Abstract
The present study demonstrates that CD4+CD25+ T cells, expanded in peripheral blood of HIV-infected patients receiving highly active antiretroviral therapy (HAART), exhibit phenotypic, molecular, and functional characteristics of regulatory T cells. The majority of peripheral CD4+CD25+ T cells from HIV-infected patients expressed a memory phenotype. They were found to constitutively express transcription factor forkhead box P3 (Foxp3) messengers. CD4+CD25+ T cells weakly proliferated to immobilized anti-CD3 monoclonal antibody (mAb) and addition of soluble anti-CD28 mAb significantly increased proliferation. In contrast to CD4+CD25– T cells, CD4+CD25+ T cells from HIV-infected patients did not proliferate in response to recall antigens and to p24 protein. The proliferative capacity of CD4 T cells to tuberculin, cytomegalovirus (CMV), and p24 significantly increased following depletion of CD4+CD25+ T cells. Furthermore, addition of increasing numbers of CD4+CD25+ T cells resulted in a dose-dependent inhibition of CD4+CD25– T-cell proliferation to tuberculin and p24. CD4+CD25+ T cells responded specifically to p24 antigen stimulation by expressing transforming growth factor β (TGF-β) and interleukin 10 (IL-10), thus indicating the presence of p24-specific CD4+ T cells among the CD4+CD25+ T-cell subset. Suppressive activity was not dependent on the secretion of TGF-β or IL-10. Taken together, our results suggest that persistence of HIV antigens might trigger the expansion of CD4+CD25+ regulatory T cells, which might induce a tolerance to HIV in vivo.
Introduction
HIV infection is mainly associated with a progressive decrease in the number of CD4+ T lymphocytes and defective CD4-specific and CD8-specific T-cell responses against HIV and other pathogens. It has been demonstrated that highly active antiretroviral therapy (HAART) results in reduced HIV-1 replication, increased CD4 cell counts in most treated patients, and progressive but incomplete recovery of CD4+ T-cell functions.1-4 Thus, treatment of chronic HIV infection does not result in most cases in a full recovery of HIV-specific helper and cytotoxic T lymphocyte (CTL) responses (reviewed in Sakaguchi et al5 ).
Recently, a CD4+ T-cell subset with regulatory properties has been characterized in humans several years after their discovery in mice.6-10 These cells, named “regulatory T” (Treg) cells express the α chain of the interleukin 2 (IL-2) receptor (CD25) and can inhibit the proliferation of CD4+ and CD8+ T lymphocytes both in vitro and in vivo.9,11 It has been demonstrated that transfer of such cells can protect neonatally thymectomized mice from autoimmune diseases, whereas their depletion results in the development of systemic autoimmune diseases.12-14 Moreover, alloantigen-induced CD4+CD25+ regulatory T cells were reported to prevent rejection initiated by CD4+ cells in both organ and bone marrow transplantation.15,16
Although CD25 is usually considered as an activation marker, it has been shown that CD4+CD25+ lymphocytes do not proliferate in response to polyclonal, allogenic, or antigen-specific stimulation8,9,17 but require activation through their T-cell receptor (TCR) to exert their suppressive function. Their anergic state could be partially reversed by IL-2 and IL-15.9
Besides CD4+CD25+ regulatory T cells, 2 other types of CD4+ cells with suppressive function have been described: type 1 T regulatory (Tr1) cells18 and T-helper 3 (Th3) cells.19 Tr1 cells were reported to suppress the immune response via the secretion of the immunosuppressive cytokines IL-10 and transforming growth factor β (TGF-β). The mechanism by which CD4+CD25+ T cells mediate suppression seems to require direct cell-cell contact. Levings et al19 have recently demonstrated, at the clonal level, that Tr1 and CD4+CD25+ T cells are distinct subsets of regulatory T cells and that the suppression mediated by CD4+CD25+ T cells is partially dependent on TGF-β.
In healthy individuals, the CD4+CD25+ T-cell subset represents 5% to 10% of peripheral CD4+ T cells and is characterized by the constitutive expression of CD152 (CTL-associated antigen 4 [CTLA-4]) and CD122 (IL-2 receptor β chain) and reduced expression of CD40L. The majority of CD4+CD25+ regulatory cells have been found to express CD45RO, thus resembling a memory T-cell phenotype.8,17
We and others have previously reported that a subset of CD4 T cells expressing CD25 was expanded in the peripheral blood of HIV-infected individuals. This expanded subset remained stable despite control of viral replication under HAART.21,22 The phenotypic and the functional characterization of these cells in HIV infection are lacking. As the decrease in CD4 T-cell proliferative responses to recall antigens and HIV proteins occurs early in HIV-infected patients before the decrease in CD4 cell counts,23,24 we postulate that peripheral CD4+CD25+ T cells are triggered by the persistence of viral antigens and may participate in the immune deficiency associated with HIV infection. The present study was aimed at characterizing the phenotype and the functions of CD4+CD25+ T cells isolated from peripheral blood of HIV-infected patients.
Patients, materials, and methods
Patients
Patients included in the study have received HAART for at least 1 year and exhibited the following at the time of the study: CD4 cell counts above 500 cells/mm3 and a plasma HIV viral load below 50 copies/mL. Blood samples from 15 HIV-infected patients were collected on EDTA (ethylenediaminetetraacetic acid) tubes and processed within 3 hours after they were drawn. Clinical characteristics of the patients are depicted in Table 1. Written informed consent was obtained from all the patients, according to human experimentation guidelines from the National Ethics Committee. Blood was also collected from healthy volunteers.
Cell isolation
CD4+ T lymphocytes were purified from whole blood using RosetteSep CD4+ enrichment antibody cocktail (StemCell Technologies, Vancouver, BC, Canada) according to manufacturer's instructions. The CD4+ cells thus obtained (> 90% purity) were washed and incubated with CD25+ magnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany) for 30 minutes at 4°C. The CD4+CD25+ cells were subsequently separated using magnetic columns (Miltenyi Biotec) according to company's guidelines. CD25+ and CD25– fractions were collected separately. This generally resulted in obtaining higher than 85% and 90% purity of CD4+CD25+ and CD4+CD25– cell populations, respectively, as assessed by flow cytometry (data not shown).
Autologous peripheral blood mononuclear cells were isolated from whole blood by Ficoll centrifugation. Monocytes (> 90% purity), obtained by plastic adherence for 1 hour at 37°C as previously described in detail,25 were used as antigen-presenting cells in proliferation and suppression assays.
Flow cytometric analysis
Phenotyping of the CD4+CD25+ and CD4+CD25– cell subsets was performed on freshly obtained whole blood samples by 4-color direct flow cytometry. The following murine monoclonal antibodies (mAbs) conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyl protein (PerCP), or allophycocyanin (APC) were used for immunostaining: anti-CD25–FITC, anti-CD4–PerCP, anti-CD3–APC, anti–HLA-DR–PE, anti-CD45RA–PE, anti-CD45RO–APC, anti-CD40L–PE, anti-CD122–PE, anti-CD95–PE, anti-CD69–PE, anti-CCR5–PE, and anti–CTLA-4–PE. All antibodies were purchased by BD Biosciences (Le Pont Claix, France). For assessing the cytokine production by CD4+, CD4+CD25–, and CD4+CD25+ cells, the following mAbs were used: anti–IL-2–PE (BD Biosciences), anti–interferon γ–FITC (anti–IFN-γ–FITC), and anti–IL-10–PE (Diaclone, Besançon, France). Isotype-matched controls were used in all staining experiments.
Whole blood samples were incubated with appropriate mAbs for 30 minutes at 4°C. All samples were stained with anti-CD3–APC, anti-CD4–PerCP, and anti-CD25–FITC and with varying PE-labeled antibodies for the markers studied. After lysis of red blood cells by fluorescence-activated cell sorter (FACS) lysis buffer (BD Biosciences), cells were washed and then fixed in 1% formaldehyde.
For detection of intracellular CTLA-4 in whole blood, samples were first incubated with mAbs against surface markers CD3, CD4, and CD25 as described in the previous paragraph. After lysis of red blood cells and subsequent washes, cells were fixed with phosphate-buffered saline (PBS) containing 4% formaldehyde for 10 minutes at 4°C before proceeding to intracellular staining. Cells were washed once with PBS containing 0.5% bovine serum albumin (BSA) and 0.5% saponin, permeabilized with 0.5% saponin, and stained with anti–CTLA-4–PE–labeled mAb for 25 minutes at room temperature. Cells were further washed twice with PBS/0.5% saponin and once with PBS before analysis by flow cytometry.
To assess the production of different cytokines by CD4+, CD4+CD25–, and CD4+CD25+ cell populations, 5 × 105 purified cells were incubated for 24 or 48 hours in 24-well cell culture plates either alone or in the presence of 5 μg/mL immobilized anti-CD3 mAb plus 5 μg/mL soluble anti-CD28 mAb or 5 μg/mL p24 protein. Brefeldin A (5 μg/mL; Sigma-Aldrich, St Louis, Missouri, MO) was added to each well for the last 4 hours of culture. Cells were then harvested, and the cytokine production of the 3 CD4+ cell subsets was assessed by intracellular staining for expression of IL-10, IFN-γ, and IL-2, as described in the previous paragraph.
Analyses were performed using FACScalibur and CellQuest software (Becton Dickinson, San Jose, CA) on at least 1000 events. Gating was restricted to the population of lymphocytes according to their light scattering properties.
Proliferation and suppression assays
All cell cultures were performed in RPMI 1640 (Bio Whittaker Europe, Verviers, France) supplemented with 10% human blood group AB serum (BioWest, Nuaille, France), penicillin-streptomycin (100 IU/mL and 100 μg/mL, respectively), 2 mM l-glutamine (all from Gibco BRL, Paisley, United Kingdom), 1 mM sodium pyruvate, and 1% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (Sigma-Aldrich). The different subpopulations (unfractionated CD4+ cells, CD4+CD25– cells, and CD4+CD25+ cells) were assessed for their proliferative capacities in response to polyclonal stimulation and to recall antigens and p24 protein.
For anti-CD3 stimulation, 5 μg/mL anti-CD3 mAb (clone UCHT1; Beckman Coulter, Marseille, France) was coated on a 96-well flat-bottomed cell culture plate overnight at 4°C. To assess the polyclonal stimulation, 1 × 105 cells/well were incubated for 3 days with plate-bound anti-CD3 mAb alone or in the presence of 5 μg/mL soluble anti-CD28 mAb (clone CD28.2; Beckman Coulter).
To assess the antigen-specific proliferation of the different CD4+ cell populations, 1 × 104 autologous monocytes were added to 1 × 105 lymphocytes/well and cultured for 5 days in the presence of the tested antigen: 5 μg/mL purified tuberculin (PPD; Statens Seruminstitut, Copenhagen, Denmark), 5 μg/mL p24 protein (Protein Science, Meriden, CT), or 1:50 dilution of cytomegalovirus (CMV) antigen (Bio Whittaker Europe); or in the presence of 100 IU/mL of recombinant human IL-2 (rhIL-2; R&D Systems, Oxon, United Kingdom) alone or in combination with p24 protein or soluble anti-CD28 mAb.
For direct suppression assays, CD4+CD25– lymphocytes were incubated for 5 days in the presence of p24 or PPD either alone or with varying numbers of CD4+CD25+ cells resulting in suppressor-responder ratios of 0:1, 1:1, 1:2, and 1:10 in a final amount of 1 × 105 cells/well. Percentage of inhibition was calculated as follows: [1–(mean cpm of coculture wells/mean cpm of CD4+CD25– cells cultured alone)]× 100; where cpm indicates counts per minute.
To assess the role of IL-10 and TGF-β in the suppressive activity of the CD4+CD25+ T-cell population, CD4+CD25– lymphocytes were cocultured with CD4+CD25+ cells as described above for 5 days with 5 μg/mL p24 alone or in the presence of 10 μg/mL neutralizing anti–IL-10 mAb, anti–TGF-β mAb (both from R&D Systems), or in the presence of both antibodies. [3H] thymidine (0.5 μCi [0.0185 MBq]; Amersham Pharmacia, Uppsala, Sweden) per well was added for the last 16 hours of culture period and [3H] thymidine incorporation was measured in a Microbeta Liquid Scintillation Counter (EG&G Wallac, Turku, Finland). Results were expressed as mean cpm of triplicate culture wells. Stimulation indexes were calculated by dividing the mean cpm of stimulated cells by the mean cpm of unstimulated cells.
Analysis of TGF-β and Foxp3 expression
Unfractionated CD4+ cells, purified CD4+CD25– cells, and purified CD4+CD25+ cells (5 × 104 each), either nonstimulated or after 48-hour stimulation in the presence of 5 μg/mL plate-bound anti-CD3 plus 5 μg/mL soluble anti-CD28 or 5 μg/mL p24 antigen, were lysed in 200 μL Trizol reagent (Invitrogen Life Technologies, Cergy Pontoise, France). Total RNA was purified by chloroform extraction (1:5 vol/vol). RNA was then reverse transcribed using 1 mM oligodeoxythymidylic acid (oligodT) and the Superscript HH Rnase H-reverse transcriptase (Invitrogen Life Technologies) according to the manufacturer's instructions. The cDNA input for each population was normalized to obtain equivalent signals with the housekeeping ribosomal S14 gene. The polymerase chain reaction (PCR) process was optimized to ensure a linear amplification. To avoid DNA amplification, primers were chosen to span introns for each transcript. Reverse transcriptase (RT)–PCR conditions for analysis of TGF-β and S14 expressions were as follows: 30 cycles, 94°C for 45 seconds, annealing at 50°C for 1 minute, 72°C for 1 minute using 15 pmol of TGF-β1 primer (sense, 5′-CGTCTGCTGAGGCTCAAGTT-3′; and antisense, 5′-GAACCCGTTGATGTCCACTT-3′) and S14 primer (sense, 5′-GGCAGACCGAGATGAATCCTCA-3′; and antisense, 5′-CAGGTCCAGGGGTCTTGGTCC-3′). Foxp3 expression was analyzed using a nested RT-PCR. A first round of PCR (40 cycles, 94°C for 45 seconds, annealing at 55°C for 1 minute, 72°C for 1 minute) was performed using the sense S1 primer (5′-TCACCTACGCCACGGTCAT-3′) and the antisense AS1 primer (5′-CACAAAGCACTTGTGCAG-3′). One thirtieth of the PCR product was amplified (25 cycles, 94°C for 45 seconds, annealing at 52°C for 1 minute, 72°C for 1 minute) using the S1 primer and an internal antisense primer AS2 (5′-ACTCAGGTTGTGGCGGAT-3′). A final PCR product of 152 bp was obtained. Ten microliters of the PCR products were analyzed by 1.5% agarose gel electrophoresis.
Statistical analysis
Data are expressed as mean ± SD for percentages and median and ranges for absolute values. Statistical comparisons were performed using Student t test. Significance was considered for P less than or equal to .05.
Results
Phenotypic and molecular characteristics of circulating CD4+CD25+ T cells in HIV-infected patients
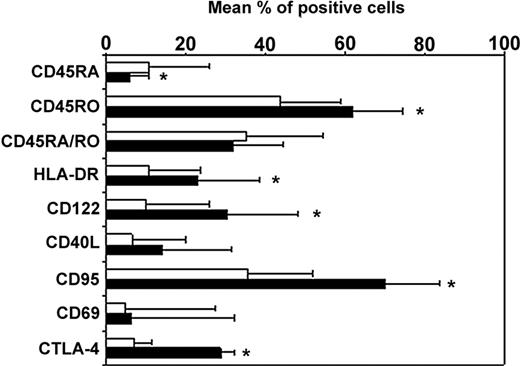
Longitudinal studies in HIV-infected patients showed an expansion of CD4+CD25+ T cells that persisted despite viral control under highly active antiretroviral therapy.21,22 In the present study, the mean percentages of CD4+ T cells expressing CD25 were 15.9% ± 3.6% and 5.9% ± 1.3% in patients (n = 15) and healthy controls (n = 5), respectively (P < .01). We sought whether these cells might exhibit phenotypic characteristics of regulatory CD4+CD25+ T cells. As expected, we found in controls that a mean of 97%, 36%, and 30% of CD4+CD25+ cells expressed CD45RO, CD122, and intracytoplasmic CTLA-4, respectively (data not shown). In HIV-infected patients, CD4+CD25+ T cells expressed a similar phenotype as in controls. As shown in Figure 1, they exhibit in their majority a phenotype of memory T cells since 94% ± 13% of the cells expressed CD45RO antigen. Among them, approximately 32% coexpressed the CD45RA marker. As previously reported,26 the majority of CD4+CD25+ T cells double-positive for CD45RO and CD45RA markers stained with intermediate fluorescence intensity. These cells have been previously referred to as a dull RA/RO double-positive population.27 The mean expression of CD122, the β chain of the IL-2 receptor, was 30% (± 18%). Twenty-three percent and 16% of CD4+CD25+ T cells from HIV-infected patients expressed HLA-DR and CD40L, respectively. In addition, we found in 3 patients that 70% of the CD4+CD25+ T cells expressed CD95 and that more than 94% of these cells did not express the early activation marker CD69. An average of 30% of CD4+CD25+ T cells were found to constitutively express intracellular CTLA-4, a negative regulator of T-cell activation. In contrast, only 10% of the CD4+CD25– T cells expressed constitutively intracellular CTLA-4.
Phenotype of the CD4+CD25+ and CD4+CD25– T-cell subsets in HIV-infected patients. The membrane or intracellular (CTLA-4) expression of the different molecules was determined in whole blood cells by 4-color direct flow cytometry after successively combining the forward scatter/side scatter (FSC/SSC), CD3+, CD4+, and CD25 gates. Analysis was performed on CD25+ (▪) and CD25– (□) T cells. Data from 15 patients studied are presented as mean percent positive cells ± SD (*P < .05).
Phenotype of the CD4+CD25+ and CD4+CD25– T-cell subsets in HIV-infected patients. The membrane or intracellular (CTLA-4) expression of the different molecules was determined in whole blood cells by 4-color direct flow cytometry after successively combining the forward scatter/side scatter (FSC/SSC), CD3+, CD4+, and CD25 gates. Analysis was performed on CD25+ (▪) and CD25– (□) T cells. Data from 15 patients studied are presented as mean percent positive cells ± SD (*P < .05).
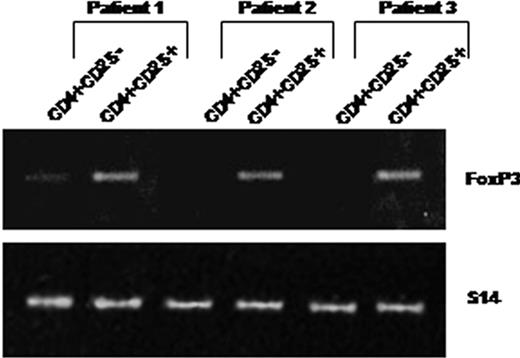
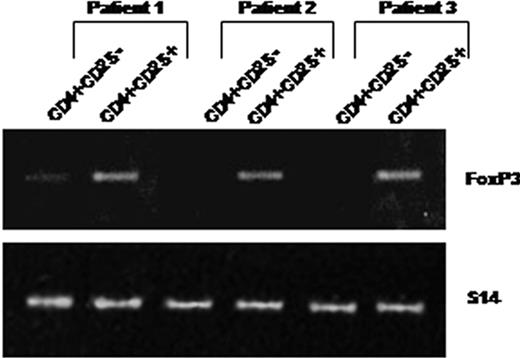
As shown in Figure 2, peripheral CD4+CD25+ purified from 3 patients constitutively expressed Foxp3 mRNAs. In contrast, Foxp3 mRNA was not expressed constitutively by purified CD4+CD25– T cells (except in one case where a faint band was detectable), whereas this expression was inducible after in vitro activation in the presence of anti-CD3 and anti-CD28 antibodies (not shown).
CD4+CD25+ T cells isolated from HIV-infected patients constitutively express Foxp3 mRNAs. RNA was extracted from purified CD4+CD25– (lanes 1) and CD4+CD25+ (lanes 2) T cells immediately after cell isolation from 3 patients. Foxp3 expression was analyzed using 2 steps of RT-PCR (see “Analysis of TGF-β1 and FoxP3 expression”). An expected 152-bp PCR product was detectable in CD4+CD25+ but not in CD4+CD25– T cells (except a faint detectable band in patient 1).
CD4+CD25+ T cells isolated from HIV-infected patients constitutively express Foxp3 mRNAs. RNA was extracted from purified CD4+CD25– (lanes 1) and CD4+CD25+ (lanes 2) T cells immediately after cell isolation from 3 patients. Foxp3 expression was analyzed using 2 steps of RT-PCR (see “Analysis of TGF-β1 and FoxP3 expression”). An expected 152-bp PCR product was detectable in CD4+CD25+ but not in CD4+CD25– T cells (except a faint detectable band in patient 1).
Overall these data showed that a high frequency of circulating CD4+CD25+ T cells from HIV-infected patients express CD45RO, CD122, CD95, and constitutive intracytoplasmic CTLA-4 antigens and Foxp3 messengers. These characteristics are highly reminiscent of those of the regulatory CD4+CD25+ T cells described in mice and healthy human individuals.8,17
Peripheral CD4+CD25+ T cells from HIV-infected patients are hyporesponsive/anergic to polyclonal and antigen-specific stimulation
In order to determine whether CD4+CD25+ T cells expanded in peripheral blood of HIV-infected patients exhibit functional characteristics of regulatory T lymphocytes, we first assessed their ability to proliferate upon stimulation with plate-bound anti-CD3 mAb, in the presence or in the absence of soluble anti-CD28 mAb, and in response to recall antigens including PPD, CMV, and p24 protein.
As illustrated in Figure 3, in the presence of immobilized anti-CD3 mAb, the proliferative capacity of CD4+CD25+ isolated from HIV-infected patients was reduced approximately 8-fold compared with CD4+CD25– cells. However, addition of soluble anti-CD28 mAb partially restored the proliferation levels of CD4+CD25+ T cells as described for regulatory CD4+CD25+ cells in healthy individuals (Figure 3A). Compared with CD4+CD25– cells isolated from HIV-infected patients, CD4+CD25+ T cells did not proliferate in the presence of either tuberculin, CMV, or HIV p24 antigens (Figure 3B). None of the studied populations proliferated spontaneously when cultured only in medium (median of background cpm was < 500 for all cell types). For 2 patients, when cells were available, we found that addition of IL-2 restores the proliferation of CD4+CD25+ T cells (data not shown). These data demonstrate that CD4+CD25+ cells in HIV-infected patients exhibit proliferative characteristics of regulatory CD4+CD25+ T cells.
CD4+CD25+ cells isolated from HIV-infected patients are hyporesponsive/anergic to polyclonal and antigen-specific stimulation. (A) Purified CD4+CD25– (□) or CD4+CD25+ (▪) T cells (1 × 105 each) were stimulated for 3 days with 5 μg/mL plate-bound anti-CD3 mAb in the presence or absence of soluble anti-CD28 mAb (5 μg/mL). (B) Freshly purified CD4+CD25– (□) or CD4+CD25+ (▪) T cells (1 × 105 each) were cultured for 5 days with 5 μg/mL purified tuberculin (PPD), 5 μg/mL p24, or 1:50 dilution of the CMV antigen. [3H] thymidine (0.5 μCi/well [0.0185 MBq]) was added during the last 16 hours of culture. Results are expressed as median [3H] thymidine incorporation from 8 HIV-infected patients (P < .05 for all of the antigens studied).
CD4+CD25+ cells isolated from HIV-infected patients are hyporesponsive/anergic to polyclonal and antigen-specific stimulation. (A) Purified CD4+CD25– (□) or CD4+CD25+ (▪) T cells (1 × 105 each) were stimulated for 3 days with 5 μg/mL plate-bound anti-CD3 mAb in the presence or absence of soluble anti-CD28 mAb (5 μg/mL). (B) Freshly purified CD4+CD25– (□) or CD4+CD25+ (▪) T cells (1 × 105 each) were cultured for 5 days with 5 μg/mL purified tuberculin (PPD), 5 μg/mL p24, or 1:50 dilution of the CMV antigen. [3H] thymidine (0.5 μCi/well [0.0185 MBq]) was added during the last 16 hours of culture. Results are expressed as median [3H] thymidine incorporation from 8 HIV-infected patients (P < .05 for all of the antigens studied).
Peripheral CD4+CD25+ T cells from HIV-infected patients suppress CD4 T-cell proliferation in response to recall antigens and HIV proteins
Next, we were interested to further investigate the potential suppressive activity of peripheral CD4+CD25+ T cells. First, we assessed the proliferative capacity of CD4+ T cells depleted from CD4+CD25+ T cells obtained from 8 HIV-infected patients. As illustrated in Figure 4A, median (range) stimulation indexes of total CD4 T cells were 6.4 (2.9-12.6), 12.9 (3.5-31.5), and 16.3 (5.5-67.0) for tuberculin, CMV, and p24 antigens, respectively. Depletion of CD4+CD25+ T cells led to an increase in proliferative indexes to 27.4 (15.3-44.1), 22.9 (7.6-64.0), and 34.8 (10.6-219.6), respectively, for the same antigens (P < .05 for all comparisons).
Peripheral CD4+CD25+ T cells from HIV-infected patients suppress CD4 T-cell proliferation in response to recall antigens and HIV proteins. (A) Unfractionated CD4+ T cells (□) or purified CD4+CD25– T cells (▪) were incubated for 5 days with 5 μg/mL tuberculin (PPD), 5 μg/mL p24 protein, or 1:50 dilution of CMV antigen before adding 0.5 μCi (0.0185 MBq) [3H] thymidine for the last 16 hours of culture. Results are expressed as median values of the stimulation indexes for 8 patients studied (*P < .05). The median (ranges) of [3H] thymidine incorporation in control cultures (medium alone) were 462 (140-824), 240 (130-377), and 136 (63-175) for unfractionated CD4+ cells, CD4+CD25– cells, and CD4+CD25+ cells, respectively. (B) Coculture of CD4+CD25– with CD4+CD25+ T lymphocytes from HIV-infected patients results in a dose-dependent suppression of antigen-specific proliferation. Purified CD4+CD25– T cells were cultured for 5 days in the presence of 5 μg/mL purified tuberculin (♦) or 5 μg/mL p24 protein (⋄) and varying numbers of purified CD4+CD25+ lymphocytes. Percentage of inhibition was calculated as described in “Proliferation and suppression assays.” Results are expressed as mean (± SD) percentage of inhibition of proliferation for 5 patients studied.
Peripheral CD4+CD25+ T cells from HIV-infected patients suppress CD4 T-cell proliferation in response to recall antigens and HIV proteins. (A) Unfractionated CD4+ T cells (□) or purified CD4+CD25– T cells (▪) were incubated for 5 days with 5 μg/mL tuberculin (PPD), 5 μg/mL p24 protein, or 1:50 dilution of CMV antigen before adding 0.5 μCi (0.0185 MBq) [3H] thymidine for the last 16 hours of culture. Results are expressed as median values of the stimulation indexes for 8 patients studied (*P < .05). The median (ranges) of [3H] thymidine incorporation in control cultures (medium alone) were 462 (140-824), 240 (130-377), and 136 (63-175) for unfractionated CD4+ cells, CD4+CD25– cells, and CD4+CD25+ cells, respectively. (B) Coculture of CD4+CD25– with CD4+CD25+ T lymphocytes from HIV-infected patients results in a dose-dependent suppression of antigen-specific proliferation. Purified CD4+CD25– T cells were cultured for 5 days in the presence of 5 μg/mL purified tuberculin (♦) or 5 μg/mL p24 protein (⋄) and varying numbers of purified CD4+CD25+ lymphocytes. Percentage of inhibition was calculated as described in “Proliferation and suppression assays.” Results are expressed as mean (± SD) percentage of inhibition of proliferation for 5 patients studied.
Furthermore, we found that addition of increasing numbers of purified CD4+CD25+ T cells (104 to 105 cells) resulted in a dose-dependent inhibition of CD4+CD25– T-cell proliferation to tuberculin and p24 protein. As illustrated in Figure 4B, experiments performed with cells isolated from 5 patients were highly reproducible and showed that addition of CD4+CD25+ T cells to CD4+CD25– T cells resulted in approximately 32% and 48% inhibition of T-cell proliferation at a ratio of 1:10 for tuberculin and p24, respectively. This inhibitory effect on proliferative responses was up to 74% for both antigens when cells were mixed at a 1:1 ratio.
Specific induction of IL-10 and TGF-β expression following stimulation of CD4+CD25+ T cells by p24 antigen
We postulated that CD4+CD25+ T cells from HIV-infected patients might exhibit a specificity bias toward HIV antigens. Therefore, we investigated the production of the cytokines IL-10, TGF-β, IL-2, and IFN-γ by CD4 T-cell subsets following p24 stimulation.
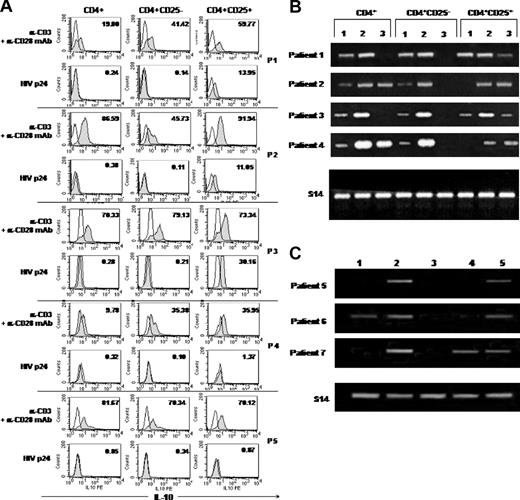
Intracellular production of IL-10, IL-2, and IFN-γ was assessed at the single-cell level on purified CD4 T-cell subsets by flow cytometry. Stimulation with anti-CD3 and anti-CD28 mAbs resulted in production of IL-2 and IFN-γ by both CD4+CD25– and CD4+CD25+ T-cell subsets. Less than 0.5% of CD4+CD25– T cells were found to produce IL-2 and IFN-γ upon p24 stimulation. No production of IL-2 and IFN-γ was detected in CD4+CD25+ T cells following p24 stimulation (data not shown).
Since regulatory T cells were reported to produce IL-10 and TGF-β, we further investigated the expression of these cytokines in CD4+CD25+ T cells from HIV-infected patients. Constitutive production of IL-10 by CD4+CD25+ T cells was not consistently found. Plate-bound anti-CD3 mAb plus soluble anti-CD28 mAb induced IL-10 production in total CD4 T cells and both CD4+CD25– and CD4+CD25+ T-cell populations. No intracellular IL-10 was detected in CD4+ and CD4+CD25– T cells after p24 stimulation. Interestingly, as illustrated in Figure 5A, p24 specifically stimulated CD4+CD25+ T lymphocytes from 3 of 5 patients to produce IL-10 (percentage of IL-10–producing cells ranged from 11% to 30%). In contrast to p24 stimulation, we found that stimulation of CD4+CD25+ T cells with CMV and PPD antigens resulted in the production of IL-10 at a lower extent (0.15%-2.0% positive cells for PPD and 1.9%-6.6% positive cells for CMV). We investigated whether CD4 T-cell populations may express TGF-β transcripts following p24 stimulation. In the 4 cases studied, we found that anti-CD3 plus soluble anti-CD28 mAbs induced TGF-β1 expression in total CD4+ T cells and both CD4+CD25+ and CD4+CD25– populations. However, p24 stimulation induced TGF-β1 mRNA expression in CD4+CD25+ T cells but not in the CD4+CD25– subset (Figure 5B). These results were confirmed in 3 other cases, where we compared TGF-β1 expression following p24, CMV, and PPD stimulations. We found that p24, but not CMV or PPD (except in one case), specifically induced TGF-β1 mRNA expression in CD4+CD25+ populations (Figure 5C).
Regulatory cells specific for p24 antigen can be found among the CD4+CD25+ T-subset in HIV-infected patients. (A) Specific induction of IL-10 production following stimulation of CD4+CD25+ cells with p24 antigen. Cells (5 × 105/well) were incubated for 48 hours alone or in the presence of 5 μg/mL plate-bound anti-CD3 (α-CD3) and 5 μg/mL soluble anti-CD28 (α-CD28) mAb or with 5 μg/mL HIV p24 antigen before assessment of intracellular IL-10 production by flow cytometry. Intracellular staining was performed using anti–IL-10–PE mAb or isotype-matched control mAb. Results from 5 patients studied are presented. The numbers in the top corner of each histogram indicate the percent of cells positive for IL-10 (gray histograms) compared with cells cultured in media alone (outlined histograms). (B) Induction of TGF-β1 mRNA expression in CD4+CD25+ cells following stimulation with p24 antigen. RNA was extracted from unfractionated CD4+ cells or purified CD4+CD25– and CD4+CD25+ T cells incubated for 48 hours alone (lane 1) or in the presence of plate-bound anti-CD3 and soluble anti-CD28 mAbs (5 μg/mL each; lane 2) or 5 μg/mL HIV p24 protein (lane 3). Data from 4 patients studied are presented. (C) Induction of TGF-β1 mRNA expression in CD4+CD25+ cells following stimulation with p24, CMV, or PPD antigens. Purified CD4+CD25+ T cells from 3 other patients were cultured for 48 hours alone (lane 1); in the presence of anti-CD3 and anti-CD28 (lane 2); in 1:50 dilution CMV antigen (lane 3); or 5 μg/mL PPD antigen (lane 4) or p24 antigen (lane 5).
Regulatory cells specific for p24 antigen can be found among the CD4+CD25+ T-subset in HIV-infected patients. (A) Specific induction of IL-10 production following stimulation of CD4+CD25+ cells with p24 antigen. Cells (5 × 105/well) were incubated for 48 hours alone or in the presence of 5 μg/mL plate-bound anti-CD3 (α-CD3) and 5 μg/mL soluble anti-CD28 (α-CD28) mAb or with 5 μg/mL HIV p24 antigen before assessment of intracellular IL-10 production by flow cytometry. Intracellular staining was performed using anti–IL-10–PE mAb or isotype-matched control mAb. Results from 5 patients studied are presented. The numbers in the top corner of each histogram indicate the percent of cells positive for IL-10 (gray histograms) compared with cells cultured in media alone (outlined histograms). (B) Induction of TGF-β1 mRNA expression in CD4+CD25+ cells following stimulation with p24 antigen. RNA was extracted from unfractionated CD4+ cells or purified CD4+CD25– and CD4+CD25+ T cells incubated for 48 hours alone (lane 1) or in the presence of plate-bound anti-CD3 and soluble anti-CD28 mAbs (5 μg/mL each; lane 2) or 5 μg/mL HIV p24 protein (lane 3). Data from 4 patients studied are presented. (C) Induction of TGF-β1 mRNA expression in CD4+CD25+ cells following stimulation with p24, CMV, or PPD antigens. Purified CD4+CD25+ T cells from 3 other patients were cultured for 48 hours alone (lane 1); in the presence of anti-CD3 and anti-CD28 (lane 2); in 1:50 dilution CMV antigen (lane 3); or 5 μg/mL PPD antigen (lane 4) or p24 antigen (lane 5).
The suppressive activity of CD4+CD25+ T cells from HIV-infected patients is not mediated by TGF-β or IL-10
Secretion of TGF-β and IL-10 (at least in mice) together with cell-cell contact have been proposed as the main mechanisms of suppressive activity of regulatory T cells.20,28,29 As we demonstrated a production of TGF-β and IL-10 by CD4+CD25+ T cells isolated from HIV+ patients, we finally investigated the effect of blocking the secretion of TGF-β or IL-10 on the suppressive activity of CD4+CD25+ T cells. We found that addition of neutralizing anti–TGF-β or anti–IL-10 mAbs, alone or in combination in a direct suppression assay, did not remove the suppressive activity of CD4+CD25+ T cells on p24-specific CD4+CD25– T-cell proliferation (Figure 6).
The suppressive activity of CD4+CD25+ T lymphocytes is not mediated by IL-10 or TGF-β. Purified CD4+CD25– and CD4+CD25+ T cells were cocultured as described in “Patients, materials, and methods” in the presence of 5μg/mL p24 protein alone or in the presence of 10 μg/mL anti–IL-10, anti–TGF-β, or both neutralizing antibodies for 5 days before adding 0.5 μCi/well (0.0185 MBq) [3H] thymidine. Percentage of inhibition was calculated as described in “Proliferation and suppression assays.” Results are expressed as mean (± SD) percentage of inhibition of proliferation (n = 3).
The suppressive activity of CD4+CD25+ T lymphocytes is not mediated by IL-10 or TGF-β. Purified CD4+CD25– and CD4+CD25+ T cells were cocultured as described in “Patients, materials, and methods” in the presence of 5μg/mL p24 protein alone or in the presence of 10 μg/mL anti–IL-10, anti–TGF-β, or both neutralizing antibodies for 5 days before adding 0.5 μCi/well (0.0185 MBq) [3H] thymidine. Percentage of inhibition was calculated as described in “Proliferation and suppression assays.” Results are expressed as mean (± SD) percentage of inhibition of proliferation (n = 3).
Discussion
In HIV infection, immune deficiency occurs before the onset of AIDS and even before CD4 T-cell depletion.23,24 CD4 T-cell–mediated proliferative responses to HIV antigens decrease early after infection, except when treated during the acute phase.1 The HIV-specific CD4 proliferative responses may be important in the control of HIV replication.30 The expansion of a subset of CD4 T cells coexpressing CD25 has been observed in HIV-infected patients. We postulated that expanded CD4+CD25+ T cells may exhibit regulatory functions and thus participate in the immune deficiency associated with HIV infection.
Here, we demonstrate that peripheral CD4+CD25+ T cells from HIV-infected patients with undetectable plasma HIV-RNA while receiving effective HAART indeed exhibit phenotypic and functional characteristics of regulatory T cells. Suppressive activity was not found to be dependent on the secretion of TGF-β or IL-10. CD4+CD25+ regulatory T cells responded specifically to p24 antigen stimulation by producing TGF-β and IL-10, indicating the presence of p24-specific CD4+ T cells among the CD4+CD25+ T-cell subset. These findings suggest that expansion of CD4+CD25+ T-cell population might be triggered by HIV antigens and might result in a tolerance to HIV in vivo.
We found that peripheral CD4+CD25+ T cells from HIV-infected patients exhibited a phenotype similar to that of CD4+CD25+ T cells from peripheral blood of healthy donors31 and cancer patients.32 Most CD4+CD25+ T cells were CD45RO+, thus being different from naive CD4+CD25+ T cells that expand in HIV-infected patients treated with IL-2.26 Compared with CD4+CD25– T cells, they expressed higher levels of CD122 and of CD95, the Fas antigen that could promote an increased susceptibility to apoptosis as described earlier.17 The percentages of T cells expressing HLA-DR and CD40L were similar in CD4+CD25+ and CD4+CD25– T-cell subsets, indicating that peripheral CD4+CD25+ T cells from HAART-treated HIV-infected patients are distinct from recently activated T cells; moreover, CD4+CD25+ T cells did not express CD69. Although there is no exclusive phenotypic marker for Treg cells, it has been previously reported that about one third of CD4+CD25+ T cells constitutively expressed intracellular CTLA-4.33 It is interesting to note that CTLA-4 was found to be up-regulated in HIV-infected patients.34 However, the relevance of this marker for the function of regulatory T cells is questionable; no or little blocking effect of anti–CTLA-4 antibodies was observed in studies of human CD4+CD25+ T cells,29,35 unlike murine CD4+CD25+ T cells.14 Interestingly, unlike CD4+CD25– T cells, peripheral CD4+CD25+ T cells from the same patients were found to constitutively express Foxp3, a factor that was shown to be crucial for the development and function of Treg cells in mice36 and more recently in humans.37 In agreement with data reported by Walker et al,35 we found that CD4+CD25– T cells may acquire expression of Foxp3 following polyclonal activation (data not shown).
Peripheral CD4+CD25+ T cells isolated from HIV-infected patients were found to be hyporesponsive/anergic to polyclonal and antigen-specific stimulation. We observed a weak proliferation of CD4+CD25+ T cells stimulated by plate-bound anti-CD3 mAb; addition of soluble anti-CD28 mAb restored T-cell proliferation, as previously described for CD4+CD25+ Treg cells.9,10 The weak proliferation of CD4+CD25+ T cells following stimulation with plate-bound anti-CD3 mAb alone probably results from the polyclonality of the peripheral CD4+CD25+ T-cell population including cells with and without suppressive function. Thus, by expanding human CD4+CD25+ T cell clones, Levings et al19 emphasize the heterogeneity of human CD4+CD25+ T cells in terms of levels of CD25 expression, proliferation in response to anti-CD3 mAbs, and of suppressive activity.
We found that isolated CD4+CD25+ T cells failed to proliferate in response to the recall antigens tuberculin and CMV and to p24 protein. Moreover, depletion of CD4+CD25+ T cells from total CD4+ T cells significantly increased the proliferation levels to these antigens. Addition of purified CD4+CD25+ T cells resulted in a dose-dependent inhibition of CD4+CD25– T-cell proliferation to tuberculin and p24. Taken together, these data demonstrate that CD4+CD25+ T cells exert a suppressive activity on proliferation of memory CD4+ T cells induced by recall antigens and p24 protein. Interestingly, a ratio of 1:10 (CD4+CD25+ T cells to CD4+CD25– T cells) resulted in 30% inhibition of tuberculin-induced proliferation and 50% inhibition of p24-induced proliferation. These results are in agreement with those of Taams et al29 that demonstrated that the human CD4+CD25+ regulatory T cells displayed a broad usage of T-cell receptor Vβ repertoire, suggesting that they recognize a wide variety of antigens. By providing evidence that human CD4+CD25+ regulatory T cells are highly differentiated CD4+ T cells that have experienced repeated episodes of antigen-specific simulation in vivo, these authors propose that suppressive CD4+CD25+ T cells may be generated in the periphery as a consequence of repeated antigen stimulation.31 Thus, besides naturally occurring CD4+CD25+ T cells generated in the thymus (Douek et al38 and Dybul et al39 and references therein), CD4+CD25+ Treg cells might be expanded in the periphery in healthy humans and probably in transplant recipients and in HIV-infected patients as a consequence of repeated antigen exposure.
Peripheral CD4+ CD25+ T cells from HIV+ patients were found to proliferate in the presence of p24 and IL-2, the proliferation level being further increased by addition of soluble anti-CD28 mAb (data not shown). Thus, we asked the question whether peripheral CD4+CD25+ T cells from HIV-infected patients might exhibit specificity for HIV proteins. Even in the absence of cloning experiments, our data strongly suggest that p24-specific CD4+CD25+ regulatory T cells, responding to p24 stimulation by producing TGF-β and IL-10, are among the CD4+CD25+ T cells expanded in HIV-infected patients. Thus, p24 protein was found to induce in a specific manner, in the CD4+CD25+ T-cell population, the intracellular production of IL-10 at the single-cell level in most patients studied and the expression of TGF-β mRNA in all patients. In contrast, no production of IL-2 and IFN-γ was detected in this cell population. In addition, when recall antigens other than p24 were tested, we found that a lower proportion of cells produced IL-10 among the CD4+CD25+ subset. Similarly, in contrast to p24 antigen, we found that stimulation of CD4+CD25+ T cells with CMV antigen did not induce the expression of TGF-β1 transcripts. Altogether, these results suggest a bias of specificity toward HIV antigens in this cell population.
CD4 proliferative responses to HIV p24 antigen are minimal or absent in many HIV-infected individuals even treated successfully by HAART. It has been suggested that the lack of enhancement of HIV-specific cellular immune responses following initiation of effective antiretroviral therapy could be the result of clonal deletion or severe depletion of HIV-specific cells early during the course of infection.1 Thus, it has recently been demonstrated that HIV-specific memory CD4+ T cells in infected individuals contained more HIV viral DNA than other memory CD4+ T cells, HIV preferentially infecting HIV-specific CD4+ T cells.40 In addition, anergy may contribute to the unresponsiveness of HIV-specific CD4+ T cells.41 Results from the present study suggest that, in HIV infection, the apparent loss of p24-specific proliferative CD4 responses may result from the suppressive effect of CD4+CD25+ T cells. The correlation between CD4+CD25+ T-cell numbers and in vitro proliferative responses to p24 protein should be confirmed in a larger cohort of patients including untreated patients. As CD4 and CD8 HIV-specific immune responses are supposed to be important in controlling HIV replication,42 we can postulate that CD4+CD25+ T cells participate in the immune deficiency associated with HIV infection. This hypothesis is further supported by data from Iwashiro et al41 who found, in a murine model, that expansion of CD4 regulatory T cells in response to a chronic retroviral infection can lead to immunosuppression.
Repeated antigen exposure in the periphery has been suggested to be sufficient to generate Treg cells.31 In contrast to naturally occurring CD4+CD25+ T cells, adaptive regulatory T cells may be generated from mature T-cell populations under certain conditions of antigen presentation. Thus, Treg cells can be induced by polyclonal or antigen-specific stimulation in vitro in the presence of IL-10 and/or TGF-β8,16,44 ; generation of adaptive Treg cells might be promoted by inappropriate antigen presentation (ie, in the absence of CD28 costimulation).45 The HIV envelope glycoprotein gp120 was found to inhibit T-cell activation by inducing a dysregulation of expression of costimulatory molecules including CD40L on T cells and CD80 on antigen-presenting cells.46 It has been demonstrated that high level of viremia suppressed HIV-specific CD4 T-cell proliferation.47 Therefore, continuous presence of HIV antigens and defective CD28 costimulation should promote the generation of CD4+CD25+ Treg cells and their survival in vivo. In addition, expansion of regulatory T cells could also be dependent on specific viral gene products and on the cytokine environment. HIV infection leads to a variety of disturbances in cytokine expression.48 Regulatory T cells (rather Tr1 cells) may be induced from naive CD4 T cells in the presence of IFN-α and IL-10,49 and exposure to TGF-β has been reported to facilitate the differentiation/expansion of suppressor CD4+CD25+ T-cell populations in vitro.44 The regulatory HIV protein transactivating factor (Tat) was reported to induce an up-regulation of TGF-β expression,50 which may participate in the generation of Treg cells. Stimulation of TCR plus exogenous IL-2 was reported to result in a loss of the suppressive function of Treg cells9 ; defective IL-2 production in HIV infection could perpetuate the suppressive function of CD4+CD25+ regulatory T cells.
The suppressive effect of murine and human Treg cells can be dependent on cell-cell contact and/or secretion of suppressor cytokines including TGF-β and IL-10.8,33,49 Despite the specific induction of TGF-β and IL-10 following p24 stimulation, we found that the suppressive activity of CD4+CD25+ T cells from HIV-infected patients was not mediated by one of these 2 cytokines. We did not assess the requirement of cell-cell contact to mediate the suppressive effect of CD4+CD25+ T cells from HIV-infected patients.
The present study demonstrates that CD4+CD25+ T cells, expanded in peripheral blood of HIV-infected patients receiving HAART, exhibit phenotypic and functional characteristics of regulatory T cells. The apparent loss of HIV-specific proliferative responses in HIV-infected patients might be related to the suppressive effect of this regulatory T-cell population. CD4+CD25+ regulatory T cells responded specifically to p24 antigen stimulation by producing TGF-β and IL-10, indicating the presence of p24-specific CD4+ T cells among the CD4+CD25+ T-cell subset. Whether CD4+CD25+ T cells expanded in HIV infection can modulate the expression of major histocompatibility complex (MHC) and costimulatory molecules on dendritic cells50 and suppress CD8+ T-cell function is currently under investigation.
Taken together, our findings suggest that the expansion of CD4+CD25+ T cells exerting a suppressive activity against HIV and other pathogens might be triggered by HIV and result in an immune tolerance to HIV antigens in vivo. Therapeutic strategies directed toward the CD4+CD25+ T-cell subset should be considered in HIV infection.
Prepublished online as Blood First Edition Paper, July 22, 2004; DOI 10.1182/blood-2004-01-0365.
Supported by Sidaction, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank all participating patients. We also gratefully acknowledge Dr N. Bengrait and Dr A. Aouba research assistants; Drs C. Piketty, G. Gonzalez-Canali, and M. Karmochkine for including patients in the study; and Corinne Jung for technical support.


![Figure 3. CD4+CD25+ cells isolated from HIV-infected patients are hyporesponsive/anergic to polyclonal and antigen-specific stimulation. (A) Purified CD4+CD25– (□) or CD4+CD25+ (▪) T cells (1 × 105 each) were stimulated for 3 days with 5 μg/mL plate-bound anti-CD3 mAb in the presence or absence of soluble anti-CD28 mAb (5 μg/mL). (B) Freshly purified CD4+CD25– (□) or CD4+CD25+ (▪) T cells (1 × 105 each) were cultured for 5 days with 5 μg/mL purified tuberculin (PPD), 5 μg/mL p24, or 1:50 dilution of the CMV antigen. [3H] thymidine (0.5 μCi/well [0.0185 MBq]) was added during the last 16 hours of culture. Results are expressed as median [3H] thymidine incorporation from 8 HIV-infected patients (P < .05 for all of the antigens studied).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-01-0365/6/m_zh80220469610003.jpeg?Expires=1768015287&Signature=p6VF0-QHM38pXzlHyj9DltheNpIhZBdSIRT~rHpgsCAUevpdobDIwuBa~~nTlthbXswHtBFuODWmRSvIfjQQVkND4A9x6y5AodoHzk2ROXdsgVK6vdBvXsRebCxgDoqKua1y5Z3FeMJbExwlqFVBT2yyqCg7nn2E36JdfcAFjf3wqprRZlmviEvtT6TqzR5z32LVKGKpsn0W8oLYyvuEvpMaBNdiFQw8vO~tcAL2zVbhX-qL6pLXoJl4X~eO7KFrm0mphBZJqdLg~li1v7qS1oBlrAr04dYI6Ert28ILIFdUDQoHG5xaxcf-~SinYcnr-mHpfRv8DhEPwHJOyrNMTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Peripheral CD4+CD25+ T cells from HIV-infected patients suppress CD4 T-cell proliferation in response to recall antigens and HIV proteins. (A) Unfractionated CD4+ T cells (□) or purified CD4+CD25– T cells (▪) were incubated for 5 days with 5 μg/mL tuberculin (PPD), 5 μg/mL p24 protein, or 1:50 dilution of CMV antigen before adding 0.5 μCi (0.0185 MBq) [3H] thymidine for the last 16 hours of culture. Results are expressed as median values of the stimulation indexes for 8 patients studied (*P < .05). The median (ranges) of [3H] thymidine incorporation in control cultures (medium alone) were 462 (140-824), 240 (130-377), and 136 (63-175) for unfractionated CD4+ cells, CD4+CD25– cells, and CD4+CD25+ cells, respectively. (B) Coculture of CD4+CD25– with CD4+CD25+ T lymphocytes from HIV-infected patients results in a dose-dependent suppression of antigen-specific proliferation. Purified CD4+CD25– T cells were cultured for 5 days in the presence of 5 μg/mL purified tuberculin (♦) or 5 μg/mL p24 protein (⋄) and varying numbers of purified CD4+CD25+ lymphocytes. Percentage of inhibition was calculated as described in “Proliferation and suppression assays.” Results are expressed as mean (± SD) percentage of inhibition of proliferation for 5 patients studied.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-01-0365/6/m_zh80220469610004.jpeg?Expires=1768015287&Signature=wA1bDfoMzjuYJhpxtNbniBHGUNB8GeGcjhyCdGGqYo9CoiqRFGqDXhoKEv9XZrFI8QVIGlN9-ExwzBZCHrPJacMp2CUYycypPNuMy0gMSAPg3yRx4HNsO-Xb0FdtfdZ2ifQIGutcmASnr7xsMw~fmFYYW5oxHMRRxvhQ9NnuLr~Cioy7qO784GlUlZcqCXNu0P8WP6TFv607SF-7h3etnV825GVgm90RhsI-llJzTfxazNhY2a8y6YMMsjTAo9bub~jhh2u8LeuTJkJp9e9P-VpeCUPaotD6d9I15~C7oujNPr-MqFIhUK6ENkpexmeOCyM2GMSCW2~SXl5jTjH3BQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. The suppressive activity of CD4+CD25+ T lymphocytes is not mediated by IL-10 or TGF-β. Purified CD4+CD25– and CD4+CD25+ T cells were cocultured as described in “Patients, materials, and methods” in the presence of 5μg/mL p24 protein alone or in the presence of 10 μg/mL anti–IL-10, anti–TGF-β, or both neutralizing antibodies for 5 days before adding 0.5 μCi/well (0.0185 MBq) [3H] thymidine. Percentage of inhibition was calculated as described in “Proliferation and suppression assays.” Results are expressed as mean (± SD) percentage of inhibition of proliferation (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-01-0365/6/m_zh80220469610006.jpeg?Expires=1768015287&Signature=cYUgKv3~cJmABwkBFML16iwXVYU8aIp6oDEsiYv67KEYRqoA73a3ZMkmPAbS-5~4sFgPeGMX5alyGmrzaISA40yo45aaQ6Wrdp5PSLVgoAbHWDys8OoJ3zpQ4JPcjkuGtnpRQwn15R1jz1A5WK3oTM9l2wfOB3ElWLcaDlbY3FFc9rNkRSMtfsc2U9tSVraMw46fUukSieepynYIvxJSpdKOpGtzDdl4haMutOsM3N1vaMqbPKSVTET7os4-T1mcOwAH-dgm32UARy-LeOnYiTXoFKn4zc0cg6AnCJzd-0mrsSZSveaAzQ49qNR~JFDJviY-JlhWY4HzCN51d1V4-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. CD4+CD25+ cells isolated from HIV-infected patients are hyporesponsive/anergic to polyclonal and antigen-specific stimulation. (A) Purified CD4+CD25– (□) or CD4+CD25+ (▪) T cells (1 × 105 each) were stimulated for 3 days with 5 μg/mL plate-bound anti-CD3 mAb in the presence or absence of soluble anti-CD28 mAb (5 μg/mL). (B) Freshly purified CD4+CD25– (□) or CD4+CD25+ (▪) T cells (1 × 105 each) were cultured for 5 days with 5 μg/mL purified tuberculin (PPD), 5 μg/mL p24, or 1:50 dilution of the CMV antigen. [3H] thymidine (0.5 μCi/well [0.0185 MBq]) was added during the last 16 hours of culture. Results are expressed as median [3H] thymidine incorporation from 8 HIV-infected patients (P < .05 for all of the antigens studied).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-01-0365/6/m_zh80220469610003.jpeg?Expires=1768015288&Signature=TIKRo5HENOW~aDenX8k4~7Kttnxf7w05CiKUlezeO-QrL7c0hQ2ZuRZnO8buNs-V9LONWYcdXsbSDlpnclXQIFKxpBvD67sq48Q~0oGIs3IRlEhpa7BymTvgU1phwsGkM5gESN2Ik4Oto6qsnKiS3NJE~BZWlHqiuVFWILkICLJ~QwmbSL38K1ULS0broHP3O1zKHqDXLVG7Y93ylZp5-yFd~MdEJIrwLL5e3LGpiTmflVp16fjUzqX7CO6FGiDyeXKnqa1cYp9yI~ZFDd16dQ5-pp5KgzbVzL-~tCZ8-m688WwZwEBro0Ygpk0o9vNsJ93r7WTH7rbCViy2tPQPzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Peripheral CD4+CD25+ T cells from HIV-infected patients suppress CD4 T-cell proliferation in response to recall antigens and HIV proteins. (A) Unfractionated CD4+ T cells (□) or purified CD4+CD25– T cells (▪) were incubated for 5 days with 5 μg/mL tuberculin (PPD), 5 μg/mL p24 protein, or 1:50 dilution of CMV antigen before adding 0.5 μCi (0.0185 MBq) [3H] thymidine for the last 16 hours of culture. Results are expressed as median values of the stimulation indexes for 8 patients studied (*P < .05). The median (ranges) of [3H] thymidine incorporation in control cultures (medium alone) were 462 (140-824), 240 (130-377), and 136 (63-175) for unfractionated CD4+ cells, CD4+CD25– cells, and CD4+CD25+ cells, respectively. (B) Coculture of CD4+CD25– with CD4+CD25+ T lymphocytes from HIV-infected patients results in a dose-dependent suppression of antigen-specific proliferation. Purified CD4+CD25– T cells were cultured for 5 days in the presence of 5 μg/mL purified tuberculin (♦) or 5 μg/mL p24 protein (⋄) and varying numbers of purified CD4+CD25+ lymphocytes. Percentage of inhibition was calculated as described in “Proliferation and suppression assays.” Results are expressed as mean (± SD) percentage of inhibition of proliferation for 5 patients studied.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-01-0365/6/m_zh80220469610004.jpeg?Expires=1768015288&Signature=SFIifEl2saVwJPfReEXcPpFO-YjtCDTkoz2IkjmV1N5ET64e7o~hCtYwO48cVokrYfvEjOOa2lgQQU5Xr61d0m~xxAfwIru3qTemMtz2OQXygr5jInd4G3-zaXKraBF2aaMfLOk26akxEO5bq-qotJllVJl8TDHp3b-I2rhyxO3O2MMG9Q2s27WNM-FOykIfmSEJQqpnSOpKDN8RND1Erp6Wl0Bhzm1SsrtsupcywkN6PV82saXcemFWmugowasv9~8mZGHmZsDEwhDIW4o~AWrigC~ZAc1L9nHrBsDuVTuQoF8U37qXDkBJqRQGeBQMowA883AfXi-j1gGal289dg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. The suppressive activity of CD4+CD25+ T lymphocytes is not mediated by IL-10 or TGF-β. Purified CD4+CD25– and CD4+CD25+ T cells were cocultured as described in “Patients, materials, and methods” in the presence of 5μg/mL p24 protein alone or in the presence of 10 μg/mL anti–IL-10, anti–TGF-β, or both neutralizing antibodies for 5 days before adding 0.5 μCi/well (0.0185 MBq) [3H] thymidine. Percentage of inhibition was calculated as described in “Proliferation and suppression assays.” Results are expressed as mean (± SD) percentage of inhibition of proliferation (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-01-0365/6/m_zh80220469610006.jpeg?Expires=1768015288&Signature=QMMML3PzYJhEa~S672ASCFEaVQxoafwP4~x77vtRj-wQuIeypVr~lCZfaC6y4Ged9PfZj-6dTbBcIIPAjMolgWBME5jxBKdZrT8ofYXM9VFa4Dadnl30PnGOZLOsjgIIS5dTEk63cYeuaJBo-y6WU-RIpHB7xl5RViyAInKJYpxME18YRm0mTuQg284S5JUPVzVWEE2xNv8d3xJi6Y0SFeuwSrDMbIqk5YdaASPOW42PJmi8fi0QKqzLQSQck5X6x0O409kWVdiPW5S3GHoz0EDvfV55wwmvq29F4HJEDw3BhWVpvRoumxfW~4CW6WTiJwUH7~zqk5MAT4chF2BIVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)