The dogma stating that the binding of immunoglobulin E (IgE) to its receptor (FcϵRI) on mast cells is simply a passive presensitization step that requires subsequent cross-linking with multivalent antigen (Ag) to elicit or biologic effects was first challenged when it was shown that IgE alone could increase the cell surface expression of FcϵRI.1,2 Then, in 2001, 2 groups showed that IgE alone could enhance the survival of cytokine-deprived, bone marrow–derived mast cells (BMMCs).3,4 However, they disagreed on how. Kalesnikoff et al,3 using the highly potent SPE-7 IgE, proposed that IgE prolonged survival via intracellular signaling that led to the maintenance of Bcl-XL levels and the production of autocrine-acting cytokines. They also showed that IgE alone stimulated more prolonged Erk phosphorylation and higher levels of inflammatory cytokines than IgE(+Ag) and proposed that this might explain why IgE could induce survival while IgE(+Ag) could not. Asai et al,4 using the less potent Liu IgE, did not observe any IgE-induced signaling and proposed a signaling-independent mechanism for IgE-induced survival. However, a more recent paper by the latter group5 confirmed that signaling was essential since IgE-induced survival required the tyrosine kinase Syk, regardless of the IgE used.FIG1
Of the IgEs tested to date, SPE-7 is by far the most potent at inducing BMMC signaling/survival, and some investigators have been reluctant to extrapolate results obtained with this potentially “oddball” IgE to other IgEs because SPE-7 was recently shown to exist in 2 interconvertible conformations in the absence of Ag: one capable of binding to its hapten (dinitrophenol [DNP]) and the other to an unrelated protein.6 How common this isomerism is amongst IgEs is not yet known. Countering this argument, other IgEs have been shown to induce BMMC survival in vitro4,5 and to increase mast cell numbers in vivo.5
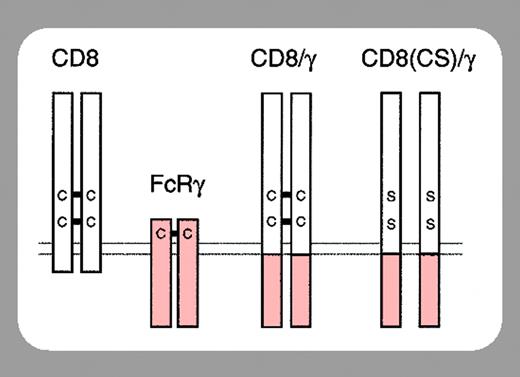
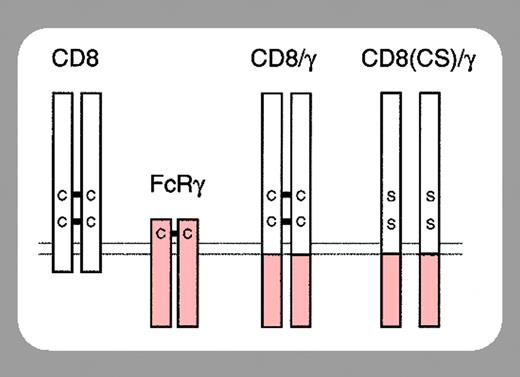
The FcϵRI on mast cells is composed of an α chain that binds IgE, a β chain that amplifies the IgE-induced signal, and 2 γ chains that transduce the signal. The β and γ chains possess immunoreceptor tyrosine-based activation motifs (ITAMs) within their cytoplasmic domains that, upon IgE-induced activation, become tyrosine phosphorylated by an associated Src kinase (Lyn/Fyn). Syk is then recruited to the phospho-ITAM of the γ chains and mediates downstream signaling.
In this issue of Blood, Yamasaki et al (page 3093) have circumvented the controversy surrounding SPE-7 by exploiting their finding that the γ ITAM is essential for both IgE-induced mast cell survival and IgE(+Ag)-induced degranulation. Specifically, they expressed a CD8/γ chimera in FcϵRIγ-/- BMMCs and found that γ engagement (by dimerizing the chimera with anti-CD8) was sufficient for the induction of BMMC survival and degranulation. However, they found that low levels of anti-CD8 stimulated survival while high levels induced degranulation. Related to this, low levels of anti-CD8 gave a much lower phosphorylation of JNK and p38 but gave a similar Erk phosphorylation to that observed with high levels. If they increased the signal by cross-linking the anti-CD8 with a secondary antibody, Erk phosphorylation was induced more strongly but peaked at 2 minutes and rapidly decreased. Importantly, this correlated with rapid internalization of CD8/γ and far less survival. From these and other studies they conclude that survival depends on sustained Erk phosphorylation.
Taken together with the work of Kalesnikoff et al3 and Kitaura et al,5 it is likely that certain highly potent IgEs are capable in vivo of increasing mast cell survival and inflammatory cytokine production via sustained Erk phosphorylation and that the severity of allergic disorders in some people may be due to the production of these IgEs.