southwestern President's Research Council Distinguished Young Investigator Award (N.J.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sterling Ortega and Bonnie Darnell for technical assistance.