Abstract
Fanconi anemia (FA) is an autosomal recessive condition associated with bone marrow failure (BMF) leading to death or hematopoietic stem cell transplantation, acute myeloid leukemia (AML), and solid tumors (STs). It is unclear which patients are most likely to develop each outcome. From a cohort of 144 North American patients with FA, we calculated individualized risks of each outcome, given the presence or absence of readily diagnosed congenital abnormalities that occur frequently in FA. Abnormal radii and a 5-item congenital abnormality score were significant risk factors for BMF. The cumulative incidence of BMF by age 10 years varied from 18% in the lowest BMF risk group to 83% in the highest. Because of competing risks, patients in the lowest BMF risk group were most likely to live long enough to develop AML or ST, and, conversely, patients in the highest BMF risk group were least likely to live long enough to develop AML or ST. By age 40, the cumulative incidence of ST ranged from 0.6% to 29% in the highest and lowest BMF risk groups, respectively. Abnormal radii are the strongest predictor of early BMF in FA; a congenital abnormality score separates patients with normal radii into distinct prognostic groups. (Blood. 2004;104:350-355)
Introduction
Fanconi anemia (FA) is an autosomal recessive condition associated with a diverse array of congenital abnormalities and multiple competing adverse events. The adverse events of FA include progressive bone marrow failure (BMF) that leads to death or hematopoietic stem cell transplantation, acute myeloid leukemia (AML), and solid tumors (STs).
FA cells are uniquely sensitive to DNA cross-linking agents,1 and this cellular phenotype defines the syndrome. FA is a heterogeneous disorder. FA currently includes at least 11 complementation groups (FANCA, B, C, D1, D2, E, F, G, I, J, and L),2-4 defined by cell fusion experiments to correct cross-linking sensitivity in vitro. There are 8 cloned FA genes (FANCA, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCL), and FANCD1 which is BRCA2.5 About 70% of patients have mutations in FANCA, 10% in FANCC, and 10% in FANCG; all other groups are rare.2 FA is considered to be a genomic instability syndrome.6
There is also genetic heterogeneity within the major complementation groups. More than 100 pathogenic mutations have been reported in FANCA,7,8 10 in FANCC,2,9,10 and 18 in FANCG.11,12 A number of founder mutations have been identified, such as the FANCC IVS4 + 4 A>T mutation in the Ashkenazi Jewish population9 and 3 founder mutations in FANCA in the Afrikaner population.13
Patients with FA manifest a broad spectrum of congenital abnormalities.1,14-17 Some patients are severely affected with numerous abnormalities, whereas others have a mild phenotype. The phenotype may be discordant between affected individuals from the same family,18 and the same genetic founder mutation may have different phenotypic effects in different populations. Notably, FANCC IVS4 + 4 A>T is associated with a severe phenotype in the Ashkenazi Jewish population19 but not in the Japanese population.20
Finally, the ages at onset of BMF, AML, and ST, the main health outcomes of FA, are also heterogeneous. At the extremes, some patients require bone marrow transplantation as early as age 3 years. Others never develop BMF or AML; these patients have a high risk of solid tumors in their mid to late 40s.21 A number of studies have identified subgroups of patients, defined by genotype or phenotype, who are at increased risk of specific adverse events.19,22,23
In a previous report,24 we showed that the risk profile of each adverse event (ie, its cause-specific hazard function) has a characteristic dependence on age. BMF is the most likely adverse outcome during childhood, whereas solid tumors are the most likely adverse outcome among patients who survive past the age of 20 years. The hazard of developing AML has an intermediate pattern, with the peak hazard rate occurring during the teenage years. The cumulative incidence of each adverse event that is observed reflects both the cause-specific hazards and the competing nature of the risks.
These risk estimates characterize the average risk of each outcome by age in the total population of North American patients. However, because the risks of FA are competing, the manifest heterogeneity between individual patients complicates risk assessment. By definition, patients at increased risk of BMF are more likely to develop BMF than patients at baseline risk of BMF. However, patients at baseline risk of BMF are more likely to develop a solid tumor than patients at increased risk of BMF, because they are more likely to live long enough to develop a solid tumor. At present, the previously identified risk factors for various outcomes are not integrated into a comprehensive model that allows a competing risks assessment to be made for each individual patient.
In this report, we calculate the cumulative incidence, by age, of each adverse outcome of FA, BMF, AML, or ST, given the presence or absence of readily diagnosed congenital abnormalities of FA that manifest early in life. Our approach provides individualized risk estimates for each end point of FA among North American patients with FA, irrespective of complementation group or mutation status, and it appears to capture a substantial amount of the heterogeneity of risk that characterizes the syndrome.
Patients, materials, and methods
Study population
We studied 145 subjects in the North American Survey (NAS), a retrospective cohort study of persons with FA.24 The Human Subjects Committee of the Johns Hopkins Bloomberg School of Public Health approved the NAS study, which was initiated while one of the authors (B.P.A.) was a graduate student at the school. The survey consisted of 4 pages of questions to be answered by the patients or their parents. After excluding one individual from our previous report who had incomplete data on physical manifestations of FA, 144 persons were available for the current analysis. We used the competing risks analytical framework and considered 3 competing events: bone marrow failure (BMF) leading to death or transplantation, acute myeloid leukemia (AML), and occurrence of a solid tumor (ST). Patients with myelodysplastic syndrome (MDS) were not considered to have leukemia.25 These outcomes are the same as those used in our previous study, except that bone marrow transplantation and death (presumably a consequence of marrow failure) prior to AML or ST are merged into the combined category of BMF.
We studied the associations between the risk of each adverse event and 10 binary (present/absent) physical manifestations (phenotypes) of FA: absent or abnormal radial ray (RAD), developmental delay, cardiopulmonary abnormality, abnormal kidney (horseshoe, pelvic, ectopic, absent), abnormal hearing or deafness, abnormal head (including microcephaly), stature below the 10th percentile, abnormal lower limbs (including dislocated hips), absent or abnormal thumbs, and other skeletal abnormalities (Table 1). Because these phenotype data derived from self-report, the 10 variables selected for association analysis were chosen because they are easy to recognize and manifest early in life, before the occurrence of adverse events. Café au lait spots and other skin pigmentation findings were excluded because they depend on age and sun exposure. Urogenital anomalies are generally identified only in males and, thus, were omitted. Eye, gastrointestinal tract, and central nervous system birth defects might have been underreported and, therefore, were also not included.
Statistical analysis
We scored each trait as a binary variable (1 if present, 0 if absent). We assessed the correlation between pairs of traits by using the odds ratio, tested for significance of the odds ratio with use of the chi-square test, and adjusted the nominal P values from the chi-square test for multiple comparisons using the Bonferroni and false discovery rate approaches described later.
For each end point (BMF, ST, and AML), and for each of the 10 binary phenotypes, we estimated the relative hazard of the end point in patients with and without the manifestation by using the Cox proportional hazards model.26 For each end point, 10 hypotheses were tested; thus, the chance of obtaining one or more false-positive findings at the 5% level of significance in the “raw” P values could be as high as 40% (or even higher if the traits are positively correlated), when in fact none of the phenotypes are associated with the end point. Therefore, we adjusted the raw P values for multiple comparisons by using the Benjamini-Hochberg false discovery rate (FDR) procedure that controls for multiple comparisons.27 FDR is the expected proportion of falsely rejected hypotheses among all those rejected. We also applied the classic Bonferroni adjustment.28 The Bonferroni approach is more stringent, because it controls the probability of making even a single false rejection, but it is also less powerful. We calculated adjusted P values for each of these approaches29 ; a hypothesis was rejected at level 0.1 if its adjusted P value was less than or equal to 0.1, etc.
We also evaluated the association between each outcome and several trait scores. These evaluations included a simplified score, which was originally developed to predict the results of chromosome breakage tests in subjects referred to genetic counseling for possible FA,14 and scores obtained by counting the number of phenotypes affecting each subject. We evaluated multivariate Cox models with effects for individual traits and trait scores by using standard methods.26
Because of competing risks, the chance that a patient will live long enough to develop one adverse event varies inversely with the chance that he or she will develop another. From the perspective of the health care provider and patient, cumulative incidence curves as defined by competing risks theory are a useful representation of the penetrance of each outcome, because they show the chance of each event occurring, given that the patient is at risk of all events.30
We calculated cumulative incidence curves specific to patients with a given phenotype by using a semiparametric procedure. First, we tabulated the data according to the numbers of events of each type and person-years at risk by single-year of age, with separate cells for each phenotypic group referenced by a particular risk model. Second, we used Poisson regression to estimate the cause-specific hazard rates. Specifically, for each end point, we fitted a generalized additive model31 for the cause-specific hazard functions by using a smoothed term for the effect of age (the baseline hazard function) and additive effects for each predictor (phenotype). We computed cumulative incidence curves from the formula for cumulative incidence30 and assessed the uncertainty of the curves by using bootstrap resampling.32
Results
Congenital abnormalities
Of the 145 subjects in NAS 144 reported complete data on the 10 components of the phenotype. The trait frequencies are given in Table 1. Abnormalities of the lower limbs were least frequent (7%), and abnormally short stature was most frequent (73%). The mean number of abnormalities was 3.3, whereas 13 subjects (9%) had none, 4 subjects (3%) had 9, and no subject had 10. As is well known, all patients with abnormal radii also had abnormal thumbs, whereas many patients with abnormal thumbs were reported to have normal radii.
All 45 pairs of traits were positively correlated with one another (ie, had odds ratios greater than 1.0). When the value of the odds ratio could be defined, the odds ratios for trait-pairs ranged from a low of 1.2 (lower limbs versus thumbs) to a high of 12.7 (abnormal head versus short stature). The very high association of short stature and microcephaly may be exaggerated because the small head sizes may have been absolute and not relative to the height. The nominal odds ratio was infinity for thumbs versus abnormal radii (RAD), because all 18 subjects with the former also had the latter. Excluding this latter pair, the mean odds ratios in the remaining 44 trait-pairs was 4.0. Among the 45 pairs, 31 were nominally significant at the α = 0.05 level, 25 remained significant after adjusting for multiple comparisons by using a 5% FDR criterion, and 11 remained significant at the same level after a Bonferroni adjustment. Therefore, at least one fourth, and likely more than one half, of the trait pairs were significantly associated with odds ratios greater than 1.0. It appears unlikely that any trait-pairs have an odds ratio significantly less than 1.0. The positive dependence of numerous traits suggests that each FA patient may have an intrinsic “frailty” or susceptibility to develop many of the disparate malformations that can occur during development and supports the construction of predictive trait scores, as described later.
Relative hazards
Six abnormalities were significantly associated with the risk of BMF at the 0.07 level or lower by using the FDR criterion: RAD; developmental delay; and abnormalities of the heart or lung, kidney, hearing, and head (Table 1). This FDR analysis is interpreted as follows: the 6 significant findings were obtained in such a way that, on average, only 7% of them (ie, none or 1 of the 6) might be a false positive. The next most significant trait after the 6 was short stature (nominal P = .18, adjusted P = .25). RAD was the strongest single risk factor for BMF (relative hazard (RH) = 5.5; 95% confidence interval [CI] = 2.8-11.0; Table 1). RAD was also the most significant (adjusted P = 3.5 × 10-4). It is extremely unlikely, therefore, that the association between BMF and RAD is spurious. The other 5 significant traits each had an RH of around 2.0.
None of the abnormalities were significantly associated with the risks of AML or ST, either nominally (ie, raw P < .05) or after adjustment for multiple comparisons. The simplified score was not significantly associated with any of the outcomes (Table 1); it was most strongly associated with the risk of AML (nominal P = .119).
We defined 2 summary scores, “CABS10,” the number of abnormalities present in the total set of 10 phenotypic features, and “CABS,” the number of abnormalities in the set composed of developmental delay, heart or lung, kidney, hearing, and head, ie, those abnormalities other than RAD that appear to be associated with BMF. CABS10 was significantly associated with the risk of BMF (P = .004), as was CABS (P = .001). CABS10 was not significant after adjustment for RAD (P = .52), whereas CABS remained significant after adjustment for RAD (P = .05).
Therefore, our best-fit model for BMF had 2 components (Table 1). The first component was a 3.63-fold increase in the hazard of BMF in persons with abnormal radii (RAD = 1) versus persons with normal radii (RAD = 0). The second component was a net 1.23-fold increase in the hazard of BMF for each unit increase in CABS, ie, for each additional abnormality in the set. Hence, compared with persons classified as CABS = 0, persons with CABS = 5 were at 1.235 = 2.8-fold higher risk of BMF. A patient's individualized risk is the product of the RAD and the CABS components. At the extremes, persons who are RAD = 1 and CABS = 5 are at 3.6 × 2.8 = 10-fold higher risk of BMF than persons who are RAD = 0 and CABS = 0.
Cumulative incidence
The cumulative incidence of each end point by a given age is the probability that an individual experiences that particular end point as the initial adverse event, given that he or she is at risk of each of the 3 possible outcomes. By competing risks, subjects at lower intrinsic risk of one event, eg, BMF, are more likely to live long enough to develop another adverse event, such as ST. We computed cumulative incidence by single-year of age for each of 12 risk groups defined by RAD (0, 1; 2 levels) and CABS (0, 1, 2, 3, 4, 5; 6 levels).
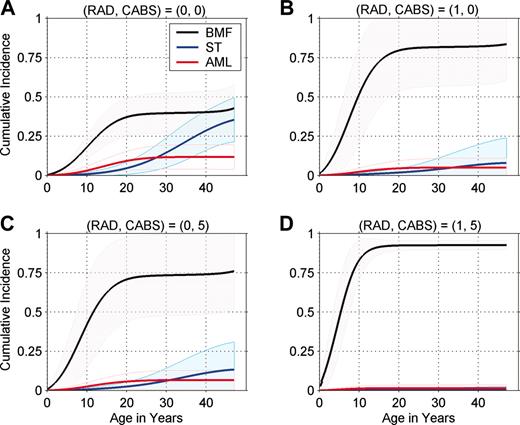
Figure 1 presents individualized cumulative incidence curves for 4 representative phenotype groups. All 12 sets of curves are available from the authors (P.S.R. or B.P.A.). The cumulative incidence of BMF by age 10 years ranges from 18% in the lowest BMF risk group (RAD = 0, CABS = 0; Figure 1A) to 83% in the highest BMF risk group (RAD = 1, CABS = 5; Figure 1D). Patients in the lower BMF risk groups were most likely to live long enough to develop AML or a solid tumor, and, conversely, patients in the higher BMF risk group were least likely to live long enough to develop ST or AML. By age 40 years, the cumulative incidence of a solid tumor ranged from 0.6% in the highest BMF risk group (Figure 1D) to 29% in the lowest BMF risk group (Figure 1A). In our study, 12.5% of patients had abnormal radii but none of the risk factors in the CABS score (RAD = 1, CABS = 0; Figure 1B). These patients were at high risk of BMF despite the favorable CABS score, and few were expected to live long enough to develop AML or solid tumors as the first event. A larger subgroup of patients, 29%, had normal radii but abnormalities in all components of the CABS score (RAD = 0, CABS = 5; Figure 1C). These patients had a high risk of BMF that was similar to those with RAD = 1 and CABS = 0 (Figure 1B).
Cumulative incidence of bone marrow failure (BMF), leukemia (AML), and solid tumor (ST), by age. Shaded regions show 95% pointwise confidence limits. CABS indicates congenital abnormality score and includes 1 point each for abnormalities in the set: developmental delay, heart or lung, kidney, hearing, and head. RAD indicates abnormal radii. The RAD = 0, CABS = 0 group (A) is at relatively low risk of BMF compared with other FA risk groups. The RAD = 1, CABS = 5 group (D) is at high risk of BMF, and the RAD = 1, CABS = 0 and RAD = 0, CABS = 5 groups (B and C, respectively) are at intermediate risk of BMF.
Cumulative incidence of bone marrow failure (BMF), leukemia (AML), and solid tumor (ST), by age. Shaded regions show 95% pointwise confidence limits. CABS indicates congenital abnormality score and includes 1 point each for abnormalities in the set: developmental delay, heart or lung, kidney, hearing, and head. RAD indicates abnormal radii. The RAD = 0, CABS = 0 group (A) is at relatively low risk of BMF compared with other FA risk groups. The RAD = 1, CABS = 5 group (D) is at high risk of BMF, and the RAD = 1, CABS = 0 and RAD = 0, CABS = 5 groups (B and C, respectively) are at intermediate risk of BMF.
The cumulative incidence of BMF by age also varied substantially by phenotype subgroup, ranging from 6% to 50% by age 5, and from 18% to 83% by age 10. Among patients in the lowest BMF risk group (Figure 1A), the cumulative incidences of BMF and ST ultimately become similar by age 50. In each group, most BMF events occur by the age of 20; BMF remains the dominant event in patients at moderate and high risk of BMF (Figure 1B,D). As shown by 95% confidence bands, estimates of cumulative incidence are uncertain, especially in the low and moderate risk groups beyond the age of 20 years, because of the small numbers of older patients.
Discussion
FA is a difficult disease for patients and their families, who early on may face hard choices about the need for therapeutic hematopoietic stem cell transplantation. However, as we show here, individuals with FA vary considerably in their expected risk of each event. The cause-specific hazard rate of BMF varies 10-fold between the lowest risk group for BMF (RAD = 0, CABS = 0) and the highest (RAD = 1, CABS = 5). As a consequence, the cumulative incidence of BMF varies by approximately 8-fold (from 6% to 50%) and by approximately 5-fold (from 18% to 83%) across phenotype subgroups, by ages 5 and 10, respectively. Absent clear signs of hematologic progression, it might be reasonable to consider deferring transplantation for patients at the lower end of this range. At the upper end, these estimates may help individuals make personalized decisions about the timing of transplantation. The cumulative incidence of BMF increases by about 7% to 8% per year in the highest risk group. These risks may be balanced against other probabilities, such as the possibility of obtaining a better-matched donor in the next year.
Because of the competing risks, patients who escape BMF and AML are most likely to live long enough to develop solid tumors. The cumulative incidence of ST varies inversely with that of BMF. By age 40, the cumulative incidence ranges from 0.6% in the highest risk group for BMF (almost none of whom are expected to develop ST as the first adverse event) to 29% in the lowest risk group for BMF. Clearly, lifelong cancer prevention and surveil-lance are critical management requirements for “mildly affected” patients with FA. The cancer risks presented here do not include tumors arising subsequent to bone marrow transplantation.33 Interestingly, in one cohort of patients with FA who received transplants, risk factors for acute graft-versus-host disease included malformations of the limbs and the urogenital tract and/or kidneys,34 similar to our finding of higher BMF risk in patients not receiving transplants with abnormal radii and kidney, respectively.
To develop our model, we used the FDR to screen a panel of candidate risk factors and to select those that remained significant after multiple hypothesis testing. FDR is a comparatively new statistical method, but it appears to be a major innovation of broad utility in genetic studies.35,36 FDR has potentially greater statistical power than traditional Bonferroni-type approaches. The FDR adjusted P values allow one to balance the false-positive and false-negative rates; for BMF, we identified 6 significant findings at the 0.063 level versus only one at the 0.05 level. For our model, because the congenital abnormalities of FA appear to be positively correlated, the classic FDR approach is proven to be valid.37 In other words, because there is only positive correlation between the traits of FA, it is likely that the actual false discovery rate in our analysis is close to the nominal levels indicated by the adjusted P values. When this is not the case, more conservative FDR procedures should be considered.37 We then used generalized additive models to estimate cause-specific hazard functions for each competing event. This approach can model distinctive agespecific risk profiles that may characterize the end points. By combining the 2 components of our model, risk factors and baseline hazard rate functions, we were able to estimate the age-specific penetrance of each adverse event for heterogeneous subgroups of patients at risk.
A major limitation of our study is its comparatively small sample size. We did not find risk factors that modulated the cause-specific hazards of ST or AML, but we did not have much power to do so because of the small numbers of these events in the NAS.24 However, our study was sufficiently well powered to detect large relative hazards of ST or AML, of 6 or 8, respectively. Another limitation is that, although we did validate AML and ST diagnoses, other data were obtained by self-report, including the physical findings. However, although these limitations may affect the validity of the specific components of our risk score, they do not invalidate the methodologic approach.
Strengths of our model are its simplicity and applicability to all North American patients with FA. Prognostic factors may differ in other populations.20 Although we did not directly identify specific FA gene mutations in the NAS, congenital abnormalities are in some cases correlated with genotype and so may indirectly reflect genetic influences. In the NAS, abnormal radii were the strongest single risk factor for BMF. In the European Fanconi Anemia Registry (EUFAR), radial ray abnormalities were significantly associated with null mutations in FANCA and with FANCC IVS4 + 4 A>T, which in turn are risk factors for younger age at hematologic onset and bone marrow failure, respectively.22 Our data appear to be consistent. We speculate that the CAB score is detecting an amalgam of genetic and epigenetic factors. Empirically, it appears capable of stratifying most patients with FA with normal radii into distinct prognostic groups. We hope that future studies will elucidate why specific genetic errors that cause specific malformations also correlate with early-onset bone marrow failure.
Our statistical approach can incorporate diverse risk factors for each adverse event, if individual-level data are available to estimate the effects. In the International Fanconi Anemia Registry (IFAR), male sex increased the risk of MDS/AML and decreased the risk of ST.23 Male sex was not significantly associated with any end point in our data. In the EUFAR, patients with FANCG were at increased risk of MDS/AML, and patients with FANCA homozygous for null mutations were at decreased risk of MDS/AML.22 The annual telomere shortening rate,38 and high levels of α-fetoprotein39 might also predict BMF. In principle, our model could be refined to include some of these other parameters. This would require a study that is both more comprehensive (with molecular data) and larger than ours, to maintain the precision of increasingly complex models.
Our methods may be broadly useful to elucidate heterogeneous risks for multiple outcomes in other cancer susceptibility syndromes. Heterogeneity appears to be a hallmark of many of the recognized autosomal recessive cancer susceptibility syndromes, including Bloom syndrome,40 Werner syndrome,41 xeroderma pigmentosum,42 and ataxia-telangiectasia.43 In these syndromes, patients are at risk of multiple competing adverse events with variable ages at onset. Our methods may also help elucidate risk in other inherited bone marrow failure syndromes, such as severe congenital neutropenia,44 a disorder that puts patients at risk of both MDS/AML and sepsis deaths.
Finally, we emphasize that “individualized” risk estimates from our model, as for other individualized risk models, are simply a sophisticated form of average risk. It is likely that genetic susceptibility, modifiable risk factors, and random events further modulate each individual's true risk over time, even in the setting of a heritable cancer susceptibility syndrome such as FA. Nonetheless, risk estimates such as ours can be useful if they help patients and their families make better-informed health care decisions. Our quantitative estimates of cumulative incidence should be interpreted cautiously because our risk predictions have not been clinically validated in an independent cohort. It is clear that clinical counseling will use all available information about a patient and not rely on any single model. With these caveats in mind, the general approach we have used to develop a risk model for FA may ultimately help inform patients affected by many syndromes that are characteristically diverse.
Prepublished online as Blood First Edition Paper, April 1, 2004; DOI 10.1182/blood-2004-01-0083.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the Fanconi Anemia Research Fund and Fanconi Canada for mailing out the questionnaires for the initial NAS study, as well as all of the families who completed them.