Abstract
Chronic infection or colonization by mycoplasma(s) could gradually and significantly alter many biologic properties of mammalian host cells in culture, including induction of malignant transformation. We examined effects of Mycoplasma fermentans infection on the continuing survival and immortality of human peripheral blood mononuclear cells (PBMCs) from healthy blood donors. Without specific supplemental growth factors, human PBMCs normally die rapidly, with few cells other than macrophages/monocytes surviving after 2 weeks in cultures. Only occasional Epstein-Barr virus (EBV)–positive B lymphocytes would continue to proliferate and undergo spontaneous immortalization. Our present study revealed that infection of human PBMCs in culture with the incognitus and PG18 strains of M fermentans, but surprisingly not with some other strains tested in parallel, markedly enhanced the rate of EBV-positive B lymphocytes to undergo immortalization (74% vs 17%). Compared with spontaneously immortalized PBMCs, the PBMCs immortalized in cultures infected with the mycoplasmas often had prominent karyotype changes with chromosomal loss, gain, or translocations. Furthermore, many of these immortalized B lymphocytes were found to be monoclonal in nature. The in vitro findings would be of relevance to lymphoproliferative disorders that occurred in patients with immune suppression. The mycoplasma-mediated promotional effect in cell immortalization and its potential clinical implications warrant further study.
Introduction
Mycoplasmas are a heterogeneous group of the smallest organisms capable of self-replication. They can cause a wide variety of diseases in animals.1 Some mycoplasmas cause respiratory or urogenital diseases in humans.2 However, mycoplasmas often chronically colonize our respiratory and urogenital tracts without apparent clinical significance.2 In this respect, wall-free mycoplasmas are among the few prokaryotes that can grow silently in close interaction with mammalian cells for a long period of time. However, prolonged interactions with mycoplasmas of seemingly low virulence could, through a gradual and progressive course, significantly affect many biologic properties of mammalian cells.3-5 We have developed a new paradigm for neoplastic processes based on our in vitro studies.3,4,6 We hypothesize that chronic infection or colonization by certain mycoplasma(s) may gradually induce malignant transformation and promote tumor growth of mammalian cells.
Previous studies reported isolation of mycoplasmas from human leukemic bone marrow.7-11 A majority of the isolated mycoplasmas were identified as Mycoplasma fermentans. Experimental inoculation of M fermentans induced leukemoid disease with myeloproliferative changes in mice.12 However, the mycoplasma-oncogenesis hypothesis failed to advance because the same mycoplasma could also be found in nonleukemic children or adults though it was most frequently isolated from patients with leukemia.13 Decades later, our understanding of disease process in chronic infections, cancer latency, and cancer-associated microbes has changed significantly.14,15 Potential biologic and clinical significance of latent infection or parasitism by prokaryotes with seemingly low virulence like mycoplasmas in human bone marrow justify further studies.
Our laboratory previously demonstrated that chronic mycoplasmal infections could induce chromosomal instability and malignant transformation of mammalian cells. This mycoplasma-mediated oncogenic process had a long latency and demonstrated distinct multistage progression.3 Overexpression of H-ras and c-myc oncogenes was found to be closely associated with both the initial reversible and the subsequent irreversible states of the mycoplasma-mediated transformation in C3H murine embryonic cells.16 More recently, we showed that infection by mycoplasmas rapidly rendered growth factor independence in otherwise strictly growth factor–dependent mouse hematopoietic progenitor 32D cells.4 Furthermore, chronic mycoplasmal infection of the 32D cells affected the fidelity of genomic transmission in cell divisions and resulted in somatic genetic abnormality and malignant transformation.
Both of the above model systems were extremely helpful in exploration of molecular mechanisms that rescue cells from apoptosis and induce continuous cell growth in mycoplasma-mediated malignant transformation. However, cells studied in both of the model systems have been maintained in laboratory for many years and no longer have a normal karyotype. These cells, when cultured under the right conditions and/or supported by proper growth factor supplements, could continue to grow without undergoing senescence. In comparison, growth of normal mammalian cells is under tight regulation to allow only a limited number of replication cycles. There are mechanisms, including telomere loss in chromosomes following each cell division, to strictly control the growth and to ensure entry into cell senescence.17,18 In this study, we have examined if the effects associated with mycoplasmal infections found in our earlier model systems (ie, antiapoptosis, induction of growth factor–independent cell growth, and chromosomal instability) can similarly be observed in normal human primary hematopoietic cells such as peripheral blood mononuclear cells (PBMCs).
Human PBMCs, mainly lymphoid cells and monocytes, when cultured in media supplemented with fetal bovine serum and without adding any other cell growth factors, usually died rapidly and disappeared from the culture within 2 to 3 weeks. In this study, we developed a culture condition to examine effects of mycoplasmal infections, if any, on continued growth or immortalization of human PBMCs. Immortalization is recognized as a crucial transition in the process of malignant cell transformation. Since M fermentans appeared to be most commonly associated with cell transformation and cancer in earlier reports, we focused our present study on M fermentans' effects on the continued growth of human PBMCs in culture. We found that infections by certain strains, but not by some other strains, of M fermentans markedly enhanced the rate of immortalization of human PBMCs in culture.
Materials and methods
Preparation of human PBMCs
Unused buffy coats of healthy blood donors were obtained from Department of Transfusion Medicine, Warren Grant Magnuson Clinical Center, National Institutes of Health (Bethesda, MD). A total of 23 blood samples were studied. Mononuclear cells were isolated from the buffy coats by gradient centrifugation at 900g for 25 minutes at room temperature using Histopaque-1077 (Sigma Chemicals, St Louis, MO). The PBMCs (1 × 106/mL) were then cultured in 30 mL of alpha-modified essential medium (alpha-MEM; GIBCO-BRL, Gaithersburg, MD) supplemented with 20% fetal bovine serum. In addition, the initial PBMC cultures also included 1 μg/mL of cyclosporine A (Sandoz, Basel, Switzerland), 10-6 M of dexamethasone (Sigma Chemicals), and 10-5 M of β-mercaptoethanol (Sigma Chemicals). All cultures were set up in T-25 culture flasks, kept upright in a humidified CO2 incubator at 37°C, and fed twice a week by replacing half of the culture medium with fresh alpha-MEM containing 20% fetal bovine serum without other supplements. Cell growth was assessed at 3- to 5-day intervals by counting cell numbers on a hemacytometer. The viability of cells was determined by trypan blue dye exclusion assay.
Mycoplasma preparation
Frozen stocks (-70°) of M fermentans (SK5, A25, PG18, and incognitus [Mi] strains) were thawed, grown in SP4 broth medium aerobically at 37°C for 3 days, and titrated before inoculation into cell cultures.3,4,19 SK5 strain of M fermentans was previously isolated from urine of a patient with AIDS,20 and A25 strain was isolated from a pediatric patient also with AIDS (S.-C. L., unpublished data, April 1997).
Experimental infections of mycoplasmas
PBMC cultures from each donor were divided into mycoplasma-infected and noninfected groups. The infected groups were inoculated with M fermentans A25, SK5, PG18, and/or Mi strains, respectively. For each infected culture, mycoplasmas in 0.5 mL of SP4 broth (107-108 color change units [CCUs]) were inoculated into a 30-mL PBMC culture (106 cells/mL). The same volume of SP4 medium was added into noninfected control cultures. The amount of PBMCs recovered from each blood sample varied. Mi strain of M fermentans and noninfected control SP4 medium were inoculated into cultures of PBMCs from all 23 blood samples. Among the 23 PBMCs samples, 18 samples were also inoculated with A25 and SK5 strains of M fermentans separately, and only 5 samples were inoculated with the PG18 strain. The cultured PBMCs with or without mycoplasmal infections were examined daily with an inverted microscope (Olympus Ck2, Tokyo, Japan) for morphologic changes. Titers of mycoplasmas in the cultures were monitored weekly.
Detection of mycoplasmas by culture and PCR
Culture isolation of mycoplasmas using SP4 broth medium was previously described in detail.19,20 One milliliter of cell culture was used for DNA isolation by the phenol/chloroform extraction method or by using the Qiagen DNA extraction kit (Valencia, CA). The isolated DNAs were tested for the presence of M fermentans DNA by polymerase chain reaction (PCR) using primers derived from a M fermentans–specific insertion sequence (IS)–like element.20,21
Characterization of the immortalized cells
Cell slides were prepared by cytocentrifugation of cell suspension from cultures on a Cytospin II centrifuge (Shandon, Pittsburgh, PA). The slides were fixed by methanol/acetone (1:1) and stored in the refrigerator. Immunocytochemical studies were performed by standard procedures using a panel of mouse monoclonal antibodies followed by detection with antimouse antibody radish peroxidase conjugate and colorizing with 3,3′-diaminobenzidine (DAB). The monoclonal antibodies against cell surface markers used in this study included leukocyte common antigen (LCA), CD20, CD3, anti-κ and anti-λ immunoglobulin light chains, and Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1). The slides were counterstained with hematoxylin.
Cytogenetic analysis
Cultured cells were arrested in metaphase by incubating cell cultures in the presence of 0.01 μg/mL of Colcemid (Sigma Chemicals) at 37°C for 1 to 2 hours. The cells were then suspended in a hypotonic solution (0.075 M KCl) for 10 to 15 minutes, fixed in acetic acid/methanol (1:3) fixative, and washed 3 times in the same fixative. Chromosome preparations were stained with Giemsa or trypsin-Giemsa (T-G) banding method.22
Telomerase assay
To prepare cell extract, 108 cells harvested from the logarithmically growing cultures were washed twice with cold phosphate-buffered saline (PBS) by centrifugation. The cell pellets were resuspended in 0.2 mL of cold lysis buffer (10 mM Tris-HCl, pH 7.5; 1 mM MgCl2; 1 mM EGTA [ethyleneglycotetraacetic acid]; 0.1 mM phenylmethylsulfonyl fluoride; 5 mM β-mercaptoethanol; and 0.5% 3-[(3-Cholamidopropyl)-dimethylammonio]-1-propane-sulfonate [CHAPS]). The sample was incubated on ice for 30 minutes and centrifuged at 13 000g for 30 minutes at 4°C. The supernatant was collected and immediately stored at -80°C. The protein concentration of the extract was between 0.5 and 1.6 μg/μL. Telomerase activity was determined using the telomeric repeat amplification protocol (TRAP) assay developed by Kim et al23 with minor modification. Briefly, telomerase-mediated primer extension was carried out in a 50-μL reaction mixture containing 1 or 2 μL cell extract; 1 × reaction buffer (20 mM Tris-HCl, pH 8.3; 68 mM KCl; 1.5 mM MgCl2; 1 mM EGTA; and 0.005% Tween-20); 0.1 μg telomerase substrates (TS) primer (5′-AATCCGTCGAGCAGAGTT-3′); 1 μg T4 gene 32 protein; 50 μM each of deoxynucleotide triphosphates; and 2 units Taq DNA polymerase at 23°C for 60 minutes. After extension, 2 μL solution containing 0.1 μg primer CX 5′-(CCCTTA)3 CCCTAA-3′ and 0.4 μL α-32P-2′-deoxycytidine5′-triphosphate (dCTP) (10 μCi [3.7 × 105 Bq]/μL, 3000 Ci [1.11 × 108 Bq]/mmol) was added to the reaction and subsequent PCR was carried out with 30 cycles at 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 90 seconds. One half of the volume of the PCR reaction was analyzed on 10% nondenaturing polyacrylamide gels in 0.5 × Tris-borate–EDTA [ethylenediaminetetraacetic acid] buffer at 150 V for 2 hours. Then gels were dried and exposed on film.
Tumorigenicity in athymic nude mice
The immortalized PBMCs were assayed for ability to form tumors in the athymic Nu/Nu mice on a BALB/c background (Harlan Sprague Dawley, Indianapolis, IN). Ten million viable cells were injected subcutaneously into the neck region of 6-to 8-week old mice and the animals were observed at regular intervals for tumor development for 8 months.
Results
Continued growth of human peripheral blood mononuclear cells in cultures infected by M fermentans
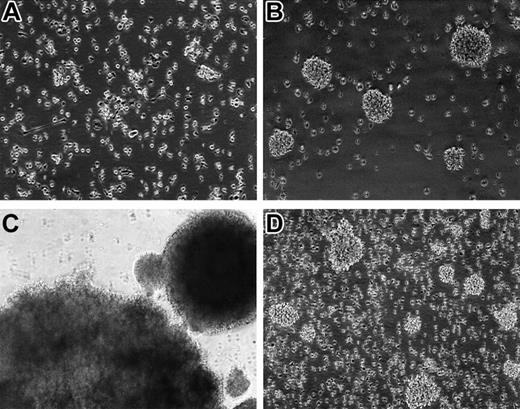
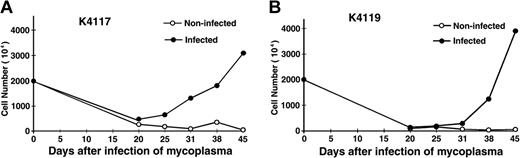
Without supplement of any cell growth factors, most of the PBMCs in cultures died rapidly within the first 2 weeks. However, in many cultures infected with M fermentans Mi strain, small aggregations of cells started to appear in 3 to 4 weeks. These cells were a bit hairy and varied in size under a reverse phase contrast microscope. After further passage, these suspension cells often grew into large aggregates or clumps (Figure 1). However, following months of continued proliferation, many cell aggregates in cultures gradually became loosely associated or individually separated (Figure 1). In the course of study, viable cells in cultures were counted weekly. As typical examples, PBMCs isolated from 2 healthy blood donors (K4117 and K4119) showed rapid cell death in the first 2 to 3 weeks followed by gradual appearance of continued proliferation of some mononuclear cells in the mycoplasma-infected cultures (Figure 2).
Photomicrographs of human PBMCs in cultures infected with M fermentans (Mi strain). (A) PBMCs cultured in suspension without infection of mycoplasma for 5 days. Some macrophages attached to the surface of the flask. (B) PBMC culture infected by mycoplasma for 4 weeks. Small clumps of cell aggregates were found in culture, and these cells later became immortalized. (C) PBMCs in a culture infected by mycoplasma for 8 weeks. The rapidly proliferating cells formed large cell aggregates. (D) PBMCs immortalized by mycoplasmal infection after 4-month culture. Many of the rapidly dividing cells became individually separated, many others aggregated into small clumps. Original magnification × 100 (A-B,D) and × 40 (C). The images were captured on an inverted phase contrast microscope Nikon Diaphot (Nikon, Tokyo, Japan) equipped with a Polaroid camera Nikon UFX-II and Photo Eyepiece objective lens CF PL 2.5× (Nikon). Original magnification was × 80 (A, B, D) and × 40 (C), under an achromatic objective lens with numerical aperture of 0.4 and 0.3, respectively.
Photomicrographs of human PBMCs in cultures infected with M fermentans (Mi strain). (A) PBMCs cultured in suspension without infection of mycoplasma for 5 days. Some macrophages attached to the surface of the flask. (B) PBMC culture infected by mycoplasma for 4 weeks. Small clumps of cell aggregates were found in culture, and these cells later became immortalized. (C) PBMCs in a culture infected by mycoplasma for 8 weeks. The rapidly proliferating cells formed large cell aggregates. (D) PBMCs immortalized by mycoplasmal infection after 4-month culture. Many of the rapidly dividing cells became individually separated, many others aggregated into small clumps. Original magnification × 100 (A-B,D) and × 40 (C). The images were captured on an inverted phase contrast microscope Nikon Diaphot (Nikon, Tokyo, Japan) equipped with a Polaroid camera Nikon UFX-II and Photo Eyepiece objective lens CF PL 2.5× (Nikon). Original magnification was × 80 (A, B, D) and × 40 (C), under an achromatic objective lens with numerical aperture of 0.4 and 0.3, respectively.
M fermentans infection induces continued growth of human peripheral blood mononuclear cells. PBMCs isolated from 2 healthy blood donors (K4117 and K4119) cultured in alpha-MEM containing 20% fetal bovine serum, 1 μg/mL of cyclosporine A, 10-6 M of dexamethasone, and 10-5 M of β-mercaptoethanol were infected with 106 CCU/mL of M fermentans (Mi strain). Noninfected control culture was inoculated with equal volume of SP4 medium. All cultures were kept in a 37°C, 5% CO2 incubator and fed twice a week by replacing the top half of the culture with the same medium without cyclosporine A, dexamethasone, and β-mercaptoethanol. Viable cells were examined by trypan blue staining and counted in a hemacytometer.
M fermentans infection induces continued growth of human peripheral blood mononuclear cells. PBMCs isolated from 2 healthy blood donors (K4117 and K4119) cultured in alpha-MEM containing 20% fetal bovine serum, 1 μg/mL of cyclosporine A, 10-6 M of dexamethasone, and 10-5 M of β-mercaptoethanol were infected with 106 CCU/mL of M fermentans (Mi strain). Noninfected control culture was inoculated with equal volume of SP4 medium. All cultures were kept in a 37°C, 5% CO2 incubator and fed twice a week by replacing the top half of the culture with the same medium without cyclosporine A, dexamethasone, and β-mercaptoethanol. Viable cells were examined by trypan blue staining and counted in a hemacytometer.
In this study, we examined mycoplasmal effects on continued growth of PBMCs from 23 healthy blood donors. Table 1 shows that PBMCs from 17 of 23 donors infected with M fermentans Mi strain became immortalized and continued to grow in cultures. In comparison, PBMCs from only 4 of 23 of our non–mycoplasma-infected control cultures maintained in parallel became immortalized. There was a marked difference in the rate of immortalization of PBMCs between the cultures with and without mycoplasmal infections (74% vs 17%). PBMCs from 18 of the 23 donors were also infected in parallel with strains SK5 and A25, 2 clinical isolates of M fermentans from patients with AIDS. However, none of the 18 PBMC cultures infected by these 2 strains of M fermentans became immortalized. Interestingly, cultures of PBMCs from 3 of 5 blood donors also became immortalized when infected with PG-18, a strain isolated previously from human urogenital tract.
Absence of mycoplasma in some immortalized human blood cell cultures
Supernatants from cultures with or without inoculation of M fermentans were examined weekly for the titer of viable mycoplasmas. It was noted that the titer of viable mycoplasmas varied significantly. Interestingly, in the first 1 to 2 weeks when most of the cultured PBMCs were rapidly dying, the titer of viable mycoplasmas had become very low (101-102) in the majority of the mycoplasma-infected cultures. Furthermore, in some of the mycoplasma-infected cultures, when proliferation of PBMCs began 3 to 4 weeks later, no viable mycoplasma could be isolated by culture, though M fermentans DNA could continue to be detected in these cultures for at least 6 weeks by PCR using M fermentans–specific primers.21 This phenomenon was particularly prominent in M fermentans Mi strain–infected cultures. We found that 9 of 17 cultures of Mi-infected PBMCs that became permanently immortalized and continued to proliferate were actually free of mycoplasma. As expected, after these immortalized cells had been successfully passed in culture for several months, M fermentans DNA could no longer be found in these cultures by PCR (Table 2). To assure the quality of our stock of the Mi strain of M fermentans, we infected cultures of CCRF-SB (human B-lymphoid cells; American Type Culture Collection [ATCC], Manassas, VA; CCL 120), CEM (human T-lymphoid cells, ATCC CCL 119), THP-1 (human monocytes/macrophages, ATCC TIB 202), and U937 (human monocytes/macrophages, ATCC CRL 1593) cell lines with the same Mi stock as controls. The titers of Mi mycoplasma in cultures of these cell lines, though fluctuating over time, always remained at a level of higher than 108 CCU/mL and never disappeared from the infected cultures after being continuously passed for more than 6 months.
Characterization of the immortalized human blood cells
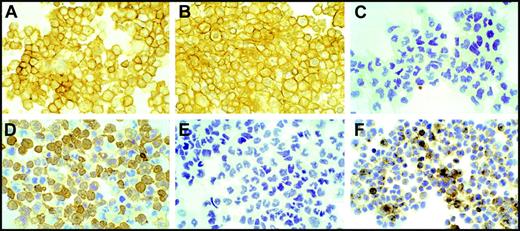
Using antibodies against specific cell molecular markers, we characterized the immortalized cells for their histogenesis. Human PBMCs that showed continued proliferation after 6 months in cultures, with or without infection by M fermentans, were centrifuged onto slides by cytospin for immunocytochemistry study. All the proliferating cells in cultures were found to be strongly positive for B-lymphocyte markers such as LCA or CD45 and CD20 (L26) B-cell antigen. No proliferating cells stained positive for CD3 T-cell marker (Figure 3). Clonality study by immunoglobulin (Ig) light-chain restriction revealed that 8 of the 17 immortalized B lymphocytes in cultures infected by Mi strain of M fermentans (47%) were monoclonal in nature, expressing either kappa or lambda light chains but not both chains (Table 2). However, lymphocytes from 1 of the 4 spontaneously immortalized non–mycoplasma-infected control cultures also appeared to be monoclonal. All the immortalized lymphocytes were also studied for evidence of EBV infection. Many cells were found to be positive for LMP1, a major transforming protein of EBV, in all of the immortalized cultures. Figure 3 shows, as a typical example, the immunocytochemistry characterization of one immortalized human PBMC (K4420). The immortalized K4420 cells (K4420-Mi) were strongly positive for LCA and CD20 B-cell markers and expressed only lambda chain but not kappa chain. They were positive for EBV LMP-1.
Characterization of human PBMCs immortalized by infection of M fermentans. Human PBMCs (K4420-Mi) that continued to proliferate for more than 5 months after infection with M fermentans (Mi) were centrifuged onto slides by cytospin for immunocytochemistry study. Practically, all cells show intensely positive staining for LCA (A) and antigen L26 (CD20, B) and negative staining for CD3 marker (C). Cells are found positive for monotypic immunostaining for lambda light chain (D) and positive for EBV latent membrane protein (LMP1, F). These cells were negative for kappa light chain staining (E). Images were captured on an Olympus BH-2 microscope (Olympus) equipped with an Olympus 35-mm film camera PM-C35DX. Original magnification was × 200 under a Splan apochromatic objective lens, numerical aperture 0.46.
Characterization of human PBMCs immortalized by infection of M fermentans. Human PBMCs (K4420-Mi) that continued to proliferate for more than 5 months after infection with M fermentans (Mi) were centrifuged onto slides by cytospin for immunocytochemistry study. Practically, all cells show intensely positive staining for LCA (A) and antigen L26 (CD20, B) and negative staining for CD3 marker (C). Cells are found positive for monotypic immunostaining for lambda light chain (D) and positive for EBV latent membrane protein (LMP1, F). These cells were negative for kappa light chain staining (E). Images were captured on an Olympus BH-2 microscope (Olympus) equipped with an Olympus 35-mm film camera PM-C35DX. Original magnification was × 200 under a Splan apochromatic objective lens, numerical aperture 0.46.
Karyotyping of the immortalized human blood cells
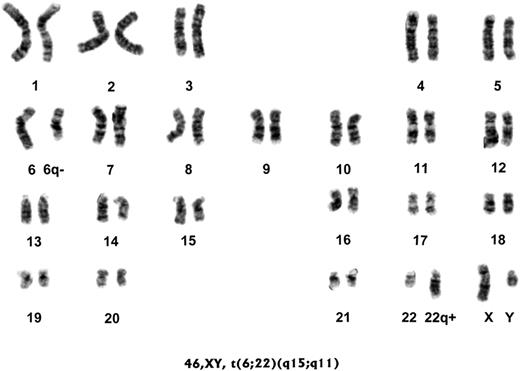
Earlier study revealed that chronic mycoplasmal infection of mammalian cells could often produce chromosomal aberrations, including chromosomal loss, gain, and translocations.24-27 We showed that chromosomal changes were associated with malignant transformation of mycoplasma-infected mammalian cells.3,4 Thus, we examined chromosomal changes in the human PBMCs that became immortalized following mycoplasmal infections as well as in those that became spontaneously immortalized in the study (Table 3). All human PBMCs from healthy donors in this study had been confirmed to have a normal karyotype before they were infected with mycoplasmas. Seven of 17 immortalized human PBMC cultures infected with Mi strain had abnormal karyotypes (Table 3). Most significantly, 90% of the immortalized K2267 cells (K2267-Mi) were monosomic for chromosome 6 and more than 95% of the immortalized K4413 cells (K4413-Mi) had a reciprocal translocation between chromosomes 6 and 22 (Figure 4). In addition, more than 30% of immortalized K611 cells (K611-Mi) were trisomic for chromosome 11. We also found that more than 75% of the immortalized K1012 cells infected with PG18 strain of M fermentans (K1012-PG) had a unique Robertsonian translocation between chromosomes 13 and 14.28 In comparison, none of the 4 non–mycoplasma-infected, spontaneously immortalized PBMCs (K351-00, K352-00, K969-00, and K1025-00) had detectable chromosomal abnormality. It was particularly interesting to note that chromosomal changes happened to many of the immortalized PBMCs that were infected initially by Mi mycoplasma but later by viable mycoplasma could no longer be recovered from the continuously proliferating cultures, such as K2267-Mi and K4413-Mi.
Abnormal karyotype of human PBMCs immortalized by infection of M fermentans. Human PBMCs (K4413-Mi) from a healthy donor with a normal karyotype were infected with M fermentans incognitus strain for 5 months. The immortalized human PBMCs were subjected to the karyotyping study. Results show that more than 95% of the immortalized K4413-Mi cells had a reciprocal translocation between chromosomes 6 and 22.
Abnormal karyotype of human PBMCs immortalized by infection of M fermentans. Human PBMCs (K4413-Mi) from a healthy donor with a normal karyotype were infected with M fermentans incognitus strain for 5 months. The immortalized human PBMCs were subjected to the karyotyping study. Results show that more than 95% of the immortalized K4413-Mi cells had a reciprocal translocation between chromosomes 6 and 22.
Telomerase activity of the immortalized human cells
Studies of various human tumors and human tumor cell lines indicated that telomerase activity might play a critical role in tumor cell growth by sustaining cellular immortality.18,23,29 We examined the telomerase activity of all the immortalized human PBMCs in cultures using TRAP assay.23 We included immortalized culture cells (transformed human embryonic kidney cell line 293 [ATCC CRL 1573] and human cervical squamous carcinoma cell line SiHa [ATCC HTB35]) as positive controls and primary culture cells of human skin fibroblasts as the negative control for the telomerase activity. We found that all immortalized human PBMCs in cultures infected by mycoplasmas as well as the spontaneously immortalized non–mycoplasma-infected PBMCs were positive for the telomerase activity (data not shown).
Tumorigenicity in nude mice
We then examined whether the immortalized human PBMCs had become tumorigenic. The ability to form tumors in nude mice has long been used as a direct indication of the true malignant transformation of cells after they have obtained the ability to continue to proliferate, although a large majority of human malignant tumor cells fail to grow when introduced into nude mice. Ten million cells of all the immortalized human PBMCs that showed properties of being monoclonal in our characterization were inoculated subcutaneously into each of 6 nude mice. The mice were followed carefully for at least 8 months. Although many had prominent karyotypic changes, none of these immortalized cells formed detectable tumors in these animals.
Discussion
Normal human somatic cells have only a limited number of cell proliferation cycles before undergoing senescence.30 The regulatory mechanisms of senescence for preventing uncontrolled cell growth are extremely stringent in human cells. Losing control of cell senescence would lead to cell immortalization, an essential transition of normal cells in the process of malignant transformation and development of cancer.18,29,30 In this study, we presented a culture condition that would facilitate our study of mycoplasmal effects on the immortalization and/or malignant transformation of human PBMCs. The alpha-MEM supplemented with 20% fetal bovine serum without adding any growth factors initially contained 1 μg/mL cyclosporine A and 10-6 M dexamethasone to suppress cell-mediated immune functions. Fresh alpha-MEM supplemented with only 20% fetal bovine serum, without cyclosporine A and dexamethasone, were then used to replace half of the culture medium in flasks twice a week. The culture condition might allow more PBMCs to undergo proliferation.
Human PBMCs in the cultures normally died quickly in the first few weeks. However, introduction of M fermentans (Mi and PG18 strains) into the PBMC cultures would markedly enhance the blood cells to undergo immortalization. We found that 17 (74%) of 23 human PBMCs continued to proliferate in the suspension cultures infected by the mycoplasmas. But 4 (17%) of the 23 human PBMCs in the control suspension cultures without infection of mycoplasma also underwent spontaneous immortalization. Interestingly, some other strains or isolates of M fermentans examined in parallel failed to induce PBMC immortalization (0/18 for both SK5 and A25 strains). It is not clear why more PBMCs in the non–mycoplasma-infected control cultures underwent spontaneous immortalization than the PBMCs in the cultures infected by these strains or isolates of mycoplasmas. However, many earlier studies revealed that mycoplasmas contain both potent mitogenic effects and cytotoxic effects on lymphoid cells. A delicate balance between these 2 effects may be crucial in ultimate successful induction of immortalization of PBMCs by the mycoplasmas. Mycoplasmas with more prominent cytopathic effects could have resulted in cell death before effective induction of PBMCs to undergo proliferation.
The human PBMCs that continued to grow for more than 6 months in cultures were considered to be immortalized. Characterization of the immortalized human PBMCs, which either occurred spontaneously or was induced by mycoplasmal infection, revealed that they were all B lymphocytes strongly positive for CD20 B-cell marker and negative for CD3 T-cell marker. Furthermore, they were all found to be positive for EBV LMP1. The association between EBV and various B-cell malignancies has long been recognized.31 Most studies indicated that EBV might be playing an essential but not sufficient role in the development of these tumors. A vast majority of people in the general population have evidence of EBV infections in the blood. Earlier studies have shown that EBV could immortalize human B lymphocytes in culture.32 Furthermore, peripheral B lymphocytes of EBV-positive individuals could undergo spontaneous immortalization in vitro. These spontaneously immortalized human peripheral B lymphocytes were always positive for EBV markers such as LMP1.33 Thus, finding EBV-positive immortalized B lymphocytes from PBMCs in our study was consistent with the earlier studies. Importantly, our study demonstrated that mycoplasmas could apparently function as a potent promoter to markedly enhance the likelihood of immortalization of EBV-infected human B lymphocytes.
Numerous studies have shown that chromosomal instability and chromosomal changes are most commonly associated with malignant properties of tumor cells. We specifically examined karyotypes of the immortalized B lymphocytes. Consistent with the finding of earlier studies that the majority of lymphoid cell lines established from healthy blood donors had a normal diploid karyotype,34-36 all 4 spontaneously immortalized EBV-positive human B lymphocytes in this study, including the one with monoclonal characteristics (K1025-00), had a normal karyotype. In comparison, more than 41% of the human PBMCs that became immortalized in the cultures infected with mycoplasmas (7 of 17) showed prominent chromosomal aberrations including chromosomal loss, gain, or translocation. Ideally, we would like to obtain a sufficient number of spontaneously immortalized B lymphocytes from healthy human PBMCs so we could demonstrate a better statistical significance of chromosomal changes induced by mycoplasmal infection. However, we would have to culture many more human PBMCs than we have presently studied, due to a much lower rate of spontaneous immortalization of normal human PBMCs. In this context, it is important to note that separate studies in our laboratory revealed that all 11 in vitro–immortalized B lymphocytes derived from PBMCs of healthy blood donors (different from the blood donors in the present study) following EBV infection had a normal karyotype (S. T. and S.-C. L., unpublished results, December 1998). Thus, the marked increase of chromosomal changes in the immortalized B lymphocytes in human PBMC cultures infected by the mycoplasma appeared to be very significant. This finding is very much consistent with the observation reported previously by us and many others that mycoplasmal infections of mammalian cells often lead to chromosomal changes.3,4,24-27
It also appeared that the human PBMCs promoted by mycoplasmal infections to undergo immortalization were more likely to be monoclonal in nature (47%). In comparison, 1 (25%) of 4 PBMCs that underwent spontaneous immortalization was found to be monoclonal. Finding of monoclonality of cells both in vivo and in culture is normally the result of selection. It simply means that a particular clone of a cell has gained a significant growth advantage and outgrown its counterparts. Thus, monoclonality is often an indication of more aggressive or true malignant property of cells. In fact, our karyotype study similarly revealed strong evidence that many of the immortalized PBMCs in cultures infected with mycoplasmas were evidently monoclonal in nature. For instance, more than 90% of immortalized K2267 PBMCs (K2267-Mi) were monosomic for chromosome 6 and more than 95% of immortalized K4413 PBMCs (K4413-Mi) had a unique translocation between chromosomes 6 and 22. On the other hand, as expected, not all the immortalized PBMCs that were found to be monoclonal by Ig light-chain restriction showed detectable abnormality in karyotyping. For example, no chromosomal changes could be identified in the immortalized K4123, K1277, or K1025 PBMCs (K4123-Mi, K1227-Mi and K1025-Mi) that were apparently monoclonal in nature.
It was surprising to find that viable mycoplasmas were no longer present in many cultures when the PBMCs had become immortalized. Since the same mycoplasma stocks introduced into the cultures of human T- and B-cell lines as well as monocyte cell lines showed excellent growth, it was not clear why the mycoplasmas failed to continuously grow in these primary PBMC cultures. Despite the fact that most of the lymphoid cells were rapidly dying, active antimicrobial phagocytosis of monocytes and leukocytes as well as many cytotoxic cytokines released in the cultures of mixed cell types might still be fully functional. It was likely that the mixed cell types of primary PBMCs still had potent microbicidal effects in culture. Furthermore, since mycoplasmas would not be able to grow by themselves in the serum-supplemented cell culture media, their growth would very much depend on the growth of the mammalian host cells in culture. In the first 2 to 3 weeks, cell growth was extremely limited in our human PBMC cultures. Most of the PBMCs in the cultures were in fact dying. Mycoplasmas might not be able to sustain their growth in this poor culture condition for 3 to 4 weeks before a significant amount of the immortalized cells begin to proliferate. However, failure of continued growth or survival of the mycoplasmas in the primary human PBMC cultures apparently did not necessarily affect their promoting effects of B-cell immortalization. Moreover, the study showed that abnormal chromosomal changes evidently occurred in many of the immortalized PBMCs that were already free of viable Mi in the cultures. The result is somewhat consistent with our earlier finding that, differing from oncogenesis induced by most oncogenic viruses, continuing presence of mycoplasma is not required for the maintenance of a malignant phenotype in mycoplasma-mediated cell transformation.37
It was not surprising to find that all the immortalized PBMCs were positive for telomerase activity. Activation of this unique polymerase to effectively cap the ends of chromosomes in cell division could be crucial for these cells to gain the ability to continue to grow and become “immortalized.” However, none of these immortalized human PBMCs were found to be tumorigenic when injected into nude mice. This could mean that, despite having prominent chromosomal aberrations and characteristics of monoclonality, these immortalized PBMCs had not gained all the malignant properties, such as the ability to form tumors in nude mice. However, it is also important to bear in mind that most of human malignant tumor cells also fail to grow into tumors when injected into nude mice.
Chronic mycoplasmal infections could induce genetic instability and chromosomal aberrations as well as prevent cells from undergoing apoptosis.3,4 Such a combination would likely lead to malignant cell transformation. We believe that the mycoplasma-oncogenesis model can be an excellent in vitro tool for studying cancer cell transformation.6 The process had a long latency and also presented progressive phases with multiple stages in the course of malignant transformation, a paradigm of multistep carcinogenesis occurring in nature.3,4,6 However, the long latency of the mycoplasma-mediated effects would require a chronic persistent infection; it is particularly challenging in studying transformation of primary human cells, such as PBMCs, that normally undergo apoptosis quickly in culture without supplement of specific growth factors. Ironically, continued growth of the mycoplasmas in the culture would depend on the continued growth and well being of the PBMCs.
As previously reported, many healthy individuals were infected by EBV, and EBV-infected human B lymphocytes could undergo spontaneous immortalization in culture. EBV is believed to play an essential but not sufficient role in causing certain B-cell malignancies. Our present study revealed that mycoplasmas could apparently markedly promote this in vitro immortalization process of human PBMCs. Moreover, differing from the human B lymphocytes that undergo spontaneous immortalization, the B lymphocytes that became immortalized in culture infected with mycoplasma were much more likely to be monoclonal in nature and showed genetic instability with prominent chromosomal aberrations. Earlier findings of mycoplasmal infection in blood and bone marrow of patients with various lymphoid malignancies by different laboratories7,8 could be meaningful. The present findings could be of particular relevance to lymphoproliferation that arises in patients with compromised immune functions,38-40 such as posttransplantation lymphoproliferative disorders (PTLDs), X-linked lymphoproliferative syndrome and Wiscott-Aldrich syndrome, and AIDS-associated lymphoproliferative diseases. Proliferating lymphoid tumors that occur in immunosuppression are most often of B-cell origin, ranging from atypical polyclonal B-cell proliferation to characteristic monormorphic non-Hodgkin lymphomas. Specifically, the vast majority of PTLD cases are EBV positive38,40,41 and often appear to closely resemble in vitro immortalization of blood B lymphocytes. Increased infection of certain mycoplasmas in the immune-suppressed transplantation patients, as already reported by some studies,42,43 by implication, might play an important promoting role in development of lymphoproliferative disorders in these patients.
Prepublished online as Blood First Edition Paper, August 26, 2004; DOI 10.1182/blood-2004-04-1245.
Supported by the research grants UBEX and UBIM (S.Z. and S.-C.L.) from American Registry of Pathology/Armed Forces Institute of Pathology.
S.Z. and S.T. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Wei-Sing Chu for his help with immunohistochemistry; Mr Jose Rodriguez for his excellent help in animal studies; and Dr Shaw-Huey Feng, Dr Nianxiang Zou, and Miss Tamara Newsome for their critical suggestions.