Abstract
Primary effusion lymphomas (PELs) represent a unique non-Hodgkin lymphoma that is consistently infected by Kaposi sarcoma herpesvirus (KSHV). PEL cells express high levels of the cell cycle inhibitor p27KIP1 and yet proliferate actively. KSHV genome encodes a viral cyclin homolog, v-cyclin, which has previously been implicated in down-regulation of p27KIP1 levels. To address how PEL cells can tolerate high p27KIP1 levels, we investigated functional interactions between v-cyclin and p27KIP1 using PEL-derived cell lines as a model system. Here we demonstrate that v-cyclin and p27KIP1 stably associate in PEL cells in vivo suggesting an attractive model by which p27KIP1 is inactivated in the actively proliferating PEL cells. Moreover, we show that v-cyclin and cyclin-dependent kinase 6 (CDK6) form an active kinase without p27KIP1 and that CDK6 is the in vivo catalytic subunit of v-cyclin in PEL cells. These findings suggest that KSHV may promote oncogenesis in PEL by expressing v-cyclin, which both overrides negative cell cycle controls present in the PEL precursor cells and induces a strong proliferative signal via CDK6 kinase activity. (Blood. 2004;104:3349-3354)
Introduction
Primary effusion lymphomas (PELs) are AIDS-related, non-Hodgkin B-cell lymphomas, where malignant effusions are found in the pleural, pericardial, or peritoneal cavities. All characterized PELs are positive for Kaposi sarcoma herpesvirus (KSHV), and several pieces of evidence suggest that KSHV may be required for AIDS-PEL pathogenesis.1,2 All KSHV-positive PEL cells express latent viral proteins,3 suggesting a role for these proteins in oncogenesis of this lymphoma. One of these proteins is v-cyclin,4,5 whose oncogenic potential was recently demonstrated by its ability to induce lymphomas in p53-/- mice.6,7 v-cyclin is a cyclin D homolog and associates with cellular cyclin-dependent kinase 6 (CDK6) upon expression in tissue culture cells.4,5 As its cellular counterparts, v-cyclin-CDK6 promotes cell cycle progression by phosphorylating specific target proteins such as retinoblastoma protein (pRb) and Histone H1. However, compared with cellular cyclin D-CDK4/6, v-cyclin-CDK6 has a broader substrate range in vitro that includes inhibitor of DNA binding protein 2 (Id-2), cdc25A, origin recognition complex-1 (ORC-1), CDC6, and Bcl-2.4,8-12
Previous studies have indicated that v-cyclin-CDK6 complexes are not sensitive to the p27KIP1 cell cycle inhibitor. p27KIP1 belongs to the complement inhibitory protein/kinase inhibitor protein (CIP/KIP) family of inhibitors, which regulate cell cycle by forming ternary complexes with cellular cyclin CDKs.13,14 When associated with cyclin E-CDK2, p27KIP1 inhibits CDK2 kinase activity and cell cycle progression. This inhibition by p27KIP1 is abrogated in situations where p27KIP1 is bound to cyclin D-CDK4/6 complexes. Sequestration of p27KIP1 by cyclin D-CDK4/6 prevents its interaction with, and the consequent inactivation of, cyclin E-CDK2.15 In overexpression models, v-cyclin can bypass the p27KIP1-induced cell cycle arrest by down-regulating the p27KIP1 levels via phosphorylation.9,11,16 However, this mechanism does not seem to be effective in PEL cells, which appear to consistently overexpress p27KIP1.17 It is also not understood how PEL cells maintain a high proliferation rate despite high levels of this negative cell cycle regulator.
In this report we have investigated the levels and functional interactions between p27KIP1, v-cyclin, and CDK6 in PEL-derived cell lines. These represent a biologically relevant model for studying the interactions between the oncogenic Kaposi sarcoma herpesvirus and its host.
Materials and methods
Cell culture and transfections
U2OS human osteosarcoma cells were routinely cultured in a humidified 5% CO2 atmosphere at 37°C in Dulbecco modified Eagle medium (DMEM), supplemented with 10% (wt/vol) fetal calf serum (FCS). PEL cell lines BC-1,18 BC-2,18 and BCBL-119 were obtained from American Type Culture Collection (ATCC; Manassas, VA). BC-3 cell line20 was kindly provided by Dr Ethel Cesarman (Cornell Medical College, New York City, NY). PEL cell lines and JOK-1 hairy cell leukemia cells (a kind gift from Dr Leif Andersson, University of Helsinki, Helsinki, Finland) were cultured in a humidified 5% CO2 atmosphere at 37°C in RPMI 1640 medium supplemented with 15% FCS (Invitrogen, Carlsbad, CA). U2OS cells were transiently transfected using Fugene 6 transfection reagent according to the manufacturer's instructions.
Expression vectors
The expression vectors used in this study and previously described were as follows: pRc-Kip1/p27KIP1 and pcDNA3-p21 (kind gifts from Rene Bernards, The Netherlands Cancer Institute, Amsterdam, the Netherlands), pcDNA3-Myc-v-cyclin (a kind gift from Sibylle Mittnacht, The Institute of Cancer Research, London, United Kingdom), and pRc-cyclin D2 (a kind gift from Philip Hinds, Harvard Medical School, Boston, MA). Hemagglutinin (HA)-CDK6 was expressed from pCMV-HA-CDK6.21
Antibodies and reagents
Mouse monoclonal antibody recognizing the Myc epitope (9E10) was from Babco (Berkeley, CA). Sheep polyclonal antibody to v-cyclin was obtained from Exalpha Biologicals (Boston, MA). Rabbit polyclonal antibodies to CDK6 (C-21), CDK4 (C-22), CDK2 (M2), cyclin D2 (C-17), p27KIP1 (C-19), p21 (C-19), and actin (C-2) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal antibody to CDK4 (DCS-35) was from NeoMarkers (Fremont, CA). Antihuman p27KIP1/Kip-1 mouse monoclonal antibody was from Upstate Biotechnology (Lake Placid, NY). The rabbit polyclonal antibody against v-cyclin was a kind gift from Emmy Verschuren (Stanford University, Stanford, CA). Bisbenzimide Hoechst 33342 was obtained from Sigma Chemical (St Louis, MO), the Fugene 6 transfection reagent was purchased from Roche Diagnostics Corporation (Indianapolis, IN), and Lipofectamine 2000 was from Invitrogen.
Western blotting
Tissue culture cells were lysed into lysis buffer (150 mM NaCl; 50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4; 0.1% Igepal; 5 mM EDTA [ethylenediaminetetraacetic acid]; 2 mM dithiothreitol [DTT]; 1 mM phenylmethylsulfonyl fluoride [PMSF]; leupeptin 2 μg/mL; pepstatin 2 μg/mL; and aprotinin 1.5 μg/mL). Forty micrograms of total proteins were analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted according to standard protocols.
Indirect immunofluorescence and apoptosis assay
Transfected cells on coverslips were fixed with 3.5% (wt/vol) paraformaldehyde (PFA), permeabilized with 0.1% TX-100 for 5 minutes, and labeled as described previously.22 DNA was stained with Hoechst 33342 (0.5 μg/mL) for 5 minutes, and the coverslips were mounted in Mowiol mounting medium (Calbiochem, La Jolla, CA) on glass slides and evaluated under a Zeiss Axioplan 2 fluorescent microscope (Carl Zeiss, Germany) equipped with Zeiss PLAN-NEOFLUOR × 20/0.50 objective lenses. 1004-989 imaging medium was used along with fixed coverslips. Images were acquired with a Zeiss Axiocam H RC, using Zeiss AxioVision and Adobe PhotoShop 7.0 (Adobe, San Jose, CA) software. Transfected cells were scored after 48 hours by expression of Myc-tagged v-cyclin (detected with 9E10) and HA-CDK6 (detected with C-21). Apoptosis in transfected cells was quantified from the Hoechst morphology of nuclei as described previously,22 and the results were displayed as the percentage of cells that were in apoptosis. At least 200 transfected cells were scored for each sample, and the results are based on at least 3 independent experiments with standard deviation (SD).
In vitro kinase assays
Primary effusion lymphoma cells were lysed into lysis buffer “Western blotting” supplemented with 25 mM β-glycerophosphate. For measuring the in vitro kinase activity toward Histone H1 (Roche Diagnostics Corporation) or glutathione-S-transferase (GST)-pRb,23 the lysates from primary effusion lymphoma cells were incubated 2 hours at 4°C with the sheep polyclonal anti-v-cyclin antibody. Immunocomplexes were coupled to protein A Sepharose beads for an additional 1 hour at 4°C and washed 3 times with the lysis buffer followed by one wash with the kinase buffer (20 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.5; 50 mM KCl; 7.5 mM MgCl2; 1 mM DTT; 25 mM β-glycerophosphate; leupeptin 2 μg/mL; pepstatin 2 μg/mL; and aprotinin 1.5 μg/mL). Immunodepletion was performed with 3 rounds of immunoprecipitation either with anti-CDK6, anti-CDK4, anti-CDK2, or anti-p27KIP1 antibody and the depleted lysates were immunoprecipitated with anti-v-cyclin antibody. Kinase reactions were performed in the presence of 2 μCi (0.074 MBq) of [32P] adenosine triphosphate (ATP) for 15 minutes at 30°C using 5 μg of Histone H1 or 5 μg of GST-pRb as a substrate. Phosphorylated proteins were analyzed by SDS-PAGE and autoradiography.
Immunoprecipitation assay
For detection of association between v-cyclin, CDK6, and p27KIP1, U2OS cells were transfected with Myc-v-cyclin, HA-CDK6, and p27KIP1 expression vectors, lysed with the lysis buffer, and 350 μg of cell extracts were incubated with anti-Myc mouse monoclonal antibody for 1 hour at +4°C. For detection of association between v-cyclin, CDK6, and p27KIP1 in PEL cells, 350 μg of cell extracts were incubated either with anti-v-cyclin sheep polyclonal antibodies or anti-p27KIP1 rabbit polyclonal antibodies for 2 hours at +4°C. Immunoprecipitations were performed as described for the in vitro kinase assay and subjected to SDS-PAGE and Western blotting analysis.
RNA interference
U2OS cells were cotransfected with 0.7 μg of pcDNA3-Myc-v-cyclin and 3.5 μg siRNA specific to human p27KIP1 (5′GAGCCAACAGAACAGAAGAtt′3; 3′UCUUCUGUUCUGUUGGCUCtt′5) using Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions. A control siRNA (to a tumor suppressor Lkb1; a kind gift from Dr Marie Aronson, University of Helsinki, Helsinki, Finland) was used as a negative control in the experiment. Cells were collected after 48 hours and lysed. Myc-v-cyclin was immunoprecipitated from 300 μg of cell lysate with anti-Myc antibodies and subjected to an in vitro kinase assay toward GST-Rb and Histone H1 as described for the in vitro kinase assays.
Results
v-cyclin associates with p27KIP1 in primary effusion lymphoma-derived cell lines
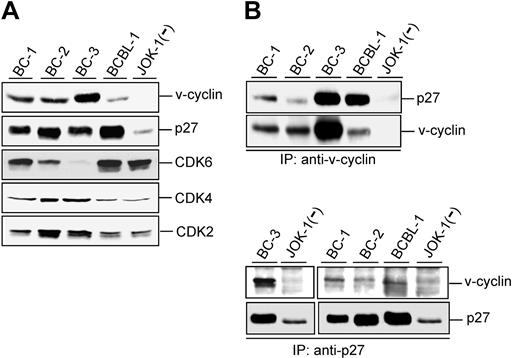
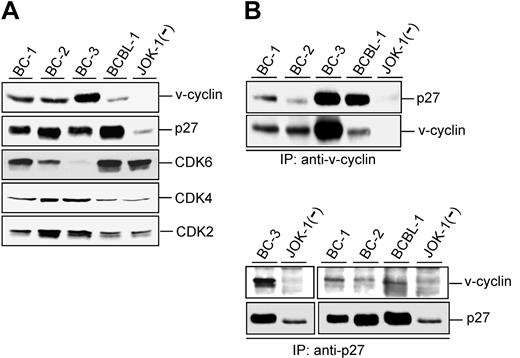
KSHV-infected PEL cell lines express unusually high levels of p27KIP1 compared with lymphomas in general.17,24 To correlate this with expression levels of other relevant cell cycle regulators, we first analyzed 4 PEL-derived cell lines (BC-1, BC-2, BC-3, and BCBL-1) for their expression of v-cyclin and CDK6, CDK4, CDK2, and p27KIP1 (Figure 1A). Consistent with previous reports,17,25 v-cyclin was expressed in all analyzed PEL cell lines, but the expression levels differed markedly between them. The p27KIP1 levels were elevated in PEL cell lines compared with JOK-1, a KSHV-negative hairy cell leukemia cell line. CDK6 expression varied considerably, with the highest levels detected in BC-1 and BCBL-1 and the lowest in BC-2 and BC-3 cells. CDK2 and CDK4 were detectable in all analyzed cell lines with slightly higher expression in BC-2 and BC-3 cells.
v-cyclin associates with p27KIP1 in PEL cells. (A) Total lysates of PEL cells (40 μg) were resolved by SDS-PAGE (12%) and immunoblotted with antibodies to v-cyclin, p27KIP1, CDK6, CDK4, and CDK2. BC-1, BC-2, BC-3, and BCBL-1 are KSHV-positive PEL cell lines, and JOK-1 cells are KSHV-negative leukemia cells. (B) Lysates of the PEL and JOK-1 cells were immunoprecipitated (IP) with anti-v-cyclin (2 top panels) or anti-p27KIP1 antibodies (2 bottom panels) and analyzed for associated proteins on SDS-PAGE by immunoblotting with anti-v-cyclin and anti-p27KIP1 antibodies. The interaction between v-cyclin and p27KIP1 after immunoprecipitation with anti-p27KIP1 antibodies is readily detectable in a short exposure from BC-3 cells (left), whereas a longer exposure was used for BC-1, BC-2, and BCBL-1 (right).
v-cyclin associates with p27KIP1 in PEL cells. (A) Total lysates of PEL cells (40 μg) were resolved by SDS-PAGE (12%) and immunoblotted with antibodies to v-cyclin, p27KIP1, CDK6, CDK4, and CDK2. BC-1, BC-2, BC-3, and BCBL-1 are KSHV-positive PEL cell lines, and JOK-1 cells are KSHV-negative leukemia cells. (B) Lysates of the PEL and JOK-1 cells were immunoprecipitated (IP) with anti-v-cyclin (2 top panels) or anti-p27KIP1 antibodies (2 bottom panels) and analyzed for associated proteins on SDS-PAGE by immunoblotting with anti-v-cyclin and anti-p27KIP1 antibodies. The interaction between v-cyclin and p27KIP1 after immunoprecipitation with anti-p27KIP1 antibodies is readily detectable in a short exposure from BC-3 cells (left), whereas a longer exposure was used for BC-1, BC-2, and BCBL-1 (right).
v-cyclin has previously been indicated to have a role in modulating p27KIP1 levels.9,11 We therefore assessed potential physical interactions between v-cyclin and p27KIP1 in the PEL cell lines. v-cyclin was immunoprecipitated from the protein extracts of the cell lines with anti-v-cyclin antibody and the immunoprecipitates were analyzed by Western blotting with anti-p27KIP1 antibodies. As shown in Figure 1B, p27KIP1 was coimmunoprecipitated with v-cyclin in all PEL cell lines. When p27KIP1 was reciprocally immunoprecipitated from the same cell lysates with an antibody against p27KIP1, the strongest signal for v-cyclin was detected from the BC-3 cell line (Figure 1B left) correlating with the highest expression of v-cyclin. v-cyclin was coimmunoprecipitated with p27KIP1 also from the other PEL cells but the signal was weaker than in BC-3 (Figure 1B right). This may be partly due to the lower levels of v-cyclin in these cell lines and/or that the p27KIP1 antibody is not as efficient to bring down the complex.
Physical association between v-cyclin and p27KIP1 was further confirmed by GST-v-cyclin affinity purification from PEL cells (data not shown). The readily detectable physical association between v-cyclin and p27KIP1 was unexpected, considering previous suggestions that v-cyclin mediates degradation of p27KIP1.9,11
Ectopic expression of p27KIP1 stabilizes v-cyclin and CDK6
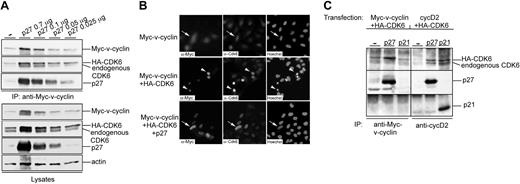
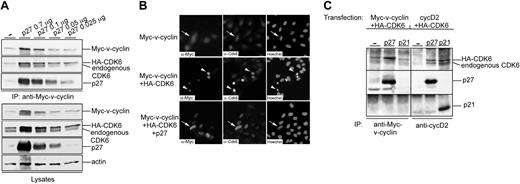
To further characterize the complex formed by v-cyclin and p27KIP1, U2OS cells were transfected with expression vectors for Myc-v-cyclin together with HA-CDK6 and p27KIP1. Notably, levels of both Myc-v-cyclin and HA-CDK6 were increased in cells cotransfected with p27KIP1 compared with cells coexpressing Myc-v-cyclin and HA-CDK6 (Figure 2A, the first two lanes at the bottom). To assess the effect of decreasing p27KIP1 levels on the stability of Myc-v-cyclin and HA-CDK6, decreasing amounts of p27KIP1 (0.7, 0.1, 0.05, and 0.025 μg, respectively) were cotransfected with Myc-v-cyclin and HA-CDK6 (Figure 2A). Proteins physically associating with v-cyclin were analyzed by performing anti-Myc immunoprecipitations from the transfected cell lysates. In accordance with our results from the PEL cells, v-cyclin associated with p27KIP1 (Figure 2A top). v-cyclin interacted with p27KIP1 even when relatively low levels of p27KIP1 were expressed. Moreover, both endogenous CDK6 and transfected HA-CDK6 were coimmunoprecipitated with v-cyclin. The levels of p27KIP1 correlated with Myc-v-cyclin and HA-CDK6 levels in total lysates as well as in immunoprecipitates (Figure 2A, the second through fifth lanes).
Ectopic expression of p27KIP1 stabilizes v-cyclin and CDK6. (A) U2OS cells were cotransfected with expression vectors for Myc-v-cyclin, HA-CDK6, and different amounts of p27KIP1 (0.7, 0.1, 0.05, and 0.025 μg). Cell lysates were immunoprecipitated with anti-Myc antibody and associated proteins were analyzed on SDS-PAGE (12%) by immunoblotting with anti-Myc, anti-CDK6, and anti-p27KIP1 antibodies (3 top panels). Total lysates of transfected cells were resolved by SDS-PAGE and immunoblotted with anti-Myc, anti-CDK6, anti-p27KIP1, and antiactin antibodies (4 lower panels). (B) Coexpression of p27KIP1 protects cells from v-cyclin-CDK6-induced cell death. U2OS cells were cotransfected with expression vectors for Myc-v-cyclin alone or together with HA-CDK6 and p27KIP1 (0.7 μg). Cells were analyzed 48 hours after transfection by indirect immunofluorescence. The cells were labeled by anti-Myc and anti-CDK6 antibodies and their nuclear morphology was visualized by Hoechst staining. Cells showing healthy Myc-v-cyclin and CDK6 (endogenous or HA-tagged)-expressing cells are indicated by arrows, whereas arrowheads are pointing at apoptotic cells. (C) U2OS cells were cotransfected with Myc-v-cyclin, HA-CDK6, and p27KIP1 or p21CIP1 (left) or with cyclin D2, HA-CDK6, and p27KIP1 or p21CIP1 (right). Cell lysates were immunoprecipitated with anti-Myc or anti-cyclin D2 antibodies and associated proteins were analyzed on SDS-PAGE (12%) by immunoblotting with anti-CDK6, anti-p27KIP1, and anti-p21CIP1 antibodies. — indicates no inhibitors (A,C).
Ectopic expression of p27KIP1 stabilizes v-cyclin and CDK6. (A) U2OS cells were cotransfected with expression vectors for Myc-v-cyclin, HA-CDK6, and different amounts of p27KIP1 (0.7, 0.1, 0.05, and 0.025 μg). Cell lysates were immunoprecipitated with anti-Myc antibody and associated proteins were analyzed on SDS-PAGE (12%) by immunoblotting with anti-Myc, anti-CDK6, and anti-p27KIP1 antibodies (3 top panels). Total lysates of transfected cells were resolved by SDS-PAGE and immunoblotted with anti-Myc, anti-CDK6, anti-p27KIP1, and antiactin antibodies (4 lower panels). (B) Coexpression of p27KIP1 protects cells from v-cyclin-CDK6-induced cell death. U2OS cells were cotransfected with expression vectors for Myc-v-cyclin alone or together with HA-CDK6 and p27KIP1 (0.7 μg). Cells were analyzed 48 hours after transfection by indirect immunofluorescence. The cells were labeled by anti-Myc and anti-CDK6 antibodies and their nuclear morphology was visualized by Hoechst staining. Cells showing healthy Myc-v-cyclin and CDK6 (endogenous or HA-tagged)-expressing cells are indicated by arrows, whereas arrowheads are pointing at apoptotic cells. (C) U2OS cells were cotransfected with Myc-v-cyclin, HA-CDK6, and p27KIP1 or p21CIP1 (left) or with cyclin D2, HA-CDK6, and p27KIP1 or p21CIP1 (right). Cell lysates were immunoprecipitated with anti-Myc or anti-cyclin D2 antibodies and associated proteins were analyzed on SDS-PAGE (12%) by immunoblotting with anti-CDK6, anti-p27KIP1, and anti-p21CIP1 antibodies. — indicates no inhibitors (A,C).
We have previously shown that v-cyclin together with high levels of CDK6 induces apoptosis in cells.22 Surprisingly, when p27KIP1 was cotransfected with v-cyclin and HA-CDK6, apoptosis was dramatically inhibited (Figure 2B). When the amount of apoptotic cells was quantitated, apoptosis decreased from 77% to 9% when p27KIP1 was present. As we have previously demonstrated,22 v-cyclin induces apoptosis only in the presence of high levels of CDK6 (Figure 2B top). The increased accumulation of Myc-v-cyclin and HA-CDK6 levels could not be due simply to the protection from apoptosis, since increased levels of Myc-v-cyclin were also seen when Myc-v-cyclin was coexpressed with p27KIP1 but in the absence of CDK6 overexpression (data not shown). These findings suggest that ectopic expression of p27KIP1 increases the abundance of Myc-v-cyclin and endogenous CDK6 as well as transfected HA-CDK6, which may result from enhanced expression, increased stability of these proteins, or both.
Both p27KIP1 and p21CIP1 inhibitors have been shown to stabilize and increase accumulation of cyclin D-CDK4 complexes in cells.26,27 Since v-cyclin is homologous to cellular cyclin D2,5 we tested and compared the interaction of v-cyclin-CDK6 with these two CDK inhibitors. To this end, U2OS cells were transfected with cyclin D2 (cycD2) or Myc-v-cyclin together with HA-CDK6 and p27KIP1 or p21CIP1. Both p27KIP1 and p21CIP1 were efficiently associated with cycD2, whereas only p27KIP1 was clearly detected to coimmunoprecipitate with v-cyclin. p21CIP1 binding to v-cyclin was apparently very weak, since the signal for p21CIP1 was only detected with very long exposures (Figure 2C; data not shown). Taken together, v-cyclin-CDK6 is readily detectable in complex with p27KIP1 in both transfected and PEL cells, whereas it is hardly detectable in complex with p21CIP1 in transfected cells.
CDK6 is the catalytic subunit of v-cyclin in PEL cells
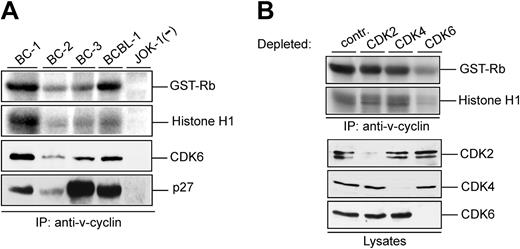
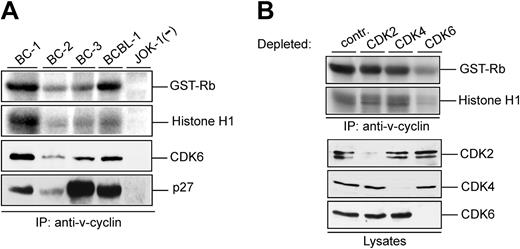
Various lines of evidence show that v-cyclin forms a functional complex with CDK6 in vitro.4,5,9,11,16 Kinase activity associated with v-cyclin in PEL cells has been demonstrated from BC-3 cells using GST-Rb as a substrate.28 To extend the analysis of v-cyclin-associated kinase activity in different PEL cell lines, cell lysates from BC-1, BC-2, BC-3, and BCBL-1 analyzed in Figure 1A were immunoprecipitated with anti-v-cyclin antibody and subjected to an in vitro kinase assay using both GST-pRb and Histone H1 as substrates. The KSHV-negative leukemia cell line JOK-1 served as a negative control (Figure 3A; 2 top panels). In the PEL cell lines tested, v-cyclin immunoprecipitates contained kinase activity toward GST-Rb and Histone H1. The highest kinase activity was detected in BC-1 and BCBL-1 cells, which correlated with the levels of coimmunoprecipitated CDK6 but not with the levels of total v-cyclin in these cell lines (Figure 3A). This indicates that total levels of proteins in cell lysates do not necessarily represent the functional complexes formed in cells. As already shown in Figure 1B, p27KIP1 was associated with the v-cyclin immunoprecipitates (Figure 3A bottom).
v-cyclin-associated kinase activity in PELs requires CDK6. (A) Lysates from PEL cell lines (BC-1, BC-2, BC-3, and BCBL-1) and a KSHV-negative control cell line (JOK-1) were immunoprecipitated with anti-v-cyclin antibody. In vitro kinase activity toward GST-Rb and Histone H1 was determined by autoradiography after SDS-PAGE (12%). The v-cyclin-associated CDK6 and p27KIP1 were analyzed by immunoblotting the nitrocellulose filter to which kinase reactions had been transferred with anti-CDK6 and anti-p27KIP1 antibodies. (B) Lysates from BC-3 cells were immunodepleted for CDK2, CDK4, and CDK6 by 3 consecutive rounds of immunoprecipitation with anti-CDK2, anti-CDK4, or anti-CDK6 antibodies. In the control (contr), lysate was immunoprecipitated with rabbit immunoglobulin G (IgG). Subsequently, depleted lysates were subjected to immunoprecipitation by anti-v-cyclin antibody and in vitro kinase assay toward GST-Rb and Histone H1. Kinase activity was determined by autoradiography after SDS-PAGE (12%). CDK-depleted lysates (40 μg) were resolved by SDS-PAGE (12%) and immunoblotted with antibodies against CDK2, CDK4, and CDK6.
v-cyclin-associated kinase activity in PELs requires CDK6. (A) Lysates from PEL cell lines (BC-1, BC-2, BC-3, and BCBL-1) and a KSHV-negative control cell line (JOK-1) were immunoprecipitated with anti-v-cyclin antibody. In vitro kinase activity toward GST-Rb and Histone H1 was determined by autoradiography after SDS-PAGE (12%). The v-cyclin-associated CDK6 and p27KIP1 were analyzed by immunoblotting the nitrocellulose filter to which kinase reactions had been transferred with anti-CDK6 and anti-p27KIP1 antibodies. (B) Lysates from BC-3 cells were immunodepleted for CDK2, CDK4, and CDK6 by 3 consecutive rounds of immunoprecipitation with anti-CDK2, anti-CDK4, or anti-CDK6 antibodies. In the control (contr), lysate was immunoprecipitated with rabbit immunoglobulin G (IgG). Subsequently, depleted lysates were subjected to immunoprecipitation by anti-v-cyclin antibody and in vitro kinase assay toward GST-Rb and Histone H1. Kinase activity was determined by autoradiography after SDS-PAGE (12%). CDK-depleted lysates (40 μg) were resolved by SDS-PAGE (12%) and immunoblotted with antibodies against CDK2, CDK4, and CDK6.
v-cyclin has been shown to complex with CDK2, CDK4, CDK5, and CDK6 in BC-3 cells.25 However, studies from transfected cells overexpressing v-cyclin imply that only association with CDK6 leads to formation of active v-cyclin-CDK complexes.4,5,22 To determine which of the associated CDKs is the in vivo catalytic subunit of v-cyclin in PEL cells, we used CDK2-, CDK4-, and CDK6-specific antibodies to remove individual CDKs from the BC-3 cells. Three consecutive rounds of immunoprecipitation with antibodies against CDK2, CDK4, or CDK6, respectively, resulted in an almost complete depletion of the protein in the lysate (Figure 3B). The CDK-depleted lysates were then subjected to immunoprecipitation with anti-v-cyclin antibody and the v-cyclin-associated kinase activity was measured in an in vitro kinase assay using GST-pRb and Histone H1 as substrates. As shown in Figure 3B, only depletion of CDK6 resulted in a significant decrease of v-cyclin-associated kinase activity. These results confirm that CDK6 is the major catalytic subunit for v-cyclin in PELs. Similar results were obtained from BCBL-1 cells (data not shown).
v-cyclin-associated kinase activity does not require p27KIP1
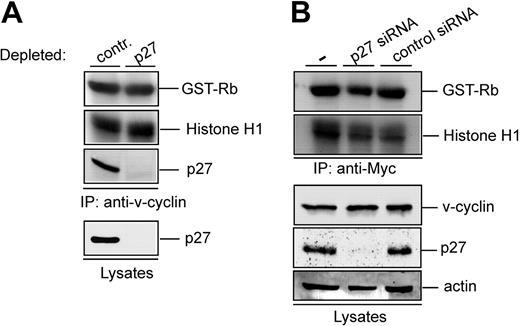
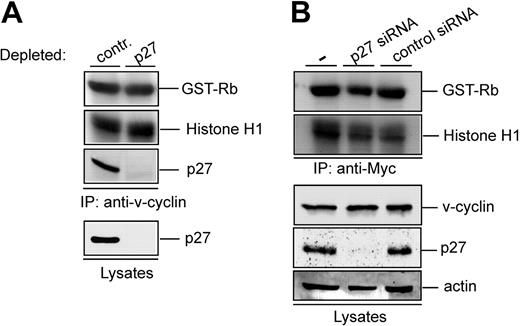
Our results show that v-cyclin is associated with p27KIP1 in PEL cells and yet v-cyclin immunocomplexes contain kinase activity. To further investigate the effect of p27KIP1 on the v-cyclin-CDK6 kinase activity, p27KIP1 was removed from PEL cell lysates with immunodepletion. The p27KIP1-depleted BC-3 lysate was subjected to in vitro kinase assay after anti-v-cyclin immunoprecipitation. Three rounds of exhaustive immunoprecipitation with anti-p27KIP1 antibody resulted in almost complete depletion of p27KIP1 from the total cell lysate and no p27KIP1 was thereafter detected in the v-cyclin complexes (Figure 4A). Significantly, p27KIP1 depletion did not have any effect on the v-cyclin-associated kinase activity. Similar results were obtained from BCBL-1 cells (data not shown). These results imply that in PEL cells, v-cyclin resides in the kinase-active v-cyclin-CDK6 complex as well as in p27KIP1-containing complex, which does not contribute to the kinase activity.
v-cyclin-associated kinase activity does not require p27KIP1. (A) Lysate from BC-3 cells was immunodepleted for p27KIP1 by 3 consecutive rounds of immunoprecipitation with anti-p27KIP1 antibody. In the control, lysate was immunoprecipitated with rabbit IgG. Subsequently, the depleted lysate was subjected to immunoprecipitation by anti-v-cyclin antibody and in vitro kinase assay toward GST-Rb and Histone H1. Kinase activity was determined by autoradiography after SDS-PAGE (12%). The p27KIP1depleted v-cyclin immunoprecipitate and lysate (40 μg) was resolved by SDS-PAGE (12%) and immunoblotted with antibody against p27KIP1. (B) U2OS cells were transfected with Myc-v-cyclin, siRNA against p27KIP1, and unrelated siRNA (Lkb1) as a control. Cells were lysed 48 hours after transfection and lysates were immunoprecipitated with anti-Myc antibody. Kinase activity was determined by in vitro kinase assay toward GST-Rb and Histone H1 and detected by autoradiography after SDS-PAGE (12%). Total protein levels of p27KIP1, v-cyclin, and actin (loading control) were determined by immunoblotting. — indicates no siRNA.
v-cyclin-associated kinase activity does not require p27KIP1. (A) Lysate from BC-3 cells was immunodepleted for p27KIP1 by 3 consecutive rounds of immunoprecipitation with anti-p27KIP1 antibody. In the control, lysate was immunoprecipitated with rabbit IgG. Subsequently, the depleted lysate was subjected to immunoprecipitation by anti-v-cyclin antibody and in vitro kinase assay toward GST-Rb and Histone H1. Kinase activity was determined by autoradiography after SDS-PAGE (12%). The p27KIP1depleted v-cyclin immunoprecipitate and lysate (40 μg) was resolved by SDS-PAGE (12%) and immunoblotted with antibody against p27KIP1. (B) U2OS cells were transfected with Myc-v-cyclin, siRNA against p27KIP1, and unrelated siRNA (Lkb1) as a control. Cells were lysed 48 hours after transfection and lysates were immunoprecipitated with anti-Myc antibody. Kinase activity was determined by in vitro kinase assay toward GST-Rb and Histone H1 and detected by autoradiography after SDS-PAGE (12%). Total protein levels of p27KIP1, v-cyclin, and actin (loading control) were determined by immunoblotting. — indicates no siRNA.
Previous studies imply that cyclin D-CDK4/6 complexes require p27KIP1 for the assembly of kinase-active complexes.29,30 To address the dependence of v-cyclin-associated kinase activity on p27KIP1, we used RNA interference to knock down p27KIP1 expression in U2OS cells. We developed and tested siRNA duplexes specifically targeting p27KIP1 (data not shown). A specific p27KIP1 siRNA duplex was cotransfected with Myc-v-cyclin into U2OS cells, which led to 95% inhibition of endogenous p27KIP1 expression as assessed by Western blotting (Figure 4B). The transfected cell lysates were thereafter subjected to immunoprecipitation by anti-Myc antibodies and in vitro kinase assay using GST-Rb and Histone H1 as substrates. Silencing of p27KIP1 expression did not have a notable effect on v-cyclin-associated kinase activity (Figure 4B), thus implying that p27KIP1 is not required for v-cyclin-associated kinase activity.
Discussion
In this study we investigated functional interactions between v-cyclin, CDK6, and p27KIP1 to assess how PEL-derived cell lines could tolerate high levels of p27KIP1. We provide compelling evidence that v-cyclin binds to p27KIP1 in PEL cells in vivo and suggest that this contributes to the abrogation of its antiproliferative function. Previous in vitro studies with recombinant proteins did not demonstrate a stable association between v-cyclin and p27KIP1.9,16 Using KSHV-infected PEL cell lines as a model system we were able to show physical interaction between v-cyclin and p27KIP1 in all 4 PEL cell lines tested.
Interaction of increasing amounts of p27KIP1 with cyclin D-CDK4 has been shown to maximize the accumulation but at the same time inhibit the activity of these complexes.26,27 Earlier studies in p27KIP1-deficient fibroblasts suggested that p27KIP1 is an essential assembly factor for active cyclin D-CDK4 complexes.29,30 However, more recent reports demonstrate that cyclin D-CDK4 complexes are formed and become active in the absence of p27KIP1,26,27 and suggest that enzymatically active binary cyclin D-CDK4 complexes comprise only a minor fraction of the total cyclin D pool in cells. p27KIP1 is also shown to maximize the accumulation of cyclin D-CDK4. Consistent with recent findings on cyclin D-CDK4, our results support that p27KIP1 is not an essential activator of v-cyclin-associated kinase complexes. Moreover, p27KIP1 expression was found to increase steady-state levels of v-cyclin and CDK6, as was the case with cyclin D-CDK4.
While several biochemical and transfection approaches have suggested that CDK6 represents the favored kinase partner for v-cyclin,4,5,9,11,16 this has not been assessed in cells naturally infected by KSHV. Results presented here demonstrate that CDK6 indeed represents the major catalytic subunit in v-cyclin immunocomplexes in PEL cell lines. Immunodepletion of p27KIP1 from PEL cell lysates did not affect the v-cyclin-associated kinase activity. Therefore, and analogous to findings from the studies with cyclin D-CDK4 complexes,26,27 our results indicate that the majority of v-cyclin-associated kinase activity in PELs resides in v-cyclin-CDK6 complexes, which do not contain p27KIP1. This is further supported by data from biochemical purification of v-cyclin complexes in PEL cells (G. Sarek, A.J., and P.M.O., unpublished data, May 2004).
In normal lymphocytes there is an inverse relationship between proliferation and p27KIP1 expression.31 p27KIP1 is expressed in nonproliferating lymphocytes, which have exited the germinal center and started to differentiate to plasma cells, whereas activated lymphocytes are consistently negative for p27KIP1 expression. The same inverse relationship is generally seen in lymphomas31,32 and yet clinical PELs and PEL-derived cell lines show exceptionally high levels of p27KIP1 expression.17 Why do PELs express such high levels of p27KIP1? One possibility relates to the apparent differentiation stage of PELs, which according to expression profiles represent a relatively late stage of B-cell differentiation including loss of BCL-6 expression and gain of the syndecan-1 expression.33 BCL-6 is a transcriptional repressor that down-regulates p27KIP1 expression,34 and accordingly high levels of p27KIP1 characterize these differentiated plasmablastic cells where no BCL-6 expression can be seen.31 This high p27KIP1 level in PEL cells may thus reflect their differentiation status.
What are the functional implications of the observed interaction between p27KIP1 and v-cyclin on PEL pathophysiology? Previous studies in transfected cells strongly suggested that v-cyclin can bypass the p27KIP1-induced cell cycle arrest by destabilizing the p27KIP1 protein via phosphorylation.9,11,16 However, since the KSHV-infected PELs retain high levels of p27KIP1, v-cyclin-CDK6-mediated degradation of p27KIP1 cannot be the sole mechanism for inhibiting p27KIP1 in PEL cells. The stable interaction between p27KIP1 and v-cyclin described here provides a plausible alternative mechanism for abrogating p27KIP1 function in PEL cells. Similar sequestration and inactivation of p27KIP1 by other D-type cyclins has been suggested in certain aggressive B-cell lymphomas, which also express high levels of p27KIP1. These lymphomas include Burkitt lymphoma (BL), certain large B-cell lymphomas (LBCLs), and the blastic variant of mantle cell lymphoma.35-37
In summary, our results demonstrate that v-cyclin and CDK6 form an active kinase excluding p27KIP1 in PEL cells. This complex is likely to provide a strong proliferative signal to KSHV-infected PEL cells. In addition, v-cyclin and p27KIP1 form stable inactive complexes, suggesting an attractive model by which p27KIP1 is inactivated in the actively proliferating PEL cells. These findings suggest that KSHV is carrying v-cyclin in its genome to both override negative cell cycle controls present in the PEL precursor cells infected by KSHV and positively influence oncogenesis.
Prepublished online as Blood First Edition Paper, July 22, 2004; DOI 10.1182/blood-2004-05-1798.
Supported by a Helsinki Graduate School in Biotechnology and Molecular Biology (A.J.) and grants from the Academy of Finland and Finnish Cancer Foundation, Biocentrum Helsinki, Sigrid Juselius Foundation, and Helsinki University Central Hospital (P.M.O., T.P.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Sibylle Mittnacht for reagents; Grzegorz Sarek for sharing unpublished results; and Birgitta Tjäder, Linda Degerth, and Evita Elfving for excellent technical assistance. Marie Aronson, Minna Taipale, Marianne Tiainen, and Tea Vallenius are acknowledged for critical reading of this manuscript. We are also grateful to the members of Mäkelä and Klefström laboratories for scientific discussions and suggestions.