Abstract
Sickle cell vaso-occlusion is a complex multistep process likely involving heterotypic interactions among sickle erythrocytes (red blood cells [RBCs]), leukocytes (white blood cells [WBCs]), and endothelial cells. Recent data using intravital microscopy in a sickle cell mouse model suggest that adherent leukocytes in postcapillary venules play a critical role in vaso-occlusion by capturing circulating sickle RBCs. In the course of studies to investigate the adhesion receptors mediating sickle RBC-WBC interactions, we found that control nonspecific immunoglobulin G (IgG) preparations displayed significant inhibitory activity. As a result, we studied the effects of commercial intravenous human immune globulin (IVIG) preparations and found that IVIG inhibits RBC-WBC interactions in cremasteric venules in a dose-dependent manner. IVIG of at least 200 mg/kg dramatically reduced these interactions, even after tumor necrosis factor-α (TNF-α) stimulation, and not only increased microcirculatory blood flow but also improved survival of sickle cell mice. These data raise the possibility that IVIG may have a beneficial effect on sickle cell–associated vaso-occlusion.
Introduction
Sickle cell disease results from a single missense mutation in the β-globin gene that alters the solubility of the deoxygenated molecule, leading to polymer formation, red blood cell (RBC) sickling, and vaso-occlusion.1 Previous studies demonstrated that sickle RBCs are more adherent to vascular endothelium and that the endothelium in sickle cell patients and mice is inflamed.2-5 Clinical studies have suggested a link between elevations in white blood cell (WBC) counts and poor outcome in sickle cell disease,6 and in vitro data indicate that sickle cells can bind neutrophils in a static adhesion assay.7 Recently, intravital microscopy studies in sickle cell mice revealed that WBC adhesion in venules played a direct role in sickle cell vaso-occlusion by interacting with sickle RBCs.8 Thus, sickle cell vaso-occlusion appears to involve multicellular interactions among RBCs, WBCs, and the venular endothelium.9 In the course of experiments using monoclonal antibodies to evaluate the role of adhesion receptors in this phenomenon, we noticed that control antibodies inhibited RBC-WBC interactions. Here, we demonstrate that high doses of commercial intravenous human immune globulin (IVIG) dramatically inhibit RBC-WBC interactions and improve blood flow and survival of sickle cell mice subjected to tumor necrosis factor-α (TNF-α)–induced vaso-occlusion.
Study design
Generation of sickle cell mice
Sickle cell (SS) mice,10 a kind gift from Dr Narla Mohandas (New York Blood Center, New York), were bred at Mount Sinai School of Medicine. Genetically identical cohorts of male sickle mice were generated by bone marrow transplantation as described previously.8 Mice carrying more than 97% donor chimerism, as assessed using acid-urea gels,11 were used for intravital microscopy studies. All experimental procedures were approved by the Animal Care and Use Committee of Mount Sinai School of Medicine.
Immunoglobulins
Human γ-globulin (IVIG) was a gift from Bayer (Elkhart, IN) and diluted in phosphate-buffered saline (PBS) for injection. Fab and Fc fragments were prepared from IVIG using a protein G column (Pharmacia, Uppsala, Sweden) as described.12
Intravital microscopy
SS mice were injected (200 μL) with either IVIG, Fab or Fc antibody fragments, immunoglobulin G (IgG)–free albumin (Sigma, St Louis, MO), or control vehicle (endotoxin-free PBS) via the lateral tail vein 20 minutes before the start of the surgery. Following anesthesia with a mixture of 2% α-chloralose and 10% urethane in PBS (6 mL/kg), a polyethylene tube was inserted via tracheotomy to facilitate spontaneous respiration, and the cremaster muscle was prepared for intravital microscopy as described.8 Fifteen minutes following surgery, venules were videotaped for 75 minutes (intravital microscopy viewing period 1: IVM 1), after which 0.5 μg murine recombinant TNF-α (rTNF-α) (R&D Systems, Minneapolis, MN) was injected intraperitoneally. After exposure to TNF-α for 90 minutes, the same venules were recorded for 90 additional minutes (IVM 2).
Image analyses
The numbers of rolling and adherent leukocytes were quantitated by playback analyses of videotapes as previously described.8 RBCs were identified based on their size and shape (discoid and sickle-shaped cells). An RBC-WBC interaction was defined as the arrest of an RBC on an adherent WBC for more than 2 video frames (more than 0.07 seconds). This time interval was selected because it correlates with a readily discernable adhesion event when the videotape is played in real time. RBC-WBC interactions were counted over a 100-μm venular segment for 3 1-minute intervals per venule.
Statistical analyses
Statistical significance for nonparametric values (RBC-WBC interactions) was assessed using the Kruskal-Wallis test followed by the Mann-Whitney test. Analysis of variance (ANOVA) with Bonferroni/Dunn correction of P was used for parametric values.
Results and discussion
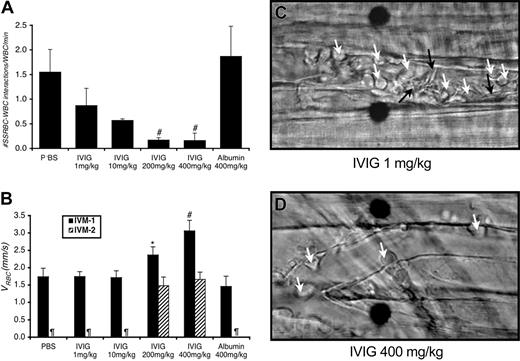
Surgical preparation of cremaster muscle for intravital microcopy invariably triggers an inflammatory response leading to rolling and adhesion of leukocytes to venular endothelium.13 Thereafter, discoid and sickle-shaped RBCs interact with adherent WBCs.8 Administration of IVIG reduced both the number of adherent WBCs attached to the endothelium (Table 1) and the number of interactions between RBCs and WBCs (Figure 1A) in a dose-dependent manner.
IVIG prevents both the interactions between sickle RBCs and WBCs and vaso-occlusion in sickle cell mice. (A) IVIG reduces RBC-WBC interactions in vivo in a dose-dependent manner; differences were significant for the groups treated with IVIG at 200 mg/kg or more. #P ≤ .01 compared with PBS, P < .05 compared with the albumin-treated group. (B) IVIG 400 mg/kg improves centerline RBC velocities (VRBC) in venules as measured during IVM 1 in real time using an optical Doppler velocimeter (Texas A&M, College Station), and the effect is sustained after TNF-α (IVM 2). *P < .05 compared with the albumin group. #P< .05 compared with both the PBS and albumin groups. ¶Groups in which the numbers of live mice and venules were too small for reliable determination. (C) Representative still frames after TNF-α administration of venules from sickle cell mice treated with 1 mg/kg or 400 mg/kg IVIG. In venules of sickle mice treated with low-dose IVIG, there are numerous interactions between RBCs (black arrows) and adherent WBCs (white arrows) accompanied by drastic reductions in blood flow in most venules. Fewer RBC-WBC interactions and adherent WBCs are observed in sickle mice treated with high-dose IVIG. Scale bar indicates 10 μm. Movie segments corresponding to these still frames (C-D) can be viewed on the Blood website. See the Supplemental Video link at the top of the online article.
IVIG prevents both the interactions between sickle RBCs and WBCs and vaso-occlusion in sickle cell mice. (A) IVIG reduces RBC-WBC interactions in vivo in a dose-dependent manner; differences were significant for the groups treated with IVIG at 200 mg/kg or more. #P ≤ .01 compared with PBS, P < .05 compared with the albumin-treated group. (B) IVIG 400 mg/kg improves centerline RBC velocities (VRBC) in venules as measured during IVM 1 in real time using an optical Doppler velocimeter (Texas A&M, College Station), and the effect is sustained after TNF-α (IVM 2). *P < .05 compared with the albumin group. #P< .05 compared with both the PBS and albumin groups. ¶Groups in which the numbers of live mice and venules were too small for reliable determination. (C) Representative still frames after TNF-α administration of venules from sickle cell mice treated with 1 mg/kg or 400 mg/kg IVIG. In venules of sickle mice treated with low-dose IVIG, there are numerous interactions between RBCs (black arrows) and adherent WBCs (white arrows) accompanied by drastic reductions in blood flow in most venules. Fewer RBC-WBC interactions and adherent WBCs are observed in sickle mice treated with high-dose IVIG. Scale bar indicates 10 μm. Movie segments corresponding to these still frames (C-D) can be viewed on the Blood website. See the Supplemental Video link at the top of the online article.
Following TNF-α administration, which is known to enhance in vivo leukocyte adhesion, sustained RBC-WBC interactions in the groups injected with PBS, IVIG 1 mg/kg, or IVIG 10 mg/kg led to progressive reductions in venular blood flow, but mice treated with at least 200 mg/kg IVIG were significantly protected from blood flow reductions (Figure 1B-C; wall shear rates in Table 1). Furthermore, most mice pretreated with PBS, IVIG 1 mg/kg, or IVIG 10 mg/kg died before the end of IVM 2 (Table 1), whereas significantly more mice treated with at least 200 mg/kg IVIG were still alive at the end of the experiment (1 of 14 alive for PBS and low-dose IVIG versus 7 of 10 alive for at least 200 mg/kg IVIG; P = .002, Fisher exact test).
To investigate whether the effects of high-dose IVIG were due to an increase in intravascular oncotic pressures by the protein or nonspecific protein coating, we treated sickle mice with 400 mg/kg IgG-free human albumin prior to the intravital microscopy protocol and evaluated its effects on RBC-WBC interactions, blood flow, and survival. We found that the number of interactions between RBCs and WBCs and effects on blood flow were similar to those observed in the PBS-treated groups (Figure 1A-B). Moreover, all 5 albumin-treated mice died during the experimental procedure (Table 1). Thus, the inhibition of RBC-WBC interactions by IgG is not due to protein-mediated improved microcirculatory hemodynamics or to non-specific protein coating of sickle RBCs or WBCs.
Previous studies revealed the presence of bound IgG on the surface of sickle cells14-17 and that IgG binding was greatest on dense16 and sickle-shaped17 RBCs. Hofstra et al found that preincubation of neutrophils with IgG inhibited their binding to sickle RBCs in a static adhesion assay, suggesting that sickle RBCs might interact with adherent neutrophils in vitro through IgG-Fc receptors.7 To investigate whether the effect of IVIG was mediated by Fab or Fc fragment of the IgG molecule, antibody fragments were prepared and injected at a dose of 200 mg/kg, which corresponds to about 400 mg/kg whole IgG on a molar basis. Neither fragment significantly affected WBC-endothelial interactions (Table 1). While both the Fab and Fc fragments tended to reduce the numbers of RBC-WBC interactions during IVM 1 (0.25 ± 0.08 and 0.82 ± 0.44 RBC-WBC interactions per minute for Fab and Fc, respectively), the reduction was only significant for the Fab group (P = .02 compared with PBS or albumin-treated). Neither fragment led to improved survival (Table 1). These results indicate that immunoglobulin fragments can influence RBC-WBC interactions, but neither can recapitulate the effects of the intact IVIG.
IVIG has been used to treat select autoimmune disorders, but its mode of action is still incompletely understood. Several mechanisms have been proposed, including blockade of Fc receptors or activation of inhibitory Fc receptors12,18 and antiinflammatory effects mediated through Fab fragments.19 Thus, both regions of IVIG may be important, explaining the partial benefit observed using individual fragments. Notably, the number of adherent leukocytes was also significantly reduced in sickle cell mice treated with high-dose (at least 200 mg/kg) IVIG but not with IgG fragments or albumin (Table 1). IVIG may thus protect sickle cell mice from vaso-occlusion by both inhibiting the RBC-WBC interactions and also by reducing the number of adherent leukocytes in venules. The latter may contribute to IVIG's antiinflammatory effects in other disorders and thus may reflect an additional mechanism of IVIG action.
Although the vaso-occlusion observed in our model may have similarities to that in patients with sickle cell disease, the possibility that our model, which uses 2 separate proinflammatory stimuli (surgery and TNF-α), may produce vaso-occlusion by mechanisms different from those causing pain crisis in humans cannot be excluded. Nevertheless, our data suggest a possible beneficial effect of IVIG in sickle cell–associated vaso-occlusion.
We identified 4 reported cases where IVIG was administered in sickle cell patients, in each case for a delayed hemolytic transfusion reaction. While IVIG was beneficial 3 cases,20,21 in the fourth case there was a temporal relationship between IVIG administration and the occurrence of a sickle cell crisis.22 Our data raise the possibility that IVIG may be beneficial in preventing or treating sickle cell vaso-occlusion. Caution is necessary, however, because IVIG administration has been associated with both renal toxicity23,24 and thrombosis,25 including an estimated 0.6% risk of stroke,26 and there is reason to believe that patients with sickle cell disease may be predisposed to such toxicities.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-07-2209.
Supported by grants from the National Institutes of Health (HL69438 and HL19278).
A.T. and P.J. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.