Abstract
Anaplastic large cell lymphoma (ALCL) designates a heterogeneous group of CD30+ (systemic or primary cutaneous) peripheral T-cell lymphomas (PTCLs). A subgroup of systemic ALCL is transformed by anaplastic lymphoma kinase (ALK). We compared 24 ALK+, 15 ALK- systemic, and 7 cutaneous ALCLs with 29 nonanaplastic PTCLs in terms of T-cell receptor (TCR) rearrangements, expression of TCRs and TCR-associated molecules (CD3, ZAP-70 [zeta-associated protein 70]). Despite their frequent clonal rearrangement for TCRβ, only 2 (4%) of 47 ALCLs expressed TCRβ protein, whereas TCRs were detected on 27 of 29 nonanaplastic PTCLs. Moreover, both TCRβ+ ALCLs lacked CD3 and ZAP-70 (ie, molecules indispensable for the transduction of cognate TCR signals). Defective expression of TCRs is a common characteristic of all types of ALCL, which may contribute to the dysregulation of intracellular signaling pathways controlling T-cell activation and survival. This molecular hallmark of ALCL is analogous to defective immunoglobulin expression distinguishing Hodgkin lymphoma from other B-cell lymphomas. (Blood. 2004; 104:3358-3360)
Introduction
Anaplastic large cell lymphoma (ALCL) of T-cell type comprises distinct entities of lymphomas expressing CD30. The World Health Organization (WHO) classification of malignant lymphomas1 distinguishes systemic and primary cutaneous forms with systemic ALCL comprising about 26% of all peripheral T-cell lymphomas (PTCLs).2 Although CD30 expression is a consistent but not defining feature of ALCL (because other PTCLs can express CD30 as well), the diagnosis of ALCL either rests on morphology or the detection of overexpressed anaplastic lymphoma kinase (ALK). Morphologically, the large and polymorphic tumor cells sometimes resemble Reed Sternberg cells, and their sheetlike growth pattern is distinct from most other PTCLs that often exhibit a minor tumor cell population in an inflammatory background. ALK is overexpressed in about 60% to 85% of systemic ALCLs and defines a clinically homogeneous group with good prognosis.1
Recent research has focused on the molecular mechanisms of ALK overexpression and its biologic consequences. In ALK- cases the exact mechanism of transformation is still unknown.3 Therefore, a unifying concept for both ALK+ and ALK- as well as systemic and cutaneous ALCL is still missing. Our finding that both ALK+ and ALK- ALCL lack T-cell receptor (TCR) expression may provide this unifying feature.
Study design
We have studied the expression of TCRs αβ (βF1; Serotec, Düsseldorf, Germany) in 46 ALCLs (24 ALK+, 15 systemic ALK-, 7 cutaneous) by immunohistochemistry. We also analyzed CD30, ALK1, CD8 (all DAKO Cytomation, Hamburg, Germany), CD3ϵ, CD4, Perforin (Novocastra, Newcastle, United Kingdom), zeta-associated protein 70 (ZAP-70; Upstate, Lake Placid, NY), T-cell intracellular antigen-1 (TIA1; Coulter Immunotech, Marsaille, France), and GranzymB (Monosan, Am Uden, The Netherlands).
Frozen material of 12 ALK+, 5 ALK- systemic, and 1 primary cutaneous ALCLs was studied in more detail, including the expression of TCRγδ (δF1; T-Cell Diagnostics, Woburn, MA) and the natural killer (NK)-cell receptor CD94 (DAKO Cytomation). All slides were evaluated using an Olympus BX50 microscope and a × 40/0.85 objective lens. Images were taken with an Olympus Cammedia C-4040 Zoom camera applying DP-Soft software version 3.2 at 2048 × 1536 pixels (all from Olympus, Hamburg, Germany). To detect weak expression levels at higher sensitivity, coexpressions of CD30 and βF1 or CD30, CD3, and ZAP-70, respectively, were investigated by double and triple immunofluorescent stains and confocal laser scanning microscopy with a HCXPL Apo × 40/1.25-0.75 oil CS objective lens and Leica Confocal Software version 2.5 (Leica, Mannheim, Germany). Rearrangements of the TCRβ and TCRγ gene families were analyzed according to the Biomed2 protocol.4
Twenty-two not otherwise specified PTCLs (PTCL-NOSs) and 7 angioimmunoblastic T-cell lymphomas (AILTs) were studied in comparison.
Immunophenotypes were compared by chi-square tests or Spearman rank correlation (ZAP-70) by using Statistica for Windows (Statsoft GmbH, Hamburg, Germany) to adopt the comparison to the scales used.
Results and discussion
Only 2 (4%) of 46 ALCLs expressed the TCRβ chain on tumor cells (Figure 1; Table 1), and all were negative for TCRδ and the NK-cell receptor CD94. In both βF1+ cases, TCRβ expression was restricted to a subpopulation of the tumor cells. By contrast, TCR molecules were detected in 20 of 22 PTCL-NOSs and in 7 of 7 AILTs.
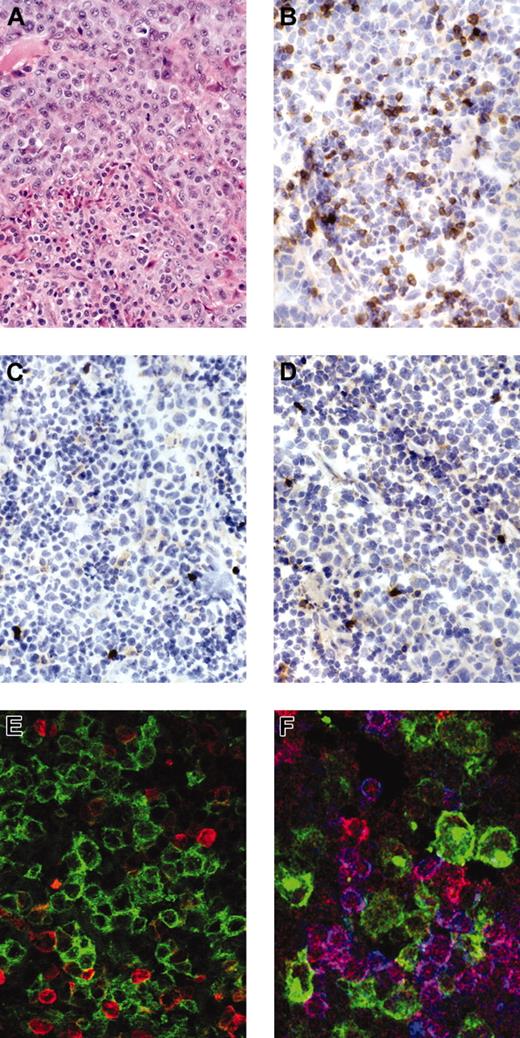
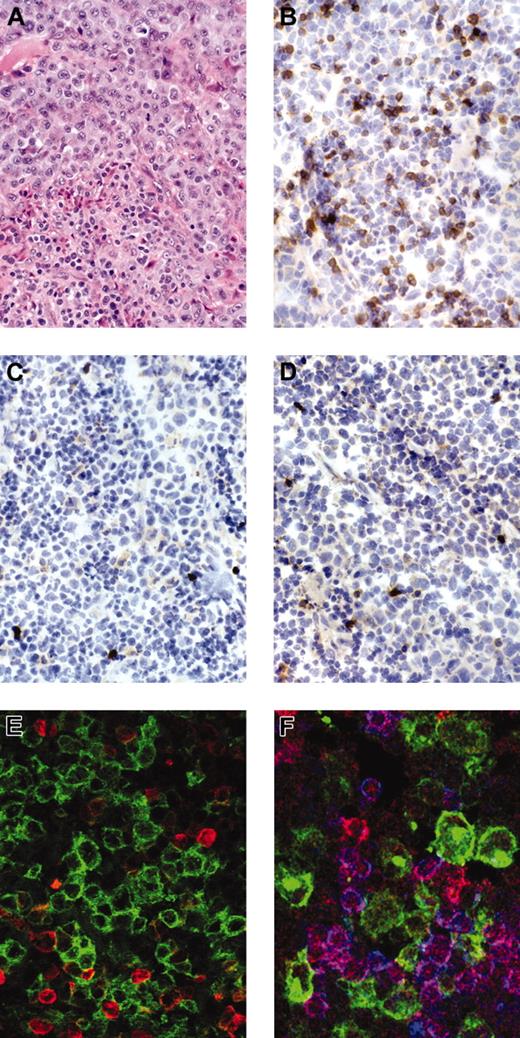
Staining for anaplastic large cell lymphoma. Anaplastic large cell lymphoma stained for H&E (hematoxylin and eosin; A), immunostains for βF1 (B), CD3 (C), and ZAP-70 (D) show negativity of the tumor cells with strong expression of the respective antigens in reactive small T cells. Immunofluorescent stains for (E) CD30 (green) and βF1 (red) (Zoom 2) or (F) CD30 (green), ZAP-70 (red), and CD3 (blue) confirm the negativity of CD30+ tumor cells for the respective antigens.
Staining for anaplastic large cell lymphoma. Anaplastic large cell lymphoma stained for H&E (hematoxylin and eosin; A), immunostains for βF1 (B), CD3 (C), and ZAP-70 (D) show negativity of the tumor cells with strong expression of the respective antigens in reactive small T cells. Immunofluorescent stains for (E) CD30 (green) and βF1 (red) (Zoom 2) or (F) CD30 (green), ZAP-70 (red), and CD3 (blue) confirm the negativity of CD30+ tumor cells for the respective antigens.
CD3 molecules are associated with the TCR and transduce the signal of TCR engagement to ZAP-70, a TCR-associated tyrosine kinase that integrates cognate and costimulatory signals to guide downstream signaling.5,6 CD3 was lacking in all but 1 systemic ALK1+ ALCL and in 40% of ALK1- systemic ALCLs in our study. In most other cases, its expression was restricted to a subset of the tumor cells and was much weaker than in accompanying small T cells. The frequent lack of TCRs and of CD3, βF1, or both has been demonstrated previously in ALCL.7 CD3 was not detected in 15 of 17,8 5 of 6,9 and 47 of 7010 cases of ALCL.
ZAP-70 was lacking in more than 70% of all ALCL cases studied. Interestingly, both TCRβ+ cases lacked both CD3 and ZAP-70, indicating that proximal TCR signaling may be impaired in all ALCLs investigated. A low protein expression of ZAP-70 has also been reported in ALCL as compared with other PTCLs.11
In the present series of 19 cases with frozen material, TCRβ and TCRγ genes were clonally rearranged in 74% each. One case each was rearranged for TCRβ but not TCRγ and vice versa. Only 3 cases, all ALK+, showed a Gaussian distribution of peak signals in gene scans. The tumor cells in these cases expressed cytotoxic granules, indicating their T-cell derivation.12 Because only a minor tumor cell population was present in an inflammatory background, existing TCR rearrangements may not have been detected.
TCR expression on the tumor cell surface in ALCL has only rarely been investigated. Early studies found TCRβ expressed in 7 of 713 and 9 of 157 ALCLs studied. In another series, TCRβ was detected in only 4 of 19 cases but TCRδ or NK cell receptor expressions were not investigated.14 Barry et al15 reported on 5 PTCLs containing small pleomorphic and large CD30+CD15+ Reed-Sternberg-like tumor cells, in which TCRβ was expressed in the small cell population but missing in the large cells. On the genomic level, TCRβ rearrangements have been detected previously in 90% of ALCLs of both T-cell and null cell type,14 and many ALCLs negative for pan-T-cell markers have somatically rearranged TCR genes.10
Until today the distinction between ALK1- ALCL and CD30+ cases of PTCL-NOS has not been conceptionally clarified. Three (14%) of our 22 PTCL-NOSs were CD30+. In contrast to the ALCL, all of them expressed the TCRβ chain. Although currently there is no difference in treatment options, the lack of TCR expression may well help to further delineate ALK- ALCL.
Considering the presence of somatic TCRβ rearrangements, the normal counterpart of ALCL appears to be an αβ T cell in most, if not all, cases. Further studies are needed to understand the mechanisms underlying the failure of ALCL to express TCRs on the cell surface. Among cell lines derived from ALK+ ALCL, Karpas299 expresses TCRβ RNA by Northern blot analysis,16 whereas both Sup-M2 and Su-DHL-1 lack full-length TCRβ RNA despite rearrangements for the TCRβ genes.17 Mutations in coding or regulatory regions of the gene or a lack of TCR-specific transcription factors may thus underlie the defective TCR expression, ie, mechanisms that have been implied to explain the lacking immunoglobulin expression in Hodgkin lymphoma.18,19 Alternatively, posttranscriptional mechanisms affecting RNA processing or protein stability are also conceivable, as described recently for the defective TCRβ protein expression in GATA-3 (GATA-binding protein-3)-deficient murine thymocytes.20
Although the effects of ALK overexpression are well studied, the exact mechanism of transformation in ALK- cases is still unknown.3 ALK mediates mitogenicity by way of phospholipase C-γ21 and Cyclin D322 and inhibits apoptosis by way of the phosphatidylinositol 3-kinase/Akt23 and the JAK/STAT (Janus kinase/signal transducer and activator of transcription) pathways.24,25 Physiologically, STAT3 and CyclinD3 activation are partially counterbalanced by the effects of TCR signaling: In activated cytokine-dependent T cells, TCR ligation may down-regulate CyclinD3 and block cytokine activity by inhibiting signal transduction by way of the JAK/STAT pathway.26 Thus, the inability of the tumor cells in ALCL to cognate TCR interactions, as suggested by our data, may perhaps contribute to the transformation in ALK- cases because of lacking feedback inhibition by way of TCR signaling.
In summary, both cutaneous and systemic as well as ALK+ and ALK- ALCLs lacked TCRs on the cell surface. Both causes and consequences of this observation require further study. The absence of TCR expression in concert with the positivity for CD30 and cytotoxic granules such as perforin12 may now allow ALCL to be delineated more precisely from large cell variants of PTCL-NOS, which can also express CD30.
Prepublished online as Blood First Edition Paper, August 5, 2004; DOI 10.1182/blood-2004-03-1037.
Supported by the German Cancer Foundation (grant 70-3131-Rü1) (T.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Dr Edgar Serfling for critical review of this manuscript.