Abstract
Autologous peripheral blood mononuclear cells (PBMC) cryopreserved from a leukapheresis collection comprise the starting cellular source for the Wave® Bioreactor-based Xcellerate III Process [
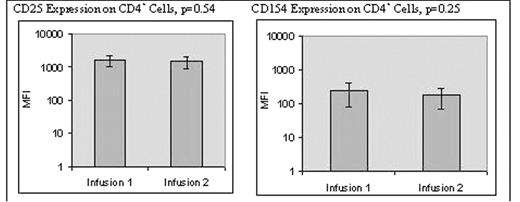
• No significant difference in the in-process T cell activation as determined by increase in cell size, up-regulation of CD25 & up-regulation of CD154 expression (refer to Figure 1).
• The total cell yield for 2nd infusion products is within one (1) standard deviation of the average for the manufacture of the 1st infusion product (refer to Table 1).
• No significant difference in cell viability, CD3+ purity, or CD4:CD8 ratio for the final Xcellerated T Cells product (refer to Table 1).
Table 1. Final Product Characteristics
| Final Product (Day 13) Average±S.D. . | p value . | 1st Infusion (n=13) . | 2nd Infusion (n=11) . |
|---|---|---|---|
| Total Cell Yield (x109) | 0.01 | 137 ± 35 | 104 ± 17 |
| Cell Viability (%) | 0.15 | 93.5 ± 3.4 | 91.6 ± 2.4 |
| CD3+ Purity (%) | <0.001 | 98.4 ± 1.1 | 99.0 ± 0 |
| CD4:CD8 Ratio | 0.32 | 8.5 ± 7.7 | 5.7 ± 4.5 |
| Final Product (Day 13) Average±S.D. . | p value . | 1st Infusion (n=13) . | 2nd Infusion (n=11) . |
|---|---|---|---|
| Total Cell Yield (x109) | 0.01 | 137 ± 35 | 104 ± 17 |
| Cell Viability (%) | 0.15 | 93.5 ± 3.4 | 91.6 ± 2.4 |
| CD3+ Purity (%) | <0.001 | 98.4 ± 1.1 | 99.0 ± 0 |
| CD4:CD8 Ratio | 0.32 | 8.5 ± 7.7 | 5.7 ± 4.5 |
These data demonstrate high reproducibility and robustness of the Xcellerate III Process when using PBMC from the same leukapheresis collection in sequential processing runs. In addition, these data demonstrate that cryopreserved PBMC can be stored for many months prior to their use as the starting material in the Xcellerate III Process. Xcyte™, Xcyte Therapies™, Xcellerate™, Xcellerated T Cells™ and the circle logo are trademarks of Xcyte Therapies, Inc.
Author notes
Corresponding author