Abstract
Upon recognition of nonpeptidic phosphoantigens, human Vδ2 T lymphocytes enter a lineage differentiation pattern that determines the generation of memory cells with a range of effector functions. Here, we show that within the effector memory Vδ2 population, 2 distinct and complementary subsets with regard to phenotype, mode of activation, and type of responses can be identified: Vδ2 TEMh cells, which express high levels of chemokine receptors, but low levels of perforin and of natural killer receptors (NKRs) and which produce large amounts of interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) in response to T-cell receptor (TCR)–specific stimulation by phosphoantigens; and Vδ2TEMRA cells, which constitutively express several NKRs, high amounts of perforin, but low levels of chemokine receptors and of IFN-γ. These NK-like cells are refractory to phosphoantigen but respond to activation via FcγRIII (CD16) and are highly active against tumoral target cells. Thus, circulating Vδ2T lymphocytes comprise 2 functionally diverse subsets of effector memory cells that may be discriminated on the basis of CD16 expression.
Introduction
Human Vδ2–T-cell receptor (TCR)+ lymphocytes constitute an unconventional lymphoid population. In striking contrast to “conventional” lymphocytes bearing the αβ TCR, Vδ2 T lymphocytes exhibit innate reactivity to major histocompatibility complex (MHC)–unrestricted microbial and tumoral nonpeptide antigens, which leads to proliferation, release of T-helper 1 (Th1) cytokines, and perforin-mediated killing.1-3 Most circulating Vδ2 cells are central memory and effector memory T cells,4,5 reflecting selective activation by common environmental antigens, such as phosphorylated metabolites. Expression of cell-surface receptors for chemokines6 and for MHC class I molecules7,8 on a large fraction of Vδ2 lymphocytes is also consistent with their memory phenotype.
Circulating naive αβ T cells use CD62L and CCR7 to bind high endothelial venules and to migrate to lymph nodes, respectively.9 They also express the CD45RA isoform and the costimulatory receptor CD27, but after primary antigen encounter surface expression of these markers is gradually switched off.10,11 On the other hand, experienced T cells comprise central memory (CD45RA–, CCR7+), effector memory (CD45RA–, CCR7–), and CD45RA+ effector memory cells (CD45RA+, CCR7–), which respectively produce increasing amounts of perforin.9,12-15 Likewise, whereas most Vγ9Vδ2 T lymphocytes from cord blood are naive (CD45RA+, CD27+), most of their mature counterparts in adult blood from healthy individuals have lost the CD45RA receptor.5 In addition, the effector functions of mature circulating Vδ2 T cells comprise a potent cytolytic activity mediated by the release of perforin,16 which is not produced by cord blood γδ T cells.17 CD45RA– CD27+ cells are abundant in lymph nodes and lack immediate effector functions. Conversely, memory CD45RA–CD27– and CD45RA+CD27– effector cells are poorly represented in lymph nodes but home in inflamed tissue and display immediate effector functions.18
Recent evidence has revealed that natural killer (NK) cells can be divided in 2 distinct subpopulations with unique effector attributes based on expression of CD16.19 This receptor binds immunoglobulin G (IgG) complexes to antigens and mediates phagocytosis, signal transduction, and antibody-dependent cellular cytotoxicity. Given the striking similarities between NK and γδ cells, we investigated whether a similar distinction applied also for the latter population. Indeed, by multiparameter phenotyping of blood Vδ2 T lymphocytes at the single-cell level using polychromatic flow cytometry, we define and characterize 2 different effector memory Vδ2 T-cell subsets on the basis of CD16 expression. We describe their distinct phenotypes, patterns of stimulation, and functional responses. Finally, we suggest that these cells represent 2 different pathways of maturation for circulating γδ T cells, which are discriminated by CD16 expression.
Materials and methods
Cell preparation and culture
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque gradient centrifugation (Pharmacia, Uppsala, Sweden) and cultured at 106 cells/mL in RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 2 mM l-glutamine, 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], and 10 U/mL penicillin and streptomycin (Life Technologies, Grand Island, NY).
Freshly collected PBMCs comprising 1% to 3% of TCRVγ9Vδ2+ cells were cultured in bulk (106 cells/mL) in complete culture medium supplemented with phosphoantigen (BrHPP; 200 nM; Innate Pharma, Marseilles, France) and interleukin 2 (IL-2; 100-400 U/mL; Sanofi-Synthélabo, Labège, France) for 2 to 4 weeks as described.20 On average, the cell lines comprised more than 95% TCRVγ9Vδ2+ after 3 weeks of culture, and were used without further separation procedure in the specified analysis.
Highly purified Vδ2 TEMh and Vδ2 TEMRA cell subsets were sorted by polychromatic flow cytometry on the MoFlo (DakoCytomation, Glostrup, Denmark). Vδ2 T-cell clones were obtained by single-cell sorting, and maintained in culture as previously described.21
Intracellular staining
Freshly isolated PBMCs were stimulated with 10 nM BrHPP and treated with brefeldin A (10 μM; Sigma-Aldrich, St Louis, MO). After 6 hours of stimulation, cells were stained following standard procedures and analyzed on the MoFlo cytometer. Results are expressed as percent of Vδ2+ IFNγ+ T cells on total Vδ2 T lymphocytes.
Flow cytometry
Seven-color fluorescence activated cell sorting (FACS) analysis was performed on a DakoCytomation MoFlo cytometer, and 5-color FACS analysis was performed on a Coulter F-500 cytometer. Data were compensated and analyzed using FlowJo software (TreeStar, Ashland, OR). In one set of experiments data were collected and acquired using RXP software (Beckman, Coulter, Fullerton, CA). PBMCs were isolated from blood samples according to standard procedures. For each staining 1 × 106 cells were used. Monoclonal antibodies (conjugated with the appropriate fluorochrome) were added at previously defined optimal concentrations. The following antibodies were used: CD3 ECD, CD62L ECD, CD27 PC5, CD16 phycoerythrin (PE)–Cy7, NKG2A PE, CD158.1 PE, CD158.2 PE, CD45RA ECD, and streptavidin PE-Cy7 from Beckman Coulter; Vδ2 fluorescein isothiocyanate (FITC) and PE, biotinylated Vδ2, CD45RA FITC, PE, and allophycocyanin (APC), CD94 FITC, NKAT2 FITC, CCR6 PE, CCR5 FITC, CXCR3 FITC, CD16 PE-Cy7, and CyChrome, perforin FITC, and interferon-γ (IFN-γ) APC from BD Pharmingen (San Diego, CA); CD27 FITC and CD62L APC-Cy7 from Caltag (Burlingame, CA).
For functional studies cells were sorted at high speed (> 30 000 cells/second) on the MoFlo.
PhosphoERK immunoblots
Highly purified (> 95%) Vδ2+ T cells and clones were stimulated with 20 nM BrHPP at 37° C at 4 time points (5, 15, 30, and 60 minutes). Stimulation through the Fc receptor was achieved by staining cells with purified α-CD16 (Pharmingen) for 30 minutes on ice, and after washing off unbound antibody (Ab), cross-linking with goat anitmouse (GAM; Southern Biotechnologies, Birmingham, AL; 1.5 μg/106 cells) for 5 minutes at 37° C. Following stimulation samples were lysed in sodium dodecyl sulfate (SDS) sample buffer. Analysis of protein components was performed on 10% polyacrylamide gels, loading the same volume of total lysate for each experimental condition. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences, Piscataway, NJ) and blots were probed overnight at 4° C with antiphospho-p44/42 mitogen activated protein (MAP) kinase (Cell Signaling, Beverly, MA), followed by horseradish peroxidase–coupled secondary antibody (Cell Signaling). Samples were analyzed using ECL Plus Western Blotting Detection Reagent (Amersham Biosciences). Quantification was performed by Kodak Image Station (KDS IS440CF 1.1; Kodak, Rochester, NY).
Extracellular cytokine release
Vδ2 T-cell clones were plated at 1 × 105 cells/well and stimulated with 100 nM BrHPP or plate bound with purified anti-CD16 antibody (10 μg/mL), anti–HLA-I (10 μg/mL) in the presence of 50 U/mL IL-2 (Roche Diagnostic, Milan, Italy). The samples were maintained at 37° C for 24 hours and then supernatants were harvested. The presence of IFN-γ was determined by a standard 2-site sandwich enzyme-linked immunosorbent assay (ELISA). Abs for IFN-γ were purchased from Pharmingen. Enhanced protein-binding ELISA plates (Nunc Maxisorb; Nunc Maxi, Roskilde, Denmark) were used.
Polychromatic synaptic transfer analysis
The Daudi lymphoma and HT29 colon carcinoma cell targets were labeled by PKH67 (Sigma-Aldrich) as described,22,23 rinsed, and added at a 5:1 cell ratio to whole PBMCs (600 000 cells per well). The 2 groups were mixed together in complete RPMI culture medium, pelleted by centrifugation (1 minute, 150 g) and left for 1 hour at 37° C in CO2 incubator for synaptic transfer. Cells were subsequently dissociated by washing with phosphate-buffered saline (PBS) containing 0.5 mM EDTA [ethylenediaminetetraacetic acid], and PBMC subsets were revealed by staining for 20 minutes at 4° C with the following monoclonal antibody (mAb) mix: TCRVδ2-PE-Cy7, CD16-Cy, CD27-APC, CD62L-ECD, CD45RA-PE. Using a polychromatic cytometric (MoFlo) analysis, after gating out of the PKH67bright tumoral targets, the following subsets were gated: Vδ2+ noneffectors (TCRVδ2+, CD62L+, CD27+, CD16–), Vδ2TEMh (TCRVδ2+, CD62L–, CD27–, CD45RA–, CD16–) and Vδ2 TEMRA (TCRVδ2+, CD62L–, CD27–, CD45RA+, CD16+). Synaptic transfer on each subset was then analyzed by comparing the mean fluorescence intensity for labeling by PKH67 at time 0 minutes and time 60 minutes of coculture.22,23
Cytotoxicity assay
Highly purified (> 95%) NK cells, Vδ2 TEMRA cells, and Vδ2 TEMh cells were sorted by high-speed cell sorting (MoFlo; DakoCytomation) from freshly isolated PBMCs. These cell subsets were used for cytotoxicity assays, as previously described,24 using Daudi cells as target cells. Briefly, target cells were labeled with 250 nM carboxyfluorescein diacetate succinimydyl ester (CFSE; Molecular Probes, Eugene, OR). The effector population were seeded with a constant number of CFSE-labeled Daudi cells (100 000) at different effector-to-target (E/T) ratios (from 10:1 to 0.3:1), and incubated for 4 hours in a 5% CO2 atmosphere at 37° C. In parallel, target cells were incubated alone to measure basal apoptosis. Cell mixture was then labeled with 20 μg/mL 7-aminoactinomycin D (7-AAD; Sigma-Aldrich) and analyzed by flow cytometry.
Results
Phenotypic and functional definition of Vγ9Vδ2 lymphocyte subsets
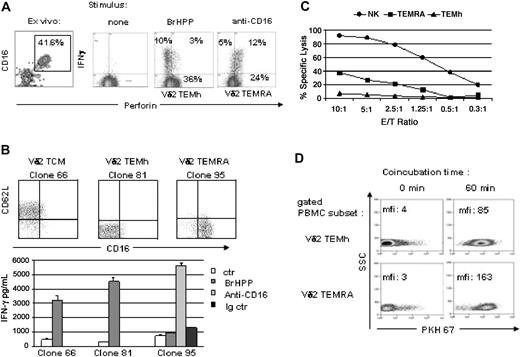
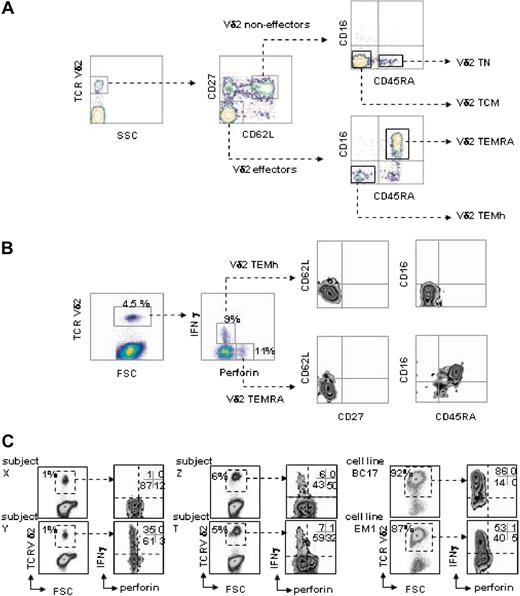
Effector memory subsets of human Vγ9Vδ2 T lymphocytes in the blood from healthy donors were defined by direct staining of freshly collected PBMCs with a panel of fluorophore-conjugated monoclonal antibodies and polychromatic flow cytometry. The panel comprised antibodies directed against TCRVδ2, CD27, CD62L, CD45RA, and CD16. All blood samples comprised 4 discrete Vδ2 subpopulations defined by the following pheno-types:18 naive (Vδ2 TN): TCRVδ2+ CD62L+ CD27+ CD45RA+ CD16–; central memory (Vδ2 TCM): TCRVδ2+ CD62L+ CD27+ CD45RA– CD16–; effector memory (Vδ2 TEMh): TCRVδ2+ CD62L– CD27–CD45RA– CD16–; and (CD45)RA+ effector memory (Vδ2 TEMRA): TCRVδ2+ CD62L– CD27–CD45RA+ CD16+ (Figure 1A). While the Vδ2TN and Vδ2TCM correspond to noneffector cells,5,18 the Vδ2 TEMh and Vδ2 TEMRA subsets display immediate effector functions. A variable percentage of CD27+CD62L– cells was found in all donors tested. This subset likely represents an intermediate stage between central memory cells and effector cells, as CD62L is rapidly down-regulated upon antigenic stimulation, whereas CD27 is down-regulated at a slower rate (data not shown). PBMCs cultured for 6 hours with the phosphoantigen BrHPP were labeled for intracellular perforin and IFN-γ in addition to the 5 surface markers previously described. As shown in Figure 1B, within Vδ2+ T cells, several subpopulations could be identified based on expression of perforin and production of IFN-γ: noneffectors, (perforin–IFN-γ–) and effectors that produce either perforin or IFN-γ. Progressive gating on each group for multiparametric analysis of surface marker expression confirmed that both TCRVδ2+ CD62L+ CD27+ CD45RA+ CD16– and TCRVδ2+ CD62L+ CD27+ CD45RA– CD16– subsets are noneffectors (not shown). By contrast, the TCRVδ2+ CD62L– CD27–CD45RA– CD16– cells were perforinlow and exploited IFN-γhigh responses, whereas the TCRVδ2+ CD62L– CD27–CD45RA+ CD16+ cells were perforinhigh but did not produce IFN-γ; these subsets were referred to as Vδ2TEMh and Vδ2 TEMRA, respectively (Figure 1B). Healthy individuals (n = 26) presented highly variable frequencies of both subsets (1-40 × 10–4 Vδ2 TEMh and 3-300 × 10–4 Vδ2 TEMRA; Figure 1C) while Vδ2 T-cell lines maintained in vitro with phosphoantigen and IL-2 prominently comprised Vδ2 TEMh cells (80% Vδ2 TEMh and 0-4% Vδ2 TEMRA; Figure 1C). Thus, by phenotypic and functional analogy with differentiation stages of αβ T lymphocytes,25 circulating human Vδ2 T lymphocytes comprise variable frequencies of naive and central memory noneffector cells, together with 2 effector memory subsets composed of helpers and cytotoxic cells.
Polychromatic profiling of the Vδ2 T-cell subsets. (A) 5-color flow cytometry discriminates between different subsets of naive and memory Vδ2 T cells from human PBMCs. Sequential gating using morphology and TCR expression defines noneffector and effector Vδ2+ cell subsets according to CD27 and CD62L expression, which are respectively subdivided according to expression of CD45RA and CD16. (B) After 6 hours of culture of PBMCs with phosphoantigen, intracellular staining for perforin and IFN-γ followed by 7-color flow cytometry as described in “Materials and methods” defines 2 functionally distinct subsets of effector memory Vδ2 T cells by showing that Vδ2TEMh are IFN-γ–producing lymphocytes with the TCRVδ2+CD27– CD62L–CD45RA– CD16– phenotype, whereas Vδ2TEMRA are perforin-producing lymphocytes with the TCRVδ2+CD27– CD62L–CD45RA+CD16+ phenotype. (C) Frequency of Vδ2TEMh and Vδ2TEMRA cells in PBMCs from 4 representative healthy donors and 2 cultured cell lines.
Polychromatic profiling of the Vδ2 T-cell subsets. (A) 5-color flow cytometry discriminates between different subsets of naive and memory Vδ2 T cells from human PBMCs. Sequential gating using morphology and TCR expression defines noneffector and effector Vδ2+ cell subsets according to CD27 and CD62L expression, which are respectively subdivided according to expression of CD45RA and CD16. (B) After 6 hours of culture of PBMCs with phosphoantigen, intracellular staining for perforin and IFN-γ followed by 7-color flow cytometry as described in “Materials and methods” defines 2 functionally distinct subsets of effector memory Vδ2 T cells by showing that Vδ2TEMh are IFN-γ–producing lymphocytes with the TCRVδ2+CD27– CD62L–CD45RA– CD16– phenotype, whereas Vδ2TEMRA are perforin-producing lymphocytes with the TCRVδ2+CD27– CD62L–CD45RA+CD16+ phenotype. (C) Frequency of Vδ2TEMh and Vδ2TEMRA cells in PBMCs from 4 representative healthy donors and 2 cultured cell lines.
Differential expression of NK and chemokine receptors on Vδ2 T-cell clones
The coexistence of 2 distinct effector subsets of memory Vδ2 T lymphocytes was confirmed by flow cytometry on isolated clones in culture. Vδ2 TEMh are perforinlow and do not express NK receptors such as CD16, CD158, and NKAT2, express low levels of NKG2A, CD94, but high levels of the chemokine receptors CXCR3, CCR5, and CCR6, thereby resembling conventional Th1 cells.6,26 On the contrary, Vδ2 TEMRA cells display an almost complementary phenotypic pattern, including high levels of perforin, several NK receptors (CD16, CD94, NKG2A, CD158, NKAT2), but low levels of chemokine receptors (CXCR3, CCR5, and CCR6; Figure 2A). Thus, the Vδ2 TEMRA phenotype depicts cytotoxic NK-like γδ T cells. Moreover, to assess induction or modulation of these receptors we stimulated Vδ2 cell clones with phosphoantigen and we measured the expression levels. Vδ2+ TEMh clones down-regulated chemokine receptor expression in response to phosphoantigens, as shown previously,27 whereas the Vδ2+ TEMRA cells showed a stable chemokine expression confirming the unresponsiveness of this subset of γδ T cells to stimulation through the TCR (Figure S1; see the Supplemental Figures link at the top of the online article on the Blood website).
Phenotype and activation patterns of Vδ2TEMhand Vδ2TEMRAlymphocytes. (A) NKR and chemokine receptors of representative TEMh (top panels) and TEMRA (bottom panels) TCR Vδ2+ clones. Cultured Vδ2 T-cell clones are characterized as Vδ2TEMh on the basis of their CD16–CD45RA– perforinlow pheno-type, or as Vδ2TEMRA when they are CD16+CD45RA+ perforinhigh (mean fluorescence intensity [MFI] for intracellular perforin is given). (B) Vδ2TEMh and Vδ2TEMRA PBMCs freshly drawn from a healthy donor with different levels of cell surface TCR Vδ2. (C) PhosphoERK immunoblots of lysates from Vδ2high and Vδ2low cells (5 × 105 cells/point). After sorting using gates shown in panel B, in vitro activation of the collected cells, and production of their lysates, the p-Erk immunoblots reveal a higher response to BrHPP in Vδ2TEMh than in Vδ2TEMRA, which respond better to stimulation with anti-CD16. The data are representative of 2 independent experiments on different donors.
Phenotype and activation patterns of Vδ2TEMhand Vδ2TEMRAlymphocytes. (A) NKR and chemokine receptors of representative TEMh (top panels) and TEMRA (bottom panels) TCR Vδ2+ clones. Cultured Vδ2 T-cell clones are characterized as Vδ2TEMh on the basis of their CD16–CD45RA– perforinlow pheno-type, or as Vδ2TEMRA when they are CD16+CD45RA+ perforinhigh (mean fluorescence intensity [MFI] for intracellular perforin is given). (B) Vδ2TEMh and Vδ2TEMRA PBMCs freshly drawn from a healthy donor with different levels of cell surface TCR Vδ2. (C) PhosphoERK immunoblots of lysates from Vδ2high and Vδ2low cells (5 × 105 cells/point). After sorting using gates shown in panel B, in vitro activation of the collected cells, and production of their lysates, the p-Erk immunoblots reveal a higher response to BrHPP in Vδ2TEMh than in Vδ2TEMRA, which respond better to stimulation with anti-CD16. The data are representative of 2 independent experiments on different donors.
TEMRA and TEMh have distinct patterns of activation and responses
Occasionally, flow cytometric analysis on freshly isolated PBMCs from healthy donors reveals the presence of 2 subsets of Vδ2 T cells with distinct levels of TCR expression. Multiparametric analysis performed on such samples revealed that TCR Vδ2high T cells are Vδ2 TEMh cells (CD45RA–CD16–), whereas the TCR Vδ2low T cells are Vδ2TEMRA cells (CD45RA+CD16+; Figure 2B). TCR Vδ2low (TEMRA) and Vδ2high (TEMh) from this donor were sorted, and functional comparison confirmed that these 2 subsets have distinct patterns of activation and responses (Figure 2C). In agreement with their higher surface expression of the phosphoantigen-specific TCR Vδ2, a stronger ERK signaling is induced in Vδ2 TEMh than in Vδ2TEMRA cells by BrHPP. Although still responding weakly to the phosphoantigen, Vδ2 TEMRA lymphocytes strongly induce their ERK signaling pathway upon the NK-like activation through FcγIII-R ligation with anti-CD16 antibody (Figure 2C). Level of expression of the Vδ2 TCR was stable in culture in both Vδ2+ TEMRA and Vδ2+ TEMh, and the differences in ERK1/2 phosphorylation were even more striking compared with those observed on freshly isolated Vδ2+ cells (Figure S2).
Accordingly, cytometric analysis from donors with large numbers of Vδ2TEMRA (Figure 3A, representative sample) showed that intracellular production of IFN-γ (and of TNF-α, data not shown) is induced by BrHPP stimulation in Vδ2TEMh but not in Vδ2TEMRA cells, whereas conversely Vδ2 TEMRA are able to produce IFN-γ following triggering through CD16. These data obtained ex vivo from PBMCs by intracellular staining were confirmed using Vδ2 T-cell clones of Vδ2 TCM, Vδ2 TEMh, or Vδ2 TEMRA in cultures stimulated with BrHPP: phosphoantigen induced the release of IFN-γ by Vδ2 TEMh cells only (Figure 3B). In agreement with results obtained with PBMCs (Figure 3A), Vδ2TEMRA cells do not produce IFN-γ in response to BrHPP but do so following cross-linking of CD16 (Figure 3B). Furthermore, we compared the cytotoxic activity of the 2 different subsets of Vδ2 effector cells by challenging Vδ2 TEMh, Vδ2 TEMRA, and NK cell clones with a tumoral cell target (Daudi). The results showed a strong lytic activity exerted by the NK cells (up to 90%), an “intermediate” lytic activity of Vδ2 TEMRA cells (up to 40%), and no cytotoxic effect by Vδ2TEMh cells. It is of note that in these experiments we analyzed spontaneous cytotoxic activity of the lymphocyte subsets, while generally γδ T-cell cytotoxicity is measured following cellular activation, and is in any case not as vigorous as we describe here.
Different functional responses of Vδ2TEMhand Vδ2TEMRAlymphocytes. (A) Cytometric panels refer to a representative healthy donor. Left panel shows cells gated for Vδ2 expressing double staining for CD16 and perforin, indicating that both CD16+perforin+ and CD16–perforin– effector cells were equally present in the chosen individual. Freshly isolated PBMCs were stimulated with BrHPP or anti-CD16 for 6 hours and intracellular staining for IFN-γ was performed (right panels). (B) Vδ2TCM,TEMh, and TEMRA T-cell clones were obtained as previously described.28 The cells were then stimulated with BrHPP (100 nM) or with anti-CD16 (10 μg/mL). Supernatants were harvested after 24 hours and IFN-γ was measured by ELISA. Anti-HLAI was used as control. The data are representative of 3 independent ELISA experiments on different donors. Data represent means ± SD. (C) NK cell, Vδ2TEMh, and TEMRA T-cell subsets, freshly isolated from a healthy donor, were used for cytotoxic assay against Daudi cells. The graph shows a strong lytic activity exerted by the NK cells (up to 90%), an “intermediate” lytic activity of Vδ2TEMRA cells (up to 40%), and no cytotoxic effect by Vδ2TEMh cells. The data are representative of 2 independent experiments. (D) Synaptic transfer by Vδ2TEMh and Vδ2TEMRA lymphocytes from bulk PBMCs coincubated for 1 hour with PKH67-labeled Daudi target cells. Using FL1 for PKH67 detection, PKH67+ Daudi cells were gated out, the Vδ2 cell subsets were defined by 5-color flow cytometry as in Figure 1, after simultaneous labeling for surface TCRVδ2, CD27, CD62L, CD45RA, and CD16. Numbers give the PKH67 MFI of the specified cells prior to and after coincubation.
Different functional responses of Vδ2TEMhand Vδ2TEMRAlymphocytes. (A) Cytometric panels refer to a representative healthy donor. Left panel shows cells gated for Vδ2 expressing double staining for CD16 and perforin, indicating that both CD16+perforin+ and CD16–perforin– effector cells were equally present in the chosen individual. Freshly isolated PBMCs were stimulated with BrHPP or anti-CD16 for 6 hours and intracellular staining for IFN-γ was performed (right panels). (B) Vδ2TCM,TEMh, and TEMRA T-cell clones were obtained as previously described.28 The cells were then stimulated with BrHPP (100 nM) or with anti-CD16 (10 μg/mL). Supernatants were harvested after 24 hours and IFN-γ was measured by ELISA. Anti-HLAI was used as control. The data are representative of 3 independent ELISA experiments on different donors. Data represent means ± SD. (C) NK cell, Vδ2TEMh, and TEMRA T-cell subsets, freshly isolated from a healthy donor, were used for cytotoxic assay against Daudi cells. The graph shows a strong lytic activity exerted by the NK cells (up to 90%), an “intermediate” lytic activity of Vδ2TEMRA cells (up to 40%), and no cytotoxic effect by Vδ2TEMh cells. The data are representative of 2 independent experiments. (D) Synaptic transfer by Vδ2TEMh and Vδ2TEMRA lymphocytes from bulk PBMCs coincubated for 1 hour with PKH67-labeled Daudi target cells. Using FL1 for PKH67 detection, PKH67+ Daudi cells were gated out, the Vδ2 cell subsets were defined by 5-color flow cytometry as in Figure 1, after simultaneous labeling for surface TCRVδ2, CD27, CD62L, CD45RA, and CD16. Numbers give the PKH67 MFI of the specified cells prior to and after coincubation.
Engagement of target cells
To confirm at the single-cell level the cytotoxic activity of the different subsets of effector Vδ2 lymphocytes, we took advantage of their ability to engage cell targets prior to lethal hit delivery. Since engagement of target cells through the immunologic synapses of Vδ2 lymphocytes,20 of NK cells22 and of CD8 αβ T lymphocytes23 leads to synaptic transfer, we used this assay with bulk PBMCs to compare at the single-cell level the ability of Vδ2 TEMh and Vδ2 TEMRA cells to engage cell targets. Vδ2 TEMh appear to engage Daudi cells (Figure 3D), but to a similar extent as the noneffector Vδ2 TN and Vδ2 TCM counterparts (not shown). Of all Vδ2 PBMC subsets, the Vδ2 TEMRA subset is the most active in establishing synapses with cancer cell targets such as the Daudi Burkitt lymphoma cell line (Figure 3D) or with HT29 colorectal cancer (data not shown).
Together, these features uncover 2 distinct subsets of effector/memory Vδ2 T cells with different antigen recognition pathways, phenotype, and functional capabilities.
Discussion
Vδ2 T cells constitute an abundant reservoir of antitumoral and anti-infectious effectors. The specific and straightforward targeting of Vγ9Vδ2 T lymphocytes by phosphoantigens, alkylamines, and aminobiphosphonates makes these cells particularly attractive for anticancer immunotherapies28 or anti-infectious vaccines.29 Thus, it is crucial to define precisely how specific activation by synthetic phosphoantigens may be exploited to tune this response. Here we show that, similar to αβ T lymphocytes, functional heterogeneity of memory cells also applies to the Vδ2 T-cell subset. Expression of CD27 and CD45RA, migratory routes, and effector functions define 4 successive differentiation steps for Vδ2 T cells.18 The complete phenotypic and functional characterization of these effector memory subsets by polychromatic flow cytometry discloses differential responses to phosphoantigens. Vδ2 human effector memory T cells comprise TEMh and TEMRA cells. The former, composed of TCR-specific, phosphoantigen-responsive helper cells is overrepresented in phosphoantigen-driven cell lines and clones in culture and corresponds to the most extensively studied Vδ2 T cells. On the other hand are the formerly described highly cytotoxic NK-like γδ T lymphocytes,21,30,31 characterized further in this study as phosphoantigen-unresponsive Vδ2 TEMRA cells and corresponding to the CD16+ γδ lymphocytes depicted elsewhere.32
By analogy with CD8 αβ TEMRA cells15,33 and considering the shorter telomeres of Vδ2 TEM cells,18 it is conceivable that Vδ2 TEMRA cells correspond to a late stage of Vδ2 differentiation. Strongly suggestive of a progressive selection for the fittest effectors,25 differentiation into Vδ2 TEMRA produces the most highly active antitumoral effectors among Vδ2 T cells. Given the negative influence of phosphoantigens on the in vitro induction of Vδ2 TEMRA cells from whole PBMCs demonstrated by this study, their presence in vivo most probably reflects a prolonged absence of stimulation by microbial phosphoantigens, as proposed for αβ TEMRA cells.33 Indeed, the pool of proliferating noneffector γδ cells must generate either TCR-dependent helpers or NK-like cytolytic effectors by some as-yet-incompletely defined terminal maturation switch. While phosphoantigen clearly drives proliferation of precursor pools18 and maturation of Vδ2 TEMh (as seen within cultured Vδ2 T-cell lines in Figure 1C and for Vδ2 PBMCs; Figure 3A), the antigen-independent generation of Vδ2 TEMRA cells remains enigmatic. Several reports have described appearance of αβ CD8 TEMRA lymphocytes following viral infections.14,34 Likewise, the frequency ex vivo of Vδ2TEMRA cells is usually very low in healthy donors18 and often appears elevated in several pathologic conditions (D.F.A. et al, unpublished observations, December 2003). However, some healthy donors having a basal Vδ2 T-cell number higher than 5% of total PBMCs may express a larger fraction of Vδ2 TEMRA probably as the consequence of subclinical infections or exposure to environmental pathogens. These Vδ2 TEMRA cells were shown to represent the main Vδ2 T-cell subset present in ascite and cerebrospinal fluids of tuberculosis patients,18 confirming the migratory capability of these end-stage effectors.
Other nonpeptide antigens than phosphoantigens, which selectively activate Vδ2 T lymphocytes, have been described, including therapeutic aminobisphosphonates35 and alkylamines derived from microbes and edible plants.36 However, these compounds appear unlikely triggers for Vδ2 TEMRA cells, since specificity for these ligands has been convincingly assigned to reactive Vδ2 TCR rather than to expression of other cell-surface receptors such as CD16. In addition, recent clinical investigations have documented the Th1-type in vivo response of human Vδ2 lymphocytes to aminobisphosphonates,29,37,38 and to an alkylamine-rich diet.39
On the other hand, we have occasionally found individuals whose Vδ2 T cells express different levels of cell-surface TCR. It is well established that surface expression of the TCR correlates to the developmental and activation status of the cell, and that its regulation affects T-cell function.39 Following encounter with antigen, the TCR is quickly internalized from the cell surface,40-43 and depending on the strength of the stimulation, T cells can become unresponsive to subsequent antigenic challenges. Indeed, while Vδ2high cells were responsive to stimulation with BrHPP, Vδ2low cells displayed low reactivity. The striking phenotypic differences between Vδ2high and Vδ2low cells tempt us to suggest that the latter are the result of a vigorous antigenic stimulation ex vivo, maybe following an infection, which determines TCR down-regulation, acquisition of NK markers, and the progression to the final steps of the differentiation to effectors. This scenario is similar to that of CD8+ αβ T cells, which have been shown to re-express the CD45RA isoform when terminally differentiated,14 and to up-regulate NKR expression.44 Effector cells are thought to have a short life span, whereas central memory cells represent the pool of long-lived cells from which effectors are generated at the time of need.25 In contrast, terminally differentiated Vδ2 TEMRA cells appear to persist in vivo for extended periods of time (D.F.A. et al, manuscript in preparation), and have been reported to be the most represented γδ T cells in inflamed tissues.18
Thus, given (1) their marked ability to engage and to lyse tumoral cell targets, (2) their high content of perforin, and (3) their ability to secrete TNF-α and IFN-γ upon CD16-mediated (but not phosphoantigen-mediated) activation, the terminally differentiated Vδ2 TEMRA cells represent a distinct and critical pool of cytotoxic effectors within the γδ T-cell population.
Our results have demonstrated that, as suggested for NK cells,19 expression of CD16 could discriminate the 2 subsets of Vδ2 T lymphocytes with different functional roles. The CD16– subset has the ability to produce high levels of cytokines, expresses low levels of killer inhibitory receptors (KIRs) and is poorly cytotoxic, while the CD16+ subset presents high levels of KIRs and is a potent cytotoxic effector cell. Therefore, we suggest that Vδ2+ T cells should not be considered as a homogeneous population, but rather as a combination of distinct effector subsets. Moreover, the similarity with NK cells is again underlined by the important role of cytokinic environment (rather than antigen recognition) in the development of distinct subsets.19
Future studies will now aim at elucidating the conditions that favor the selective generation of human Vδ2 TEMRA lymphocytes, due to the need of generating cytotoxic effectors for anticancer immunotherapy purposes.
Prepublished online as Blood First Edition Paper, June 3, 2004; DOI 10.1182/blood-2004-01-0331.
Supported by institutional grants from the Italian Ministry of Health (L.B., F.P.), the Italian Ministry of Scientific Research, the Ministero dell'Instruzione, dell'Universitè e della Ricerca (MIUR), the Progetti di Ricerca di Interesse Nazionale (PRIN), and the Fondo per gli Investimenti della Ricerca di Base (FIRB) (L.B.), INSERM l'Association pour la Recherche sur le Cancer, Association pour la Recherche sur le Cancer (ARC) no. 3283 (J.J.F.), and an Hoechst Marion Roussel–Groupment d'Interêt Public (HMR-GIP) Aventis fellowship (M.P.).
D.F.A. and G.B. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge Innate Pharma (Marseille, France) for providing BrHPP, and Sanofi-Synthélabo (Labège, France) for generous supply of IL-2.

![Figure 2. Phenotype and activation patterns of Vδ2TEMh and Vδ2TEMRA lymphocytes. (A) NKR and chemokine receptors of representative TEMh (top panels) and TEMRA (bottom panels) TCR Vδ2+ clones. Cultured Vδ2 T-cell clones are characterized as Vδ2TEMh on the basis of their CD16–CD45RA– perforinlow pheno-type, or as Vδ2TEMRA when they are CD16+CD45RA+ perforinhigh (mean fluorescence intensity [MFI] for intracellular perforin is given). (B) Vδ2TEMh and Vδ2TEMRA PBMCs freshly drawn from a healthy donor with different levels of cell surface TCR Vδ2. (C) PhosphoERK immunoblots of lysates from Vδ2high and Vδ2low cells (5 × 105 cells/point). After sorting using gates shown in panel B, in vitro activation of the collected cells, and production of their lysates, the p-Erk immunoblots reveal a higher response to BrHPP in Vδ2TEMh than in Vδ2TEMRA, which respond better to stimulation with anti-CD16. The data are representative of 2 independent experiments on different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/6/10.1182_blood-2004-01-0331/6/m_zh80180466780002.jpeg?Expires=1766005302&Signature=wVNj362lu0lCec~E-xGaUME2TWedD~mb66Tb0rmP1uSljiYYiLHEo1zVogXvoO1X~jyenB7I0bpJ2LsB-vnZlFMQhNSvw2RBWZMaLn3mSz1~ezC5i3JhPeLdZ-0ZZeeTXqwqUx38J8fZnT9fZ7xrpbsuSvm7QzsyA7w~pWj~WzK9ZYQZqOaBZyadKK4TbKL4ErviUZIm2-sLHPmIoEVuoJ3TS2MEVwm469dY0bn8~ZSKPOkArksilO1L1OL5WkttoJivQmkfPeJgCfl0oH8jpYTJFRjHAwwv6pkc39NfopBnC8mgYHnygulM7RZhLwbxlx3u6pO8cVFKLrRkl8yhKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)