Abstract
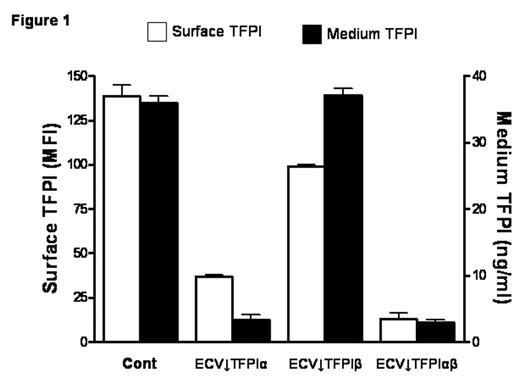
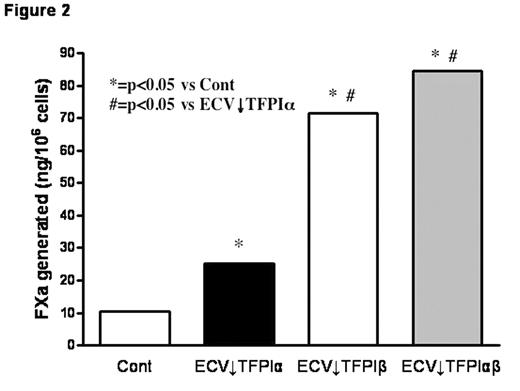
TFPI is mainly produced by endothelial cells and alternative mRNA splicing generates two forms, TFPIα and TFPIβ. A small proportion of expressed TFPI remains associated with the cell surface through both direct (TFPIβ) and indirect (TFPIα) glycosylphosphatidylinositol (GPI)-mediated anchorage. TFPIβ lacks the C-terminus and Kunitz-3 (K3) domains of TFPIα and, instead, contains an unrelated C-terminus that directs the attachment of a GPI anchor. TFPIα and TFPIβ migrate with similar MWs (~45,000) on SDS-PAGE due to differing levels of glycosylation; removal of carbohydrate produces protein MWs of 36,000 and 28,000, respectively. Stable clones of ECV304 cells, a human cell line with some endothelial properties, that express reduced levels of TFPIα, TFPIβ, or both were produced using a plasmid-based small interfering RNA gene-silencing technique (siRNA) (Table 1); empty vector transfected clones served as controls. Total TFPI on the cell surface and in the medium of the selected clones was evaluated by flow cytometry (expressed as mean fluorescence intensity) and immunoassay, respectively (Fig. 1). Surface TFPI activity was determined by a two stage chromogenic assay based on the ability of each form to inhibit FX activation by FVIIa on cells with comparable levels of tissue factor (TF). Although TFPIβ accounted for only ~20% of the cell surface TFPI, it was responsible for most of the anti-FVIIa/TF activity (Fig. 2), suggesting a potential alternative role for surface TFPIα. At physiologic concentrations, rTFPIα expressed by ECV304 and mouse C127 cells binds to ECV↓TFPIα cells, but rTFPIα produced by mammalian A-293, HepG2, and CHO cells, and by E. coli does not, implying that post-translational modifications are important for the cell binding of TFPIα. Experiments in C127 cells show that optimal cell binding of TFPIα requires its basic C terminus and the P1 residue (R199) of its K3 domain; mutations within the putative heparin binding region of K3 (K232A, K240A, K241A) do not affect binding. Additional preliminary studies suggest that the cell binding of TFPIα requires N-linked glycosylation and is also reduced by mutation of serine 2, which is phosphorylated in C127 cells. Overall, these results call into question the physiologic relevance of previous cell binding experiments that relied on the use of high concentrations of rTFPIα expressed in E. coli.
table 1
| . | Surface TFPIα (ng/107 cells) . | Surface TFPIβ (ng/107 cells) . |
|---|---|---|
| Each result represents the average of five independent experiments | ||
| Cont | 2.3 | 0.5 |
| ECV↓TFPIα | 0.4 | 0.5 |
| ECV↓TFPIβ | 2.4 | 0.04 |
| ECV↓TFPIαβ | 0.3 | 0.05 |
| . | Surface TFPIα (ng/107 cells) . | Surface TFPIβ (ng/107 cells) . |
|---|---|---|
| Each result represents the average of five independent experiments | ||
| Cont | 2.3 | 0.5 |
| ECV↓TFPIα | 0.4 | 0.5 |
| ECV↓TFPIβ | 2.4 | 0.04 |
| ECV↓TFPIαβ | 0.3 | 0.05 |
Author notes
Corresponding author