Abstract
The active role of angiogenesis during disease progression is well recognized in solid tumors. In hematologic malignancies such as multiple myeloma (MM), it is not known whether tumor neovascularization is an epiphenomenon or whether it is actively involved in disease progression. At clinical presentation, myeloma disease and the associated angiogenesis are both well established. Here the 5T2MM murine model was used to analyze angiogenesis during preclinical myeloma stages. Bone marrow (BM) of 5T2MM-inoculated mice was analyzed at weekly intervals until the end stage of the disease. Histologic analysis and assessment of microvessel density (MVD) by CD31 staining demonstrated a preangiogenic stage of small tumor aggregates followed by an angiogenic switch and subsequently an angiogenic stage of progressive tumor growth and large, confluent tumor nodules. Flow cytometric analysis that indicated an increase in percentage CD45- MM cells preceded the angiogenic switch. Real-time polymerase chain reaction (RT-PCR) of sorted CD45+ and CD45- MM cells indicated higher vascular endothelial growth factor 120 (VEGF120) and VEGF164 transcripts in CD45- MM cells. VEGF enzyme-linked immunosorbent assay (ELISA) revealed high secretion by CD45- MM cells but no protein secretion by CD45+ MM cells, indicating angiogenic heterogeneity among the MM cells. These data suggest that, like in solid tumors, angiogenic switch and angiogenic heterogeneity exist in MM. (Blood. 2004;103:3131-3137)
Introduction
Angiogenesis or neovascularization is a multistep process of new blood vessel formation from existing blood vessels and is indispensable for physiologic growth, tissue healing, and regeneration. Neovascularization is also an essential process during tumorigenesis. Like normal tissues, tumor cells require an adequate supply of oxygen, nutrients, and an effective way to dispose of toxic metabolites. Generally, tumor masses beyond 0.5 mm in diameter require neovascularization for their survival and progressive growth.1-3 For few tumors, however, the preexisting vessels are sufficient.4-6 In solid tumors, the active role of neovascularization during tumorigenesis is well recognized.7 Increased angiogenesis in several human solid tumors has been associated with poor clinical outcome8-14 and plays a crucial role in their progressive growth and metastasis.15-17 Solid tumor progression is characterized by an avascular phase of slow tumor growth and a vascular phase of rapid tumor progression and metastasis. In the dormant, avascular stage, the tumor nodules are small enough to allow diffusion of oxygen from preexisting capillaries to the cancer cells; however, for their further progression the induction of a tumor vasculature must take place. This initiation of tumor vasculature termed “angiogenic switch” is orchestrated by a balance between proangiogenic and antiangiogenic molecules.7,18 From the several angiogenic activators described,7 vascular endothelial growth factor (VEGF) is an essential regulator of vascularization and is indispensable for both developmental and tumor angiogenesis.19-22 VEGF acts as an endothelial cell survival, mitogenic, and chemotactic factor23 and induces vessel destabilization by the induction of angiopoietin-2 in endothelial cells, resulting in angiogenic sprouting.24 Three isoforms of VEGF occur by alternative splicing of the mRNA: 121-, 165-, and 189–amino acid proteins (in mouse 120, 164, and 188 residues, respectively).25 In humans, VEGF206, a very rare form, has also been described.26 Although angiogenesis has been reported in hematologic (“liquid”) malignancies,27 it is not clear whether the neovascularization is actively involved in the disease progression or is merely an epiphenomenon. Multiple myeloma (MM) is such a “liquid” malignancy and is characterized by expansion and accumulation of monoclonal plasma cells in the bone marrow (BM). Several groups have reported an increased angiogenesis during clinical MM progression associated with proliferation of the MM cells and adverse clinical prognosis.28-32 In addition, the transition from monoclonal gammopathy to MM, accounting for one third of the new MM cases, is associated with an increased neovascularization.28,33 Myeloma cells express VEGF,34,35 and elevated serum levels of VEGF have been associated with increased BM angiogenesis29 and a higher MM cell labeling index.29,36 However, at clinical presentation myeloma disease as well as the associated angiogenesis are both well established, and whether the angiogenesis is an epiphenomenon or an important step in myeloma pathogenesis is unclear. In a previous work our group demonstrated in the 5T2MM experimental mouse model that preclinical myeloma disease progression is a multistage and dynamic process of differentiation, proliferation, invasion, and apoptosis.37 In this work the 5T2MM model was used to investigate angiogenesis during the initial, preclinical, stages of MM. Based on histologic, microvessel density (MVD), and flow cytometric analyses, we herein report a preangiogenic stage of slow myeloma progression, followed by an angiogenic switch and subsequent angiogenic stages of progressive tumor growth. The angiogenic switch was preceded by an increase in percentage CD45- MM cells. Real-time polymerase chain reaction (PCR) of flow cytometric–sorted CD45+ and CD45- MM cells indicated a higher number of VEGF mRNA copies in the CD45- MM cells. Analysis of conditioned media from sorted CD45+ and CD45- MM cells by enzyme-linked immunosorbent assay (ELISA) revealed high VEGF secretion by the CD45- MM cells but no detectable secretion by the CD45+ MM cells.
Materials and methods
5T2MM myeloma model
5T2 multiple myeloma cells originate from spontaneously developed myeloma in elderly C57BL/KaLwRijHsd mice.38,39 The model was initiated and is continued by intravenous injection of diseased BM cells into young (6- to 10-week-old) syngeneic recipients (Harlan, Horst, The Netherlands) as described previously.40
Study design
Thirty-three mice were intravenously injected with 2 × 106 5T2MM cells, and from the onset of the experiment 3 mice were killed each week until the terminal stage of the disease. From each mouse, paraprotein (serum concentration of monoclonal antibodies secreted by the MM cells) and tumor load were quantified and the MM cells were phenotyped. One hind leg was processed for histologic analysis and CD31 staining. In a second part of the study, MM cells were sorted for in vitro experiments.
Serum paraprotein quantification and isolation of BM cells
Mice were bled before killing, and serum paraprotein concentration was quantified by electrophoresis.41 BM cells were flushed out from femora and tibiae, and BM mononuclear cells (BMNCs) were isolated by centrifugation of the cell suspension on Lympholyte M (Cedarlane, Hornby, ON, Canada).
Histologic analysis and assessment of microvessel density
One hind of each mouse was fixed by immersion in zinc fixative (0.1 M Tris [tris(hydroxymethyl)aminomethane] buffer, pH 7.4, recipe above 1000 mL, 0.5 g calcium acetate, 5.0 g zinc acetate, and 5.0 g zinc chloride) for 48 hours and decalcified in a decalcification solution (100 g EDTA [ethylenediaminetetraacetic acid], 12 g NaOH, 50 mL formalin 40%, 950 mL demineralized water) for 4 days. Samples were embedded in paraffin, and 5-μm sections were made by a rotation microtome (Microm HM335, Walldorf, Germany). Of each sample step, sections were made. One slide was stained with hematoxylin and eosin. On the next slide, an immunostain for CD31 was performed. On the hematoxylin and eosin–stained slides, the MM cell infiltration and the growth pattern was assessed. On the CD31-immunostained slide, the microvessel density (MVD) was determined.
CD31 immunostaining. For CD31 immunostaining the sections were deparaffinized. The endogenous peroxidase was quenched by incubating the slides in 50 mL methanol, 0.5 mL H2O2 (30%) solution for 30 minutes. Trypsinization (Trypsin 0109819; Roche, Manheim, Germany) for 20 minutes at 37°C was used for antigen retrieval. The slides were preincubated with normal goat serum for 30 minutes. The primary antibody CD31 (platelet endothelial cell adhesion molecule-1 [PECAM-1]; Becton Dickinson PharMingen, San Jose, CA) was incubated overnight at 4°C at a dilution of 1:10. As secondary antibody biotin-conjugated goat antirat-specific polyclonal IgG (554014; Becton Dickinson PharMingen) was used in a dilution of 1:75. The Tyramide Signal Amplification (TSA; NEN Life Science Products, Boston, MA) was used to enhance the signal intensity. Chromogenic visualization was accomplished through the use of a streptavidin–horseradish peroxidase conjugate, followed by diaminobenzidine. The sections were counterstained with Carrazi hematoxylin. As negative controls, isotype-matched irrelevant antibodies were used.
Determination of the MVD. On the CD31-immunostained section, the areas with the highest density of blood vessels (hot spots) were selected. In these areas, the number of blood vessels was counted per 0.20 mm2 using a microscope eyepiece graticule (× 10 ocular and × 20 objective) on a light microscope (Jenamed2; Zeiss, Jena, Germany). All sections were scored independently by 2 individuals, and no significant differences were observed.
Flow cytometry
Tumor load in the BM was quantified by staining of the BMNCs with anti-5T2MM idiotype-specific antibodies (18B9, mouse immunoglobulin G1 [IgG1]).41 Rat antimouse IgG1–peridinin chlorophyll protein (IgG1-PerCP) (Becton Dickinson) was used as a second step. Rat antimouse CD45–fluorescein isothiocyanate (CD45-FITC; clone AMS4508; Biosource International, Camarillo, CA) was used to analyze CD45 expression on the MM cells. Isotype-matched irrelevant antibodies were used as negative controls. All samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson), and all cell sortings were performed on a FACSVantage-SE flow cytometer (Becton Dickinson). Myeloma cells were selected by sequential gating of 5T2MM idiotype-positive cells as described in detail previously.37 Sorted 5T2MM idiotype-positive cells were examined by light microscopy after May-Grünwald-Giemsa staining to confirm their identity as true MM cells.37
Bromodeoxyuridine incorporation
Bromodeoxyuridine incorporation was performed to analyze the proliferation of the MM cells during disease progression. Total MM cell populations from different disease stages were sorted as described previously.37 A quantity of 0.25 × 106 cells was incubated for 1 hour in Dulbecco modified essential medium (DMEM) supplemented with penicillin-streptomycin, glutamine, minimum essential medium (MEM) (GIBCO, Merelbeke, Belgium), and 10% bovine serum (Hyclone, Logan, UT) with 12.5 μg/mL bromodeoxyuridine (BrdU) (Sigma, St Louis, MO). Cells incubated in medium without BrdU were used as control. After incubation, the cells were stained for flow cytometric analysis with anti-BrdU monoclonal antibodies (clone BRD.3, mouse IgG1) (Biosource International) according to the manufacturer's instructions. Rat antimouse IgG1-phycoerythrin (IgG1-PE) (Becton Dickinson) was used as secondary reagent.
Quantitative RT-PCR
To analyze VEGF mRNA expression by MM cells, real-time (RT)–PCR was performed. Total RNA was extracted from flow cytometric–sorted CD45+ and CD45- MM cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and converted into cDNA using the SuperScript First-Strand Synthesis System (Invitrogen Life Technologies, Merelbeke, Belgium), according to the manufacturer's instructions. Primers and probes used for VEGF120 and VEGF164 have been described by others.42 For both isoforms the common probe 5′-ACAGCAGATGTGAATGCAGACCAAAGAAAG-3′ and the common forward primer 5′-GCCAGCACATAGAGAGAATGAGC-3′ were used. As reverse primers, 5′-CGGCTTGTCACATTTTCTGG-3′ and 5′-CAAGGCTCACAGTGATTTTCTGG-3′ were used for VEGF120 and VEGF164, respectively. Glyceradehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous reference gene to standardize for the amount of sample RNA. GAPDH primers and probes were purchased from ABI (Foster City, CA) and used according to the manufacturer's instructions. VEGF and GAPDH probes were labeled with tetramethylrhodamine (TAMRA) quencher dye and FAM (6-carboxyfluorescein) (for VEGF) or VIC (for GAPDH) reporter dyes. Taqman PCR was performed in a 25-μL reaction mix containing 12.5 μL 2 × Master Mix (ABI), 200 nM probe, 300 nM forward primer, 300 nM reverse primer, and 50 ng sample cDNA. PCR cycles consisted of an initial denaturation step at 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Each sample was amplified in triplicate. For relative standard curves cDNA from 5T33MMvivo cells (50, 10, 2, 0.4, and 0.083 ng) were used. Measurement of gene expression was performed using the ABI PRISM 7700 Sequence Detector. The relative standard curve method43 was used to quantitate the relative VEGF mRNA expression.
VEGF ELISA
Secretion of VEGF by MM cells was analyzed by ELISA. Flow cytometric–sorted CD45+ and CD45- MM cells were incubated in DMEM supplemented with penicillin-streptomycin, glutamine, and MEM at a cell concentration of 1 × 106/mL. After 24 hours, conditioned media were collected after centrifugation of the cells. VEGF ELISA was performed using the Quantikine M, mouse VEGF ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. With this ELISA, VEGF164 and VEGF120 are detected with a lower detection limit of 3.0 pg/mL.
Statistical analysis
StatView 5.0.1 software (SAS Institute, Cary, NC) was used for statistical analysis. Simple regression analysis was performed to calculate correlations. Mann-Whitney U test was used for the comparison of 2 means. P values .05 or less were considered as significant.
Results
Angiogenesis and tumor growth during 5T2MM disease progression
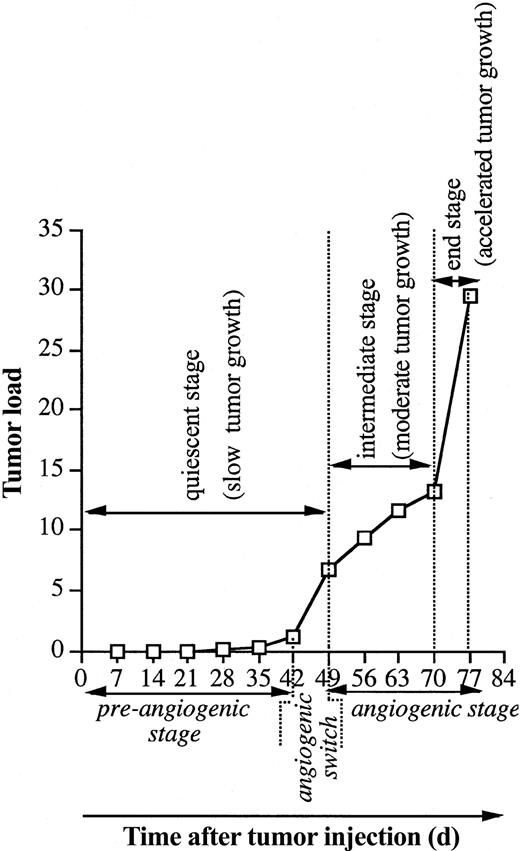
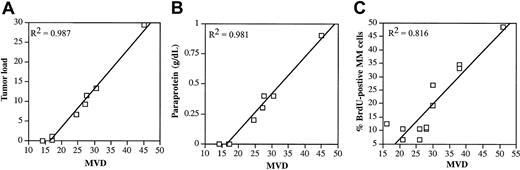
Naive mice were intravenously injected with 5T2MM cells, and each week 3 animals were analyzed until all animals were terminally diseased. In Figure 1 the evolution of the tumor load during the disease progression is illustrated. Myeloma cells were detectable by flow cytometry 4 weeks after tumor inoculation. As recently described by our group,37 3 disease stages were distinguished: a quiescent stage, an intermediate stage, and an end stage of slow, moderate, and accelerated tumor growth, respectively. MVD during the disease progression was analyzed by CD31 staining of the BM samples. Normal BM vasculature consists of a complex sinusoidal network that becomes part of the tumor vasculature during progressive tumor growth. Because in this stage it is difficult to distinguish between the newly formed blood vessels (angiogenesis) and the preexisting blood vessels, the latter were included in the assessment of MVD. There was a good correlation between the MVD and the tumor load, paraprotein concentration, and percentage BrdU-positive MM cells (Figure 2). These correlations are in line with clinical observations in human MM28-30 confirming the strength of the 5T2MM model.
Evolution of the tumor load during 5T2MM disease progression. Naive mice were injected with 5T2MM cells, and each week 3 animals were killed until all remaining mice were terminally diseased. BMNCs were isolated, and the tumor load (total MM population in the BM) was assessed by flow cytometric staining and analysis for 5T2MM idiotype-positive cells, as described previously.33 Each point represents mean value of 3 mice. The different growth stages and corresponding angiogenic phases are indicated.
Evolution of the tumor load during 5T2MM disease progression. Naive mice were injected with 5T2MM cells, and each week 3 animals were killed until all remaining mice were terminally diseased. BMNCs were isolated, and the tumor load (total MM population in the BM) was assessed by flow cytometric staining and analysis for 5T2MM idiotype-positive cells, as described previously.33 Each point represents mean value of 3 mice. The different growth stages and corresponding angiogenic phases are indicated.
Correlation between MVD and the tumor load, paraprotein, and BrdU incorporation. (A) Correlation between MVD and tumor load during 5T2MM disease progression. Tumor load was assessed as described in the legend of Figure 1. BM samples from the same mice were stained for CD31, and MVD (number of blood vessels per 0.20 mm2) was quantified. Each point represents mean value of 3 animals. (B) Correlation between MVD and paraprotein during 5T2MM disease progression. MVD was measured as described in “Materials and methods.” Before killing, the animals were bled and serum paraprotein concentration was quantified by protein electrophoresis. Each point represents mean values of 3 mice. (C) Correlation between MVD and BrdU incorporation during 5T2MM disease progression. MVD was quantified as described in panel A. 5T2MM cells from different disease stages were sorted by flow cytometry. Cells were incubated with BrdU for 1 hour followed by flow cytometric analysis of nuclei. Each point represents data from one mouse.
Correlation between MVD and the tumor load, paraprotein, and BrdU incorporation. (A) Correlation between MVD and tumor load during 5T2MM disease progression. Tumor load was assessed as described in the legend of Figure 1. BM samples from the same mice were stained for CD31, and MVD (number of blood vessels per 0.20 mm2) was quantified. Each point represents mean value of 3 animals. (B) Correlation between MVD and paraprotein during 5T2MM disease progression. MVD was measured as described in “Materials and methods.” Before killing, the animals were bled and serum paraprotein concentration was quantified by protein electrophoresis. Each point represents mean values of 3 mice. (C) Correlation between MVD and BrdU incorporation during 5T2MM disease progression. MVD was quantified as described in panel A. 5T2MM cells from different disease stages were sorted by flow cytometry. Cells were incubated with BrdU for 1 hour followed by flow cytometric analysis of nuclei. Each point represents data from one mouse.
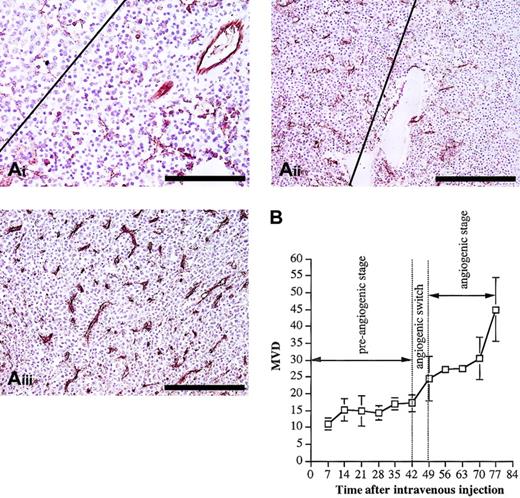
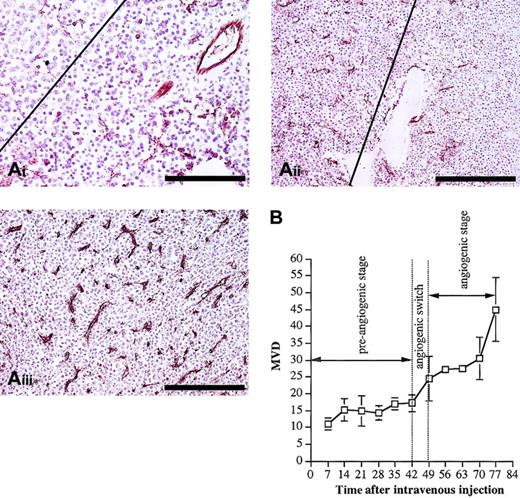
Histologic analysis (Figure 3A) indicated different patterns of tumor infiltration in the different disease stages. In the quiescent stage, individual cells were observed from day 28, and from day 35 small (fewer than 50 cells) aggregates of MM cells were formed. By the end of this dormant phase, larger (more than 50 cells) tumor aggregates were found. These aggregates did not disturb the architecture of the preexisting sinusoidal vessels. The major part of the quiescent stage was characterized by a constant MVD at the level of normal BM vascularization (Figure 3B). At the end of this stage, between day 42 and day 49, a clear onset in the increase of the MVD was observed, indicating an angiogenic switch. During the subsequent angiogenic stage (intermediate and end stage), progressive tumor growth was observed, as indicated by flow cytometric analyses of the tumor load (Figure 1). This was confirmed by histologic examination of animals in intermediate and end stage, demonstrating the presence of confluent tumor nodules, followed by subtotal infiltration and diffuse infiltration of the BM (Figure 3B). During this angiogenic stage, the MVD further increased parallel with the tumor growth (Figure 3). Compared with the preangiogenic sinusoids, the microvessels of the tumor-infiltrated areas were smaller with a slitlike lumen and immunostained more intense for CD31. All together, these data clearly indicate that 5T2MM disease progression is characterized by a preangiogenic stage of slow tumor progression, followed by an angiogenic switch and a subsequent angiogenic stage of progressive tumor growth.
Histologic analysis and evolution of the MVD. (A) Histologic analysis and CD31 staining of BM samples during 5T2MM disease progression. From the onset of the tumor inoculation 3 mice were analyzed each week until the end stage of the disease was reached. From each mouse one hind leg was processed for CD31 staining. (i) Preangiogenic stage: Small tumor aggregates were formed (upper left corner) without increase of the MVD. The right part of the figure illustrates tumor-free normal BM tissue. This section was made from a mouse killed at day 35. Bar = 100 μm. (ii) Early angiogenic stage: The angiogenic stage started when large confluent aggregates of MM cells were formed (left). On the right side, in the noninvaded part of the BM, the MVD is lower than on the left side. This section was made from a mouse killed at day 49. Bar = 200 μm. (iii) Late angiogenic stage: Massive BM invasion was accompanied by a high MVD. Most of the microvessels are small and intensely stained for CD31. This section was made from a mouse at day 77. Bar = 200 μm. Each section is representative for 3 mice. (B) Evolution of the MVD during 5T2MM disease progression. MVD (number of blood vessels per 0.20 mm2) was quantified as described in “Materials and methods.” Each point represents mean values of 3 mice ± SD.
Histologic analysis and evolution of the MVD. (A) Histologic analysis and CD31 staining of BM samples during 5T2MM disease progression. From the onset of the tumor inoculation 3 mice were analyzed each week until the end stage of the disease was reached. From each mouse one hind leg was processed for CD31 staining. (i) Preangiogenic stage: Small tumor aggregates were formed (upper left corner) without increase of the MVD. The right part of the figure illustrates tumor-free normal BM tissue. This section was made from a mouse killed at day 35. Bar = 100 μm. (ii) Early angiogenic stage: The angiogenic stage started when large confluent aggregates of MM cells were formed (left). On the right side, in the noninvaded part of the BM, the MVD is lower than on the left side. This section was made from a mouse killed at day 49. Bar = 200 μm. (iii) Late angiogenic stage: Massive BM invasion was accompanied by a high MVD. Most of the microvessels are small and intensely stained for CD31. This section was made from a mouse at day 77. Bar = 200 μm. Each section is representative for 3 mice. (B) Evolution of the MVD during 5T2MM disease progression. MVD (number of blood vessels per 0.20 mm2) was quantified as described in “Materials and methods.” Each point represents mean values of 3 mice ± SD.
Increase in percentage CD45- 5T2MM cells precedes the angiogenic switch
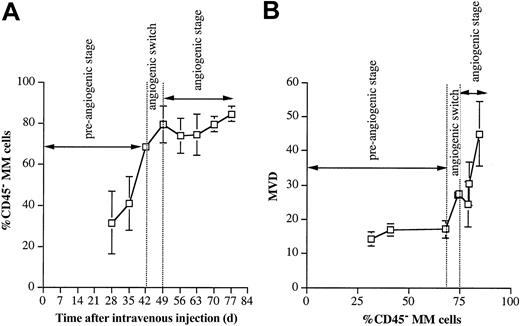
In analogy to human MM, 5T2MM cells have a heterogeneous CD45 expression.44 Recently we reported that preclinical 5T2MM disease progression is a process of ongoing differentiation of CD45+ MM cells into CD45- cells.37 In the end stage of the disease (corresponding to the human situation at clinical presentation) most 5T2MM cells are CD45-. Because angiogenic heterogeneity among cancer cells has been reported for solid tumors,45,46 we analyzed whether the angiogenic switch was associated with the presence of CD45- MM cells. Flow cytometric analysis (Figure 4A) indicated that the angiogenic switch was preceded by an increase in the percentage CD45- MM cells. Most of the myeloma cell population during the angiogenic stage consisted of CD45- MM cells. When the MVD was plotted against the percentage CD45- MM cells, it became clear that the angiogenic switch did not occur until the percentage CD45- MM cells had reached a threshold value (Figure 4B). There was also a significant difference between the percentage CD45- MM cells and between the MVD before and after the angiogenic switch (Table 1). These data demonstrate that the angiogenic switch coincides with an increased proportion of CD45- MM cells in the myeloma population.
CD45- cell evolution. (A) Evolution of percentage CD45- 5T2MM cells during disease progression. MM cells were detectable above background levels by flow cytometry 4 weeks after tumor injection and were phenotyped for CD45 expression. Percentages of CD45- MM cells in the total myeloma population are shown. Each point represents mean value of 3 mice ± SD. (B) Evolution of the MVD in function of percentage CD45- 5T2MM cells. MVD represents the number of blood vessels per 0.20 mm2. Percentages of CD45- MM cells in the total myeloma population are shown. Each point represents mean value of 3 animals ± SD.
CD45- cell evolution. (A) Evolution of percentage CD45- 5T2MM cells during disease progression. MM cells were detectable above background levels by flow cytometry 4 weeks after tumor injection and were phenotyped for CD45 expression. Percentages of CD45- MM cells in the total myeloma population are shown. Each point represents mean value of 3 mice ± SD. (B) Evolution of the MVD in function of percentage CD45- 5T2MM cells. MVD represents the number of blood vessels per 0.20 mm2. Percentages of CD45- MM cells in the total myeloma population are shown. Each point represents mean value of 3 animals ± SD.
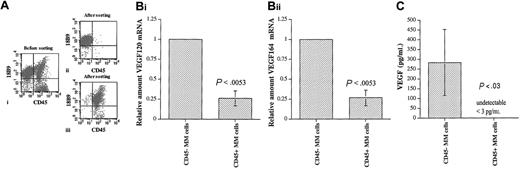
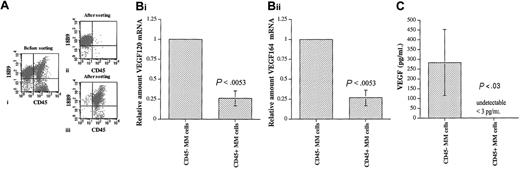
Expression of VEGF by CD45+ and CD45- 5T2MM cells
VEGF is an essential factor for both normal and tumor-associated angiogenesis19-21 ; therefore, we analyzed whether there was any difference in the expression of this key regulator of neovascularization between the CD45+ and the CD45- MM cells. In analogy to human MM cells,34,35 5T2MM cells express VEGF120 and VEGF164.47 5T2MM cells were sorted into CD45+ and CD45- MM cells by flow cytometry, as illustrated in Figure 5A. Microscopic analysis of the sorted cells37 confirmed their identity as true MM cells (not shown). Quantitative real-time PCR was performed to analyze the expression of VEGF120 and VEGF164 transcripts by the CD45+ and CD45- MM cells. For both VEGF120 and VEGF164, their mRNA expression by CD45- MM cells was 4-fold higher compared with the CD45+ MM cells (Figure 5B). Twenty-four–hour conditioned media from CD45+ and CD45- MM cells were analyzed by ELISA to assess the secretion of VEGF protein by these 2 populations. As described in “Materials and methods,” this ELISA detects both VEGF120 and VEGF164 isoforms with a lower detection limit of 3.0 pg/mL. There was a high VEGF secretion by the CD45- MM cells (range, 179 to 478 pg/mL), while no VEGF was detected in conditioned media obtained from CD45+ MM cells (Figure 5C). These data demonstrate a higher VEGF mRNA expression by CD45- MM cells and VEGF protein secretion by CD45- MM cells only and clearly indicate an angiogenic heterogeneity among the myeloma cell population.
VEGF secretion by CD45+ and CD45- MM cells. (A) Sorting of CD45+ and CD45- 5T2MM cells. BMNCs isolated from tumor-bearing mice were stained with anti-5T2MM idiotype antibodies (18B9) and CD45 (i). The 5T2MM cell population was sorted into CD45- (ii) and CD45+ (iii) fractions. Sorted cell populations with a purity of at least 95% were used for further experiments. Dot plots from one representative cell sorting are illustrated. (B) Expression of VEGF transcripts by CD45+ and CD45- 5T2MM cells. Flow cytometric–sorted CD45- and CD45+ MM cells were analyzed for VEGF120 (i) and VEGF164 (ii) mRNA expression by quantitative RT-PCR as described in “Materials and methods.” Values are expressed relative to the expression by CD45- MM cells. Mean ± SD values of 3 independent experiments are shown. (C) Secretion of VEGF by CD45+ and CD45- 5T2MM cells. VEGF secretion by CD45- and CD45+ MM cells in 24-hour conditioned media was analyzed by ELISA. Mean ± SD values of 3 independent experiments are illustrated.
VEGF secretion by CD45+ and CD45- MM cells. (A) Sorting of CD45+ and CD45- 5T2MM cells. BMNCs isolated from tumor-bearing mice were stained with anti-5T2MM idiotype antibodies (18B9) and CD45 (i). The 5T2MM cell population was sorted into CD45- (ii) and CD45+ (iii) fractions. Sorted cell populations with a purity of at least 95% were used for further experiments. Dot plots from one representative cell sorting are illustrated. (B) Expression of VEGF transcripts by CD45+ and CD45- 5T2MM cells. Flow cytometric–sorted CD45- and CD45+ MM cells were analyzed for VEGF120 (i) and VEGF164 (ii) mRNA expression by quantitative RT-PCR as described in “Materials and methods.” Values are expressed relative to the expression by CD45- MM cells. Mean ± SD values of 3 independent experiments are shown. (C) Secretion of VEGF by CD45+ and CD45- 5T2MM cells. VEGF secretion by CD45- and CD45+ MM cells in 24-hour conditioned media was analyzed by ELISA. Mean ± SD values of 3 independent experiments are illustrated.
Discussion
Unlike in solid tumors, the role of angiogenesis in the pathogenesis of hematologic malignancies such as MM is a subject of debate.28-30 At the time of diagnosis, the myeloma disease as well as the associated angiogenesis are both well established. Insights into the early, preclinical tumor stages and neovascularization would clarify the role of angiogenesis in myeloma pathobiology. Such an investigation requires an in vivo model, and for this purpose we used the 5T2MM model in this work. This murine model has clinical symptoms similar to human myeloma.47-49 In addition, we demonstrated in this work that, like in the human situation,28-30 a good correlation exists between the MVD and the tumor load, the serum paraprotein levels, and the proliferation of the 5T2MM cells.
As reported in a previous work,37 a quiescent stage of slow tumor progression and 2 progressive stages of moderate and accelerated tumor growth—intermediate and end stage, respectively—could be distinguished during 5T2MM tumorigenesis. The early, quiescent stage was a preangiogenic phase of slow tumor progression within the network of preexisting BM sinusoidal capillaries. During this stage the MM cells were found as single cells, small aggregates of few cells, or as larger but still separated aggregates at the end of the preangiogenic stage. These aggregates did not interfere with the architecture of the sinusoidal vessels. The myeloma cell population consisted of mainly CD45+ MM cells, which exhibited undetectable VEGF secretion. In this stage BrdU incorporation by MM cells was low, indicating a low proliferative rate, as reported previously.37 Myeloma disease progression is a process of ongoing differentiation of CD45+ MM cells into CD45- MM cells.37 By the end of the preangiogenic stage, most of the MM cells were CD45- with high levels of VEGF secretion. At this time point the angiogenic switch was observed as a significant increase in the MVD. In the subsequent angiogenic stage a progressive expansion of the tumor was observed. The tumor load increased progressively, and the MM growth pattern consisted of confluent tumor nodules, followed by subtotal and diffuse infiltration of the BM. In line with these findings, we reported that MM cells in the intermediate and end stage exhibit a high proliferation as indicated by BrdU incorporation.37 These data are completely in line with the occurrence of angiogenesis during solid tumorigenesis. In early stages of solid tumor growth there is a preangiogenic stage of dormant, slow tumor progression. In this stage the preexisting vessels of the host tissue are sufficient to maintain the tumor (co-option). During the preangiogenic phase the tumor population consists of only few angiogenic cancer cells (cancer cells with the capacity to induce neovascularization). When enough cancer cells become angiogenic, angiogenic switch occurs, followed by a vascular stage of progressive tumor expansion and metastasis.7,50 Most, if not all, cases of myeloma are preceded by monoclonal gammopathy of undetermined significance (MGUS) or smoldering myeloma. Our data demonstrate that in myeloma the preclinical stage is a preangiogenic phase that is followed by an angiogenic stage of overt (clinical) myeloma. In addition to other key events, angiogenic switch might contribute to the transformation of MGUS/smoldering MM to symptomatic myeloma.
Recently the heterogeneity of angiogenic activity among cancer cells in solid tumors has been recognized45 whereby only specific subsets in the tumor population are able to induce angiogenesis. Achilles et al45 demonstrated that human liposarcoma contains subpopulations with high angiogenic capacity, giving rise to fast-growing, aggressive tumors; subpopulations with weakly angiogenic activity, resulting in slow-growing tumors; and nonangiogenic subsets that remain stable tumors.46 Our data suggest that, in MM, CD45- MM cells are the angiogenic subpopulation and indicate that the concept of angiogenic heterogeneity is also applicable for myeloma disease. In keeping with these findings, we recently reported that a minority of 5T2MM-bearing mice have a slow disease progression associated with the presence of high proportions of CD45+ MM cells.51
Angiogenic switch is induced when the balance of angiogenic inhibitors and activators is changed by overexpression of proangiogenic factors.7 VEGF is one of the most essential activators of both normal and tumor angiogenesis and acts on the endothelial cells via VEGF receptors (VEGFRs). VEGFR-2 is the most important for the angiogenic switch.52 Like human BM endothelial cells,53 mouse BM endothelial cells also express VEGFR-2, as indicated by flow cytometric analysis of immortalized BM endothelial cells, STR-4, STR-10, and STR-12 54 (data not shown), suggesting that endothelial cells in the BM are prone to VEGF. The loss of a single VEGF allele results in severely impaired vascularization and early embryonic lethality.19,20 Cancer cells knocked out for the VEGF gene have a dramatically decreased vascular density and a greatly reduced tumor growth when inoculated in vivo.21,22 From these latter works it became clear that tumor-derived VEGF plays a crucial role in tumor angiogenesis, and these works settled the debate of the relative contribution of cancer cell versus normal host cell VEGF expression to tumor growth.21,22 The angiopoietin/Tie-2 system plays also a crucial role in angiogenesis.55 Angiopoietin-1 and -2 have Tie-2 as common receptor on the endothelial cells. Angiopoietin-1 maintains and stabilizes mature vessels, while its natural antagonist, angiopoietin-2, destabilizes the vessels and induces angiogenic sprouting. Recent insights indicate that angiopoietin-2 is induced in the endothelial cells by tumor-derived VEGF determining the angiopoietin/Tie-2 balance in favor of angiogenesis.24 The expression of angiopoietin-2 and Tie-2 by BM endothelial cells in human myeloma has been well documented.53,56
At clinical presentation, most human MM cells are CD45-, which is also observed in the 5T2MM model.37 5T2MM cells express VEGF120 and VEGF164 isoforms, another analogy to the human MM cells.34,35 Overexpression of specific VEGF isoforms in fibrosarcomas knocked out for the VEGF gene has demonstrated that each isoform has a unique role in tumor angiogenesis.22 VEGF-null fibrosarcomas failed to induce tumor vascularization, resulting in a significant reduction of tumor size compared with the wild type. VEGF120 overexpression recruited peripheral host blood vessels to the periphery of the tumor but induced only little vascularization of the tumor itself, also resulting in highly decreased tumor growth. VEGF164 was the only isoform that recruited host blood vessels and induced tumor microvasculature, fully restoring the tumor-associated angiogenesis and tumor expansion. When mice were inoculated with a mixture of different VEGF isoform transfectants, VEGF164-expressing cancer cells exhibited the most rapid expansion. These data indicated that during tumorigenesis selective pressure favors the cancer cells expressing VEGF164. Our observation of increasing CD45- MM cell compartment during the disease progression is also in line with this finding in solid tumors. Recently, CD45- phenotype appeared to be a predictor of poor clinical outcome in human57 and 5T2MM myelomas.51 Antiangiogenic therapy in MM has been suggested by several groups33,35,58,59 ; however, our data indicate the existence of a preangiogenic stage. This raises the possibility that antiangiogenic drugs alone may not be fully curative because MM cells in this stage are angiogenic independent and can survive within the network of normal BM vasculature.
All together, the data in this work indicate important similarities between angiogenesis in solid tumor and angiogenesis in MM. The existence of a preangiogenic stage of slow tumor progression, mainly consisting of MM cells with no detectable VEGF secretion, and the increase in VEGF-producing MM cells preceding the angiogenic switch and the subsequent angiogenic stage of progressive tumor expansion suggest that, as in solid tumors, angiogenesis is an active and important process in myeloma disease progression.
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-08-2946.
Supported by Onderzoeksraad-VUB, the Fund for Scientific Research-Flanders (Belgium) (FWO-Vlaanderen), Belgian Federation against Cancer, Fortis-viva and International Myeloma Foundation (IMF) Ashley Barit Research grant (K.A.). K.A. and K.V. are postdoctoral fellows of FWO-Vlaanderen. H. De R. has a Clinical Doctoral Grant from FWO-Vlaanderen.
K.A. and H. De R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Gunther Vrolix, Liliane Moeneclaey, and Frank Rylant for the histologic sections and immunostainings.