Abstract
Serum ferritin has been used widely in clinical medicine chiefly as an indicator of iron stores and inflammation. Circulating ferritin also can have paracrine effects. Despite the clinical significance of serum ferritin, its secretion remains an enigma. The consensus view is that serum ferritin arises from tissue ferritins— principally ferritin light—which can be glycosylated. Ferritin heavy and light chains are cytosolic proteins that form cages of 24 subunits to store intracellular iron. We show that ferritin light is secreted when its expression is increased in stable, transfected HepG2 cells or adenovirus-infected HepG2 cells. Export occurs through the classical secretory pathway and some chains are N-glycosylated. Ferritins do not need to form cages prior to secretion. Secretion is blocked specifically, effectively, and rapidly by a factor in serum. The timing of this inhibition of ferritin secretion suggests that normally cytosolic ferritin L is targeted to the secretory pathway during translation despite the absence of a conventional signal sequence. Thus, secretion of glycosylated and unglycosylated ferritin is a regulated and not a stochastic process.
Introduction
Serum ferritin has wide clinical utility primarily as an indicator of intracellular iron stores1-3 and as an acute-phase reactant useful for the diagnosis and follow-up of adult Still disease4 and systemic lupus erythematosis.5,6 Elevated serum ferritin also has been used as a harbinger of tissue masses including ovarian carcinoma,7 Hodgkin disease,8 renal cell carcinoma,9 and melanoma,10 and preterm deliveries.11,12 Ferritin has been linked to insulin resistance13-15 and, controversially, to atherosclerosis.16 Thus, serum ferritin has been used for the diagnosis and follow-up of numerous disorders, although it is most strongly linked to intracellular iron status and adult Still disease.
Despite the frequent use in clinical medicine of measurements of serum ferritin, most aspects of its secretion remain unknown. An understanding of the mechanism and regulation of the secretion of ferritin would inform the use of this commonly used clinical test. Moreover, the trafficking of normally cytosolic ferritins is relevant to the physiology of iron homeostasis and the pathophysiology of many disorders in which intracellular ferritin could play a role.17-20
There are 2 forms of ferritin that function together to store iron in cells.2 In humans, ferritin light (L) has a molecular mass of 19 kDa and consists of 175 amino acids. Human ferritin L is 53% identical to ferritin heavy (H), which contains 183 amino acids and is 21 kDa.21 Unlike ferritin L, ferritin H contains a ferroxidase site. Cages that store iron atoms are formed from 24 ferritin monomers in varying ratios of H/L.21-23 Both ferritin H and L subunits are necessary for efficient iron storage; although ferritin L lacks a ferroxidase site, the protein nucleates iron mineralization.21,24
Ferritin is secreted from liver25,26 and lymphoid cells.27 Some studies suggest that serum ferritins are distinct from tissue ferritins and encoded by different genes.26,28 Studies of serum ferritin in other animals, however, suggest that the circulating forms are derived from tissue ferritin H and L.29,30 In knock-out mice that are heterozygous ferritin H (-/+), the compensatory rise in tissue levels of ferritin L is accompanied by a similar increase in serum ferritin L.31 Serum ferritin in humans appears to consist of ferritin L, a glycosylated form of L termed ferritin G,32,33 and trace amounts of ferritin H.34 Extracellular glycosylation appears unlikely because of the short half-life of ferritin in humans, and incubation of cytosolic ferritin in serum for 48 hours did not result in any glycosylation.33
The hereditary hyperferritinemia-cataract syndrome provides additional evidence that the product of the ferritin L gene can be secreted in humans. People with this autosomal dominant disorder have markedly elevated levels of serum ferritin L despite normal iron stores.35-37 This disorder arises from mutations in the iron response element (IRE) that prevent stem loop formation or otherwise inhibit iron regulatory protein (IRP) binding in the 5′ untranslated region of ferritin L.37-42 The translational repressors, IRP1 and IRP2, are unable to bind the IRE efficiently, synthesis of ferritin L is increased, and levels of ferritin L are increased in tissues and in serum. Much of the serum ferritin is glycosylated.39 Thus, in these patients, serum ferritin L and G appear to result from dysregulation of tissue ferritin L synthesis.
In this study, we investigated the secretion of ferritin from hepatocytes. We find that HepG2 cells can secrete glycosylated, tissue ferritin L and that secretion is an active process. Secretion of ferritin L can be inhibited specifically by the rapid action of a factor in serum. These findings indicate that secretion of ferritin L is regulated and that the decision to direct newly synthesized ferritin to either the secretory pathway or cytosol occurs cotranslationally.
Materials and methods
Reagents
Alpha modification of Eagle medium (αMEM), Dulbecco modified Eagle medium (DMEM), minimum essential medium (MEM) without methionine/cysteine, penicillin/streptomycin, l-glutamine, and fetal bovine serum were purchased from Mediatech (Herndon, VA). Human serum was prepared from a volunteer. Restriction enzymes, endoglycosidase H (endoH), peptide:N-glycosidase F (PNGase F), RNase B, and sialidase were purchased from New England BioLabs (Beverly, MA). Brefeldin A (BFA) was purchased from Sigma (St Louis, MO). Protein G–agarose beads and LumiGLO chemiluminescent substrate were purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD). Polyclonal and monoclonal antibodies against ferritins and purified human liver ferritin were purchased from Fitzgerald Industries International (Concord, MA). Most other chemicals were purchased from Sigma or Fisher (Hampton, NH).
Cell culture
The HepG2 cell line was obtained from the American Type Culture Collection (Manassas, VA) and maintained as monolayer cultures in αMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine at 37°C with 5% CO2. The HEK293 cell line was obtained from Clontech (Palo Alto, CA) and maintained in DMEM supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine at 37°C with 5% CO2.
Generation of recombinant adenoviruses
The Kozak consensus sequence and the coding regions for ferritin heavy and light chains were excised from the plasmids pcFerritinLight and pcFerritinHeavy,43 using BamHI and XbaI and inserted into the same sites in a shuttle plasmid termed pShuttleBus. This plasmid is a modified version of pShuttle that is part of the Adeno-X system from Clontech. The polylinker region of pShuttle from NheI to KpnI was replaced with a new polylinker resembling that of pcDNA3 encoding sites for NheI-KpnI-BamHI-EcoRV-NotI-XbaI-ApaI. This replacement recreated the original NheI site but not the original KpnI site; a new KpnI site was created.
Similarly, the Kozak sequence and coding region for green fluorescent protein (GFP) was excised from pcDNA344 using BamHI and XbaI and ligated into the same sites in pShuttleBus. The 3 constructs were then ligated into Adeno-X viral DNA (Clontech). Recombinant adenoviruses were obtained by transfecting HEK293 cells and amplified according to the instructions of the manufacturer up to 3 generations prior to use.
Generation of cage mutant adenovirus
The coding region for ferritin light in pcFerritinLight was used as a template to generate the L165R cage mutant by polymerase chain reaction (PCR). Since amino acid residue 165 is encoded near the 3′ end of the ferritin L coding region, a modified downstream primer was used to generate the mutation by PCR. The primers used for the amino-terminal and carboxy-terminal ends were as follows: CGGGATCCGCCACCATGAGCTCCCAGATTCGTC (encoding a BamHI site) and GCTCTAGATTAGTCGTGCTTGAGAGTGAGCCTTTCGAAGCGATACTCGCCC (with a XbaI site and a single altered base encoding L165R shown underlined). The PCR product was digested with BamHI and XbaI and cloned into the same sites in pShuttleBus.
Infection and pulse-chase labeling
HepG2 cells at 70% confluence in 60-mm cell culture dishes were infected with adenoviruses overnight in 4 mL medium. Infected hepatocytes were used for metabolic labeling 72 hours following infection. Cells were washed twice in DMEM without cysteine, methionine, and serum, and starved for methionine in 1 mL of this medium for one hour unless otherwise indicated. Cells were then pulse-labeled with 100 μCi/mL (3.7 MBq) 35S-methionine for 15 minutes (unless otherwise indicated) and chased in 1 mL serum-free αMEM for 1 or 2 hours. In some experiments, BFA was added to the medium at 1 μg/mL during the starvation, labeling, and chase periods.
Immunoprecipitation
After the chase period, the media were collected and cells were lysed with Triton X-100 salt wash buffer (TXSWB) (1% Triton X-100, 100 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 8.0, 100 mM NaCl, 10 mM EDTA [ethylenediaminetetraacetic acid], 1 mM phenylmethylsulfonyl fluoride) for 10 minutes at 4°C. Cell lysates and media were spun in a microcentrifuge at 14 000 g for 10 minutes at 4°C. The clarified supernatants were collected in new microfuge tubes and incubated with antiferritin antibodies at 1:500 dilution at 4°C for one hour. Then, 10 μL protein G–agarose beads was added, and samples were rotated overnight at 4°C. The beads were washed twice with TXSWB and twice with wash buffer (100 mM Tris-HCl, pH 8.0, 100 mM NaCl). In many cases, the washed immunoprecipitates were rotated in 500 μL of 100-μM FeSO4 for 30 minutes at room temperature and washed twice with wash buffer.
Electrophoresis and fluorography
The washed immunoprecipitates were solubilized in sodium dodecyl sulfate (SDS) sample buffer at 37°C for 10 minutes, boiled for 10 minutes, and resolved on 15% gels. Gels were soaked in destain buffer for 30 minutes, washed twice with water, and soaked in 1 M sodium salicylate for 30 minutes. Gels were dried and exposed to films, and in some cases, bands were quantified on a phosphor imager (Molecular Dynamics, Sunnyvale, CA).
Immunoblotting
After immunoprecipitation, samples were resolved by electrophoresis and proteins were transferred overnight onto nitrocellulose membranes (Hybond-C Super; Amersham, Arlington Heights, IL) at 14 volts at 4°C. Proteins were probed using a 1:1000 dilution of primary antibody and revealed with secondary antibodies (from BioRad [Hercules, CA] or Pierce [Rockford, IL]) using a chemiluminescent substrate kit.
Deglycosylation of proteins
After immunoprecipitation of the media of radiolabeled AdX-FL–infected HepG2cells, the washed beads were combined in one tube and the supernatant was removed. Then, 40 μL denaturing buffer (0.5% SDS, 1% β-mercaptoethanol) was added and boiled for 10 minutes with brief vortexing occasionally. The supernatant was recovered and divided into 2 aliquots, and the beads were discarded. One aliquot was left untreated in sample buffer. The second aliquot was treated with either 1 μL endoH in G5 buffer (50 mM sodium citrate, pH 5.5) or 1 μL PNGaseF in G7 buffer (50 mM sodium phosphate, pH 7.5) with 0.1% nonidet P-40 and incubated at 37°C overnight. After deglycosylation, sample buffer was added and the proteins were resolved by SDS–polyacrylamide gel electrophoresis (PAGE) and fluorography.
Results
Stable, transfected HepG2 cells secrete ferritin L
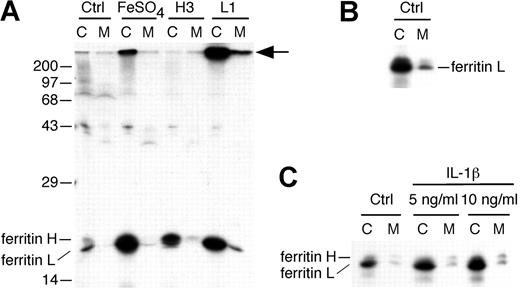
We first assayed the secretion of ferritins from HepG2 cells. Radiolabeled ferritins have been shown to be representative of total ferritins.26 When labeled with radioactive methionine, untreated HepG2 cells synthesize a relatively small amount of ferritins (Figure 1A). A longer exposure of another autoradiograph shows that some ferritins are secreted from untreated HepG2 cells (Figure 1B). We increased the synthesis of ferritins in HepG2 cells by loading the cells with iron to cause release of IRPs from the IREs of ferritin mRNAs and permit increased translation. When HepG2 cells are treated with ferrous sulfate prior to labeling, synthesis of ferritin H and L is increased greatly (FeSO4; Figure 1). This procedure for iron loading is not toxic to cells.45 Ferritin L and a trace amount of ferritin H are found in the media, but the amounts are minimal.
More ferritin L is secreted when its synthesis is elevated. (A) Untransfected HepG2 cells were left untreated (Ctrl) or treated with 10 μM FeSO4/10 μM 8-hydroxyquinoline (FeSO4) during the first 30 minutes of the methionine starvation period. H3 and L1 cells are stable, transfected HepG2 cells expressing high levels of ferritin H or L, respectively. Cells were radiolabeled for 30 minutes and chased in complete medium overnight. The cell lysate (C) and media (M) were immunoprecipitated using polyclonal antibodies to ferritins and resolved by SDS-PAGE and fluorography. Treatment with FeSO4 increased synthesis of both ferritin H and L chains, but little secretion occurs. The stable cell line H3 synthesizes increased ferritin H, but only a trace amount is secreted. The stable cell line L1 synthesizes an increased amount of ferritin L, and some is secreted. A high-molecular-weight protein coimmunoprecipitates with ferritin L (arrow). The position of molecular mass markers is shown on the left. (B) A darker exposure of a separate labeling experiment (as in A) shows that untreated HepG2 cells (Ctrl) secrete some ferritin L. (C) HepG2 cells were left untreated (Ctrl) or treated with IL-1β at either 5 or 10 ng/mL at the outset of methionine starvation (60 minutes prior to labeling) and continuing throughout the labeling (30 minutes) and chase (overnight) periods. Note that this exposure is darker than in panel A, as some ferritin L is visible in the media of Ctrl cells.
More ferritin L is secreted when its synthesis is elevated. (A) Untransfected HepG2 cells were left untreated (Ctrl) or treated with 10 μM FeSO4/10 μM 8-hydroxyquinoline (FeSO4) during the first 30 minutes of the methionine starvation period. H3 and L1 cells are stable, transfected HepG2 cells expressing high levels of ferritin H or L, respectively. Cells were radiolabeled for 30 minutes and chased in complete medium overnight. The cell lysate (C) and media (M) were immunoprecipitated using polyclonal antibodies to ferritins and resolved by SDS-PAGE and fluorography. Treatment with FeSO4 increased synthesis of both ferritin H and L chains, but little secretion occurs. The stable cell line H3 synthesizes increased ferritin H, but only a trace amount is secreted. The stable cell line L1 synthesizes an increased amount of ferritin L, and some is secreted. A high-molecular-weight protein coimmunoprecipitates with ferritin L (arrow). The position of molecular mass markers is shown on the left. (B) A darker exposure of a separate labeling experiment (as in A) shows that untreated HepG2 cells (Ctrl) secrete some ferritin L. (C) HepG2 cells were left untreated (Ctrl) or treated with IL-1β at either 5 or 10 ng/mL at the outset of methionine starvation (60 minutes prior to labeling) and continuing throughout the labeling (30 minutes) and chase (overnight) periods. Note that this exposure is darker than in panel A, as some ferritin L is visible in the media of Ctrl cells.
We found that ferritin L is secreted from stable, transfected hepatocytes. Previously, we created stable HepG2 cell lines that express high levels of ferritin H (H3 cells) or ferritin L (L1 cells) that are not subject to regulation by iron.43 Ferritin L is synthesized in large amounts in L1 cells, and the protein is readily detected by immunoprecipitation using polyclonal antibody against human liver ferritin H and L (Figure 1). Furthermore, 10% to 16% of the labeled ferritin L is secreted into the medium overnight (Figure 1). The secreted ferritin L is of similar mobility by electrophoresis to intracellular ferritin L. Moreover, assays of lactate dehydrogenase (LDH)44 indicated less than 1% lysis of L1 cells overnight (equivalent to untransfected HepG2 cells). Therefore, the ferritin L in the media of L1 cells is the result of secretion. We also found that H3 cells secrete a trace amount of ferritin H. These cells have increased synthesis of ferritin H compared with untransfected HepG2 cells (Figure 1). Some of this ferritin H is secreted into the media; however, the amount of ferritin H secreted is minimal. This result may mimic the trace amounts of ferritin H found in normal human serum and the greater secretion of ferritin L from control HepG2 cells (Figure 1B).
Treatment of rat hepatocytes with the cytokine interleukin-1β (IL-1β) increases the secretion of ferritins approximately 3-fold.26 We found that radiolabeled HepG2 cells treated with IL-1β at the same concentrations used in rat hepatocytes26 likewise exhibit increased ferritin L synthesis and secretion (Figure 1C). (A second band of slightly slower mobility than ferritin H also is visible; this band is discussed in Figure 2.) Thus, ferritin L is secreted from untreated HepG2 cells, and the amount secreted is increased when the synthesis of the protein is elevated. Even when synthesis and secretion are increased by these experimental manipulations, however, ferritin L secreted into the media is detected only after a prolonged chase period. To define cotranslational and posttranslational effects, an experimental system is needed in which a larger amount of ferritin L is secreted rapidly.
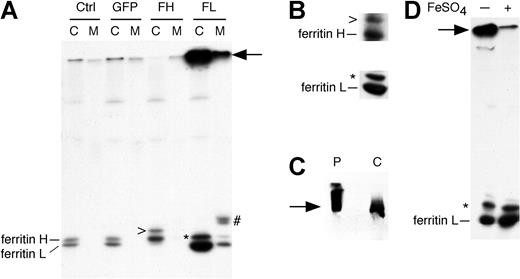
Adenovirus-transduced HepG2 cells synthesize and secrete increased amounts of ferritin L. (A) HepG2 cells were uninfected (Ctrl) or infected with AdX-GFP (GFP), AdX-FH (FH), or AdX-FL (FL). After 72 hours, the cells were labeled with 35S-methionine for 15 minutes and chased in complete medium for one hour. The cell lysate (C) and media (M) were immunoprecipitated using polyclonal antibodies to ferritins. Adenovirus infection per se does not affect synthesis or secretion of ferritins (compare GFP with Ctrl). Increased synthesis and secretion of ferritin L occurs from AdX-FL–infected cells. A high-molecular-weight protein coimmunoprecipitates with ferritin L (arrow). There are 2 proteins of slightly higher molecular weight that coimmunoprecipitate with ferritin H (>) and L (*) in cell lysates, and 2 other proteins coimmunoprecipitate with ferritin L in the medium (#). (B-D) Identification of proteins that coimmunoprecipitate with ferritin H and L in AdX-FH– and AdX-FL–infected HepG2 cells. (B) In the top panel, HepG2 cells infected with AdX-FH were lysed and immunoprecipitated using polyclonal antibodies against ferritins before Western blotting using polyclonal antibodies. In the bottom panel, HepG2 cells infected with AdX-FL were lysed and immunoprecipitated using monoclonal antibody against ferritin L before Western blotting using polyclonal antibodies. The coimmunoprecipitated proteins (denoted by > and *) are detected by immunoblotting. (C) Western blot showing that the high-molecular-mass protein (arrow) in AdX-FL–infected HepG2 cells (labeled C) comigrates with commercially available purified human liver ferritin (P). The cell lysate of HepG2 infected with AdX-FL was immunoprecipitated with polyclonal antibodies against ferritin, electrophoresed alongside 2 μg purified ferritin, and subjected to Western blotting using polyclonal antibody against ferritins. (D) The high-molecular-mass protein migrates as ferritin L monomers after treatment with ferrous sulfate. HepG2 cells infected with AdX-FL were labeled with 35S-methionine, and the cell lysate was immunoprecipitated with polyclonal antibodies against ferritins. The beads containing the washed immunoprecipitates of the cell lysates were separated into 2 aliquots. One aliquot was left untreated (-) while the other aliquot was rotated in 0.5 mL of 100-μM FeSO4 for 30 minutes at room temperature (+). After treatment with ferrous sulfate, the amount of the high-molecular-weight protein (arrow) is decreased and the amount of the ferritin L monomer is increased.
Adenovirus-transduced HepG2 cells synthesize and secrete increased amounts of ferritin L. (A) HepG2 cells were uninfected (Ctrl) or infected with AdX-GFP (GFP), AdX-FH (FH), or AdX-FL (FL). After 72 hours, the cells were labeled with 35S-methionine for 15 minutes and chased in complete medium for one hour. The cell lysate (C) and media (M) were immunoprecipitated using polyclonal antibodies to ferritins. Adenovirus infection per se does not affect synthesis or secretion of ferritins (compare GFP with Ctrl). Increased synthesis and secretion of ferritin L occurs from AdX-FL–infected cells. A high-molecular-weight protein coimmunoprecipitates with ferritin L (arrow). There are 2 proteins of slightly higher molecular weight that coimmunoprecipitate with ferritin H (>) and L (*) in cell lysates, and 2 other proteins coimmunoprecipitate with ferritin L in the medium (#). (B-D) Identification of proteins that coimmunoprecipitate with ferritin H and L in AdX-FH– and AdX-FL–infected HepG2 cells. (B) In the top panel, HepG2 cells infected with AdX-FH were lysed and immunoprecipitated using polyclonal antibodies against ferritins before Western blotting using polyclonal antibodies. In the bottom panel, HepG2 cells infected with AdX-FL were lysed and immunoprecipitated using monoclonal antibody against ferritin L before Western blotting using polyclonal antibodies. The coimmunoprecipitated proteins (denoted by > and *) are detected by immunoblotting. (C) Western blot showing that the high-molecular-mass protein (arrow) in AdX-FL–infected HepG2 cells (labeled C) comigrates with commercially available purified human liver ferritin (P). The cell lysate of HepG2 infected with AdX-FL was immunoprecipitated with polyclonal antibodies against ferritin, electrophoresed alongside 2 μg purified ferritin, and subjected to Western blotting using polyclonal antibody against ferritins. (D) The high-molecular-mass protein migrates as ferritin L monomers after treatment with ferrous sulfate. HepG2 cells infected with AdX-FL were labeled with 35S-methionine, and the cell lysate was immunoprecipitated with polyclonal antibodies against ferritins. The beads containing the washed immunoprecipitates of the cell lysates were separated into 2 aliquots. One aliquot was left untreated (-) while the other aliquot was rotated in 0.5 mL of 100-μM FeSO4 for 30 minutes at room temperature (+). After treatment with ferrous sulfate, the amount of the high-molecular-weight protein (arrow) is decreased and the amount of the ferritin L monomer is increased.
Ferritin L expressed by adenovirus transduction is efficiently secreted
The L1 cell line demonstrates that genetically driven overexpression of ferritin L can increase secretion of the protein. Since integration of genes to generate stable cell lines using a plasmid such as pcDNA3 is inefficient, probably only one copy of the constitutively expressed ferritin L coding region is present in L1 cells. We reasoned that synthesis and secretion of ferritin L would be increased by adenovirus-mediated transfer of multiple copies of the coding region. Using the Adeno-X system, we created adenoviruses bearing the coding region of ferritin L (AdX-FL), ferritin H (AdX-FH), or green fluorescent protein (AdX-GFP).
We found that HepG2 cells infected with adenoviruses encoding ferritin L efficiently secrete the protein. We infected dishes of HepG2 cells that were about 75% confluent with AdX-FL, AdX-FH, or AdX-GFP viruses. Expression of GFP, as indicated by fluorescence microscopy, is detected within 24 to 48 hours and increased by 72 hours. The majority of cells—but not all cells— fluoresce at 72 hours after infection. The cells were then labeled with radioactive methionine for 15 minutes and incubated in chase medium for one hour. Uninfected, control HepG2 cells synthesize relatively little ferritin H and L, and secretion of these proteins is not detected (Figure 2A). Similarly, AdX-GFP–infected HepG2 cells show the same pattern of synthesis and secretion of ferritin, indicating that adenovirus infection does not alter ferritin translation or export (Figure 2A). Overexpression of ferritin H, seen in cells infected with AdX-FH, did not result in appreciable secretion over the one-hour chase period. However, AdX-FL–infected cells synthesize and secrete significantly increased amounts of ferritin L. After one hour of chase, up to 25% of ferritin L can be found in the media as measured by a phosphor imager. Assays of LDH indicate that less than 0.2% lysis occurs in these adenovirus-infected cells (equivalent to the lysis of uninfected HepG2 cells), indicating that the ferritin detected in the media is secreted rather than released from lysis.
A second protein can be coimmunoprecipitated with ferritin L from the AdX-FL–infected HepG2 cells, and some of this protein is secreted (denoted by an asterisk in Figure 2A). This protein appears to migrate above ferritin L and ferritin H. This protein is immunoprecipitated with monoclonal antibody against ferritin L and is specifically recognized by antiferritin antibody by Western blotting (denoted by an asterisk in Figure 2B, bottom panel). Hence, this protein appears to be a modified form of ferritin L. This modification does not appear to be glycosylation since treatment with endoglycosidases (endoH or PNGaseF) or sialidase did not affect its mobility on SDS-PAGE (data not shown). Similarly, another protein coimmunoprecipitates with ferritin H that is slower in mobility (denoted by “>” in Figure 2A). This band is also recognized by Western blotting (denoted by “>” in Figure 2B, top panel), indicating that it is a modified form of ferritin H. The precise nature of these modified ferritin proteins remains unknown.
Ferritin L forms cages that are resistant to dissolution
In the cell lysate and media of L1 and AdX-FL–infected HepG2 cells, a prominent band is noted near the top of the gel (denoted by an arrow in Figures 1 and 2A). To identify this band, we examined its mobility compared with purified liver ferritin in intact cages. The topmost band seen with immunoprecipitated ferritin L migrates at around the same position as the oligomeric cages of commercial, purified human liver ferritin (Figure 2C). Apoferritin (iron-free) shells are known to be stable to heat.2 We reasoned that ferritin L in AdX-FL–infected HepG2 cells forms iron-poor cages because their synthesis is not stimulated by iron. To load these cages with iron, we incubated them with ferrous sulfate, which has been shown to enter apoferritin shells.46 This topmost band is diminished when the washed immunoprecipitates are incubated in 100 μM ferrous sulfate (note the band denoted by the arrow in Figure 2D), and the bands corresponding to ferritin L and modified ferritin L (denoted by asterisk) increase in intensity. Thus, the topmost band seen in immunoprecipitates when ferritin L is overexpressed appears to be in major part the cage form of ferritin L. The addition of iron appears to change their physical properties to cause the cages to separate into monomers when heated in SDS with dithiothreitol.
Ferritin L is secreted via the classical secretory pathway
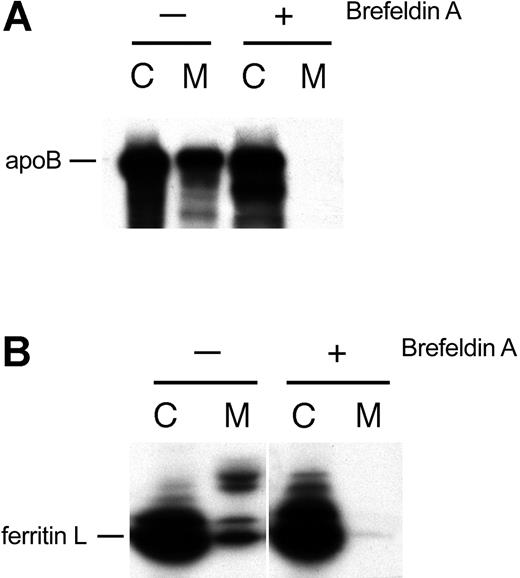
If ferritin L is actively secreted from AdX-FL–infected HepG2 cells and not the result of cell lysis, then the protein would be expected to traverse the endoplasmic reticulum (ER) and Golgi apparatus.26 We investigated whether the export of ferritin L from adenovirus-infected hepatocytes uses this classical secretory pathway. We treated AdX-FL–infected HepG2 cells with 1 μg/mL brefeldin A beginning one hour prior to labeling to cause resorption of the Golgi apparatus. As expected, this treatment blocks the classical secretory pathway as evidenced by the lack of secretion of apolipoprotein B (Figure 3A). The secretion of ferritin L in these cells was also blocked (Figure 3B). Therefore, ferritin L appears to pass through the Golgi apparatus en route to being secreted. This inhibition of secretion is further evidence that ferritin L is actively secreted and not released by lysis of adenovirus-infected cells.
Ferritin L traverses the classical secretory route. (A) BFA inhibits the secretion of apolipoprotein B (apoB), which is secreted via the classical secretory route. AdX-FL–infected HepG2 cells were left untreated (-) or treated with 1 μg/mL BFA (+). The cell lysates (labeled C) and medium (M) were collected, and one aliquot of each was subjected to immunoprecipitation with polyclonal antibodies against apolipoprotein B. (B) BFA inhibits the secretion of ferritin L. Aliquots of the cell lysate (labeled C) and medium (M) from panel A were immunoprecipitated with polyclonal antibodies against ferritin.
Ferritin L traverses the classical secretory route. (A) BFA inhibits the secretion of apolipoprotein B (apoB), which is secreted via the classical secretory route. AdX-FL–infected HepG2 cells were left untreated (-) or treated with 1 μg/mL BFA (+). The cell lysates (labeled C) and medium (M) were collected, and one aliquot of each was subjected to immunoprecipitation with polyclonal antibodies against apolipoprotein B. (B) BFA inhibits the secretion of ferritin L. Aliquots of the cell lysate (labeled C) and medium (M) from panel A were immunoprecipitated with polyclonal antibodies against ferritin.
Some of the secreted ferritin L is N-glycosylated
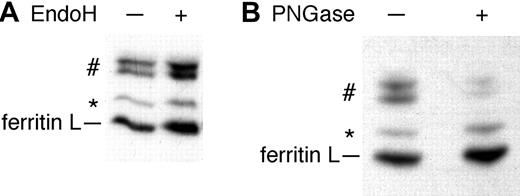
The 2 prominent bands of slower mobility are seen only in the media of AdX-FL–infected HepG2 cells (denoted by “#” in Figure 2). In view of the difference in mobility of these proteins compared with 19 kDa ferritin L, we hypothesized that some of the secreted ferritin L is glycosylated. Ferritin L contains one potential N-glycosylation site. In human serum, glycosylated ferritin G is postulated to be related to ferritin L because of their overlapping immunoreactivity.32,33 We treated ferritin L immunoprecipitated from the media of AdX-FL–infected HepG2 cells with endoH or PNGase F. Although endoH did not affect the distribution of labeled proteins (Figure 4A), it did deglycosylate a control protein, RNase B (data not shown). PNGaseF treatment, however, did cause a decrease in the 2 bands of about 23 kDa (denoted by “#”) and a corresponding increase in ferritin L (Figure 4B). These data are the first demonstration of glycosylated, secreted ferritin arising from high levels of expression of cytosolic ferritins in hepatocytes. Deglycosylation by PNGaseF but not endoH indicates that the oligosaccharides on ferritin L have matured in the Golgi apparatus.
Some ferritin L is N-glycosylated. (A) HepG2 cells infected with AdX-FL were labeled with 35S-methionine. The media was immunoprecipitated with polyclonal antibodies against ferritins and the washed beads were divided into 2 aliquots. One aliquot was treated with endoH (+) while the other aliquot was left untreated (-). EndoH does not affect the mobility of the proteins. (B) Radiolabeled ferritins were immunoprecipitated from the media of AdX-FL–infected HepG2 cells. The washed immunoprecipitates were boiled in 1% SDS and the supernatant was diluted in reaction buffer and divided into 2 aliquots. One aliquot was treated with PNGaseF for one hour while the other aliquot was left untreated. After treatment with PNGaseF, the proteins of higher molecular mass in the medium (#) decreased in intensity, whereas the intensity of bands corresponding to ferritin L and modified ferritin L (*) increased.
Some ferritin L is N-glycosylated. (A) HepG2 cells infected with AdX-FL were labeled with 35S-methionine. The media was immunoprecipitated with polyclonal antibodies against ferritins and the washed beads were divided into 2 aliquots. One aliquot was treated with endoH (+) while the other aliquot was left untreated (-). EndoH does not affect the mobility of the proteins. (B) Radiolabeled ferritins were immunoprecipitated from the media of AdX-FL–infected HepG2 cells. The washed immunoprecipitates were boiled in 1% SDS and the supernatant was diluted in reaction buffer and divided into 2 aliquots. One aliquot was treated with PNGaseF for one hour while the other aliquot was left untreated. After treatment with PNGaseF, the proteins of higher molecular mass in the medium (#) decreased in intensity, whereas the intensity of bands corresponding to ferritin L and modified ferritin L (*) increased.
Ferritin L can be secreted without forming a cage
In the cytosol, ferritins assemble into oligomeric structures that store iron atoms. This assembly can occur rapidly in vitro, and happens when ferritins are overexpressed in bacteria or mammalian cells. Hence, wild-type ferritins have a propensity to form cages. At the ER, improperly folded proteins (eg, alpha1 antitrypsin mutants) or polypeptides that fail to assemble with other protein subunits (eg, T-cell receptor) or lipids (eg, apolipoprotein B) are degraded rather than secreted. To be secreted, ferritin L probably undergoes the same molecular scrutiny at the ER. We examined whether ferritin L must assemble into a cage to be secreted.
Ferritin proteins with specific alterations form cages poorly. A specific mutation at the 4-fold axis of symmetry (L169R) in ferritin H drastically impairs formation of 24-subunit cages.47 The L169R mutant ferritin H can form dimers, and it can be induced to form 24-subunit cages when mixed with an excess of wild-type ferritin H. The ability of the mutant ferritins to form dimers on their own and cages with wild-type ferritin H indicates that the tertiary structure of the mutant ferritin monomers is preserved. On their own, however, L169R dimers require a half-time of 2 days to form 24-subunit cages compared with a few minutes for wild-type ferritin H. These studies were performed using mutated ferritin H, but they should extend to ferritin L also. Ferritin L is virtually identical in sequence throughout this region of the 4-fold axis, and the 4 amino acids (EYLF) around the 4-fold axis are identical between ferritin H and L. Ferritin H has 4 extra amino acid residues at its amino-terminus compared with ferritin L. Therefore, a L165R mutant of ferritin L (accounting for the 4 fewer amino-terminal residues in ferritin L) would be expected to impair the formation of ferritin L cages.
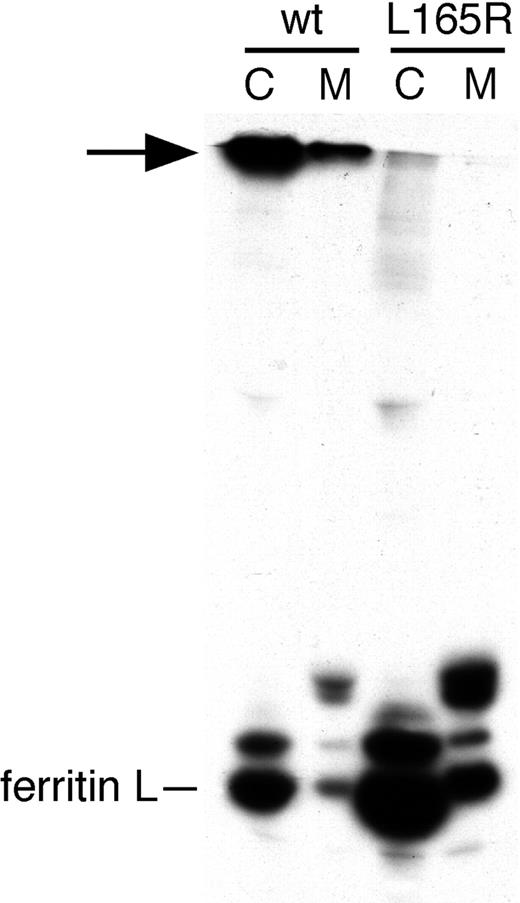
We studied the effect of the L165R mutation in ferritin L on cage formation and secretion in hepatocytes. We engineered an adenovirus encoding ferritin L with this mutation termed AdX-FL(L165R). HepG2 cells were infected with AdX-FL(L165R) and metabolically labeled with radioactive methionine. As expected, the mutant ferritin L did not form cages in HepG2 cells under conditions in which we observed oligomer formation by wild-type ferritin L (bands denoted by the arrow in Figure 5) or under nonreducing conditions (data not shown). This cage-mutant ferritin L, however, was secreted (Figure 5) and in a proportion comparable with wild-type ferritin L (by phosphor imager, data not shown). Therefore, the assembly into cages is not necessary for ferritin L to exit the ER and become secreted. Furthermore, the secretion of ferritin L (L165R) provides further proof that it is the adenovirus-transduced gene product that is being secreted.
A 4-fold axis cage mutant of ferritin L is secreted. HepG2 cells were infected with AdX-FL (wt) or the AdX-FL(L165R) cage mutant (L165R), and 72 hours after infection were labeled with 35S-methionine and chased for 2 hours. Cell lysates (C) and media (M) were immunoprecipitated with polyclonal antibodies against ferritin L. In contrast to wild-type ferritin L, mutant ferritin L chains did not form high-molecular-weight oligomeric cages (arrow), but both forms are secreted.
A 4-fold axis cage mutant of ferritin L is secreted. HepG2 cells were infected with AdX-FL (wt) or the AdX-FL(L165R) cage mutant (L165R), and 72 hours after infection were labeled with 35S-methionine and chased for 2 hours. Cell lysates (C) and media (M) were immunoprecipitated with polyclonal antibodies against ferritin L. In contrast to wild-type ferritin L, mutant ferritin L chains did not form high-molecular-weight oligomeric cages (arrow), but both forms are secreted.
A factor in serum inhibits secretion of ferritin
The release of ferritin from hepatocytes is increased by cytokines and iron.26 Iron and cytokines, such as tumor necrosis factor alpha and IL-1β, also increase the synthesis of ferritins.20 Therefore, these stimuli might simply boost synthesis of the protein rather than affect secretion of ferritin independently. All of these factors up-regulate the secretion of ferritin; no factors are known to decrease secretion directly.
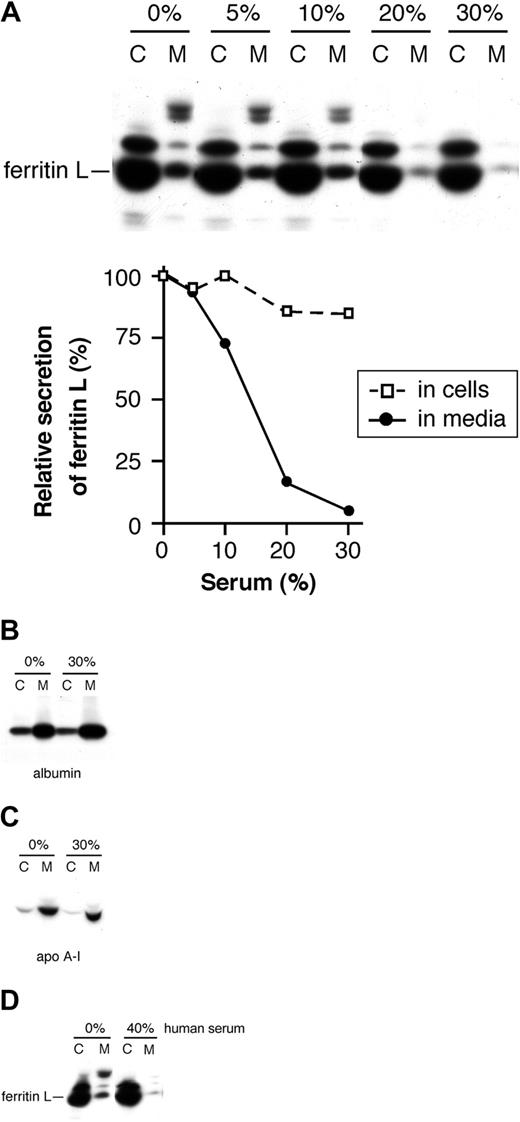
In the course of optimizing our assays, we discovered that serum contains a factor that inhibits the secretion of ferritin L. Fetal bovine serum was added in concentrations ranging from 0% to 30% to the media of AdX-FL–infected HepG2 cells during the periods of methionine starvation, radioactive labeling, and chase (Figure 6A). Increasing amounts of serum result in marked inhibition of ferritin L secretion despite only a slight effect on its synthesis; with 30% serum, secretion of ferritin L was reduced more than 94%, whereas synthesis decreased 15% (Figure 6A). This reduction of ferritin L in the media is not due to competition of ferritin in serum during immunoprecipitation (below). We also investigated whether serum blocks the secretion of all proteins from hepatocytes. The secretion of albumin (Figure 6B) and apolipoprotein A-I (Figure 6C) is not affected by the highest concentration of fetal bovine serum used (30%). Therefore, the serum-induced inhibition of ferritin secretion is specific for ferritin L rather than a global effect on the secretory pathway. Furthermore, human serum likewise inhibits the secretion of ferritin L (Figure 6D). The nearly complete inhibition of ferritin secretion by serum furnishes additional proof that the protein is actively secreted rather than released by cell lysis.
A factor in serum specifically inhibits the secretion of ferritin L. (A) Fetal bovine serum inhibits secretion of ferritin L. Fetal bovine serum was added to the media of AdX-FL–infected HepG2 cells in increasing concentrations (0%, 5%, 10%, 20%, and 30%) from the outset of the starvation period, during radiolabeling, and throughout the chase period. The bands were quantified as described in “Materials and methods.” The relative synthesis and secretion of ferritin L compared with the sample without serum are plotted in the graph. Increasing concentrations of serum are associated with a slight decrease in synthesis and marked drop in secretion of ferritin L. (B-C) Serum does not affect the secretion of albumin or apolipoprotein A-I. AdX-FL–infected HepG2 cells were incubated with media containing either 0% or 30% fetal bovine serum as in panel A. The cell lysate (labeled C) and media (M) were divided into 2 aliquots and immunoprecipitated with antibodies against albumin (B) or apolipoprotein A-1 (C). No difference in the secretion of albumin or apolipoprotein A-1 occurs with 30% serum. (D) Human serum inhibits ferritin L secretion. AdX-FL–infected HepG2 cells were incubated in media containing 0% or 40% human serum as in panel A. The cell lysates (labeled C) and media (M) were immunoprecipitated using polyclonal antibodies against ferritins. The secretion of ferritin L is markedly reduced with human serum.
A factor in serum specifically inhibits the secretion of ferritin L. (A) Fetal bovine serum inhibits secretion of ferritin L. Fetal bovine serum was added to the media of AdX-FL–infected HepG2 cells in increasing concentrations (0%, 5%, 10%, 20%, and 30%) from the outset of the starvation period, during radiolabeling, and throughout the chase period. The bands were quantified as described in “Materials and methods.” The relative synthesis and secretion of ferritin L compared with the sample without serum are plotted in the graph. Increasing concentrations of serum are associated with a slight decrease in synthesis and marked drop in secretion of ferritin L. (B-C) Serum does not affect the secretion of albumin or apolipoprotein A-I. AdX-FL–infected HepG2 cells were incubated with media containing either 0% or 30% fetal bovine serum as in panel A. The cell lysate (labeled C) and media (M) were divided into 2 aliquots and immunoprecipitated with antibodies against albumin (B) or apolipoprotein A-1 (C). No difference in the secretion of albumin or apolipoprotein A-1 occurs with 30% serum. (D) Human serum inhibits ferritin L secretion. AdX-FL–infected HepG2 cells were incubated in media containing 0% or 40% human serum as in panel A. The cell lysates (labeled C) and media (M) were immunoprecipitated using polyclonal antibodies against ferritins. The secretion of ferritin L is markedly reduced with human serum.
Serum inhibits the secretion of ferritin L rapidly and cotranslationally
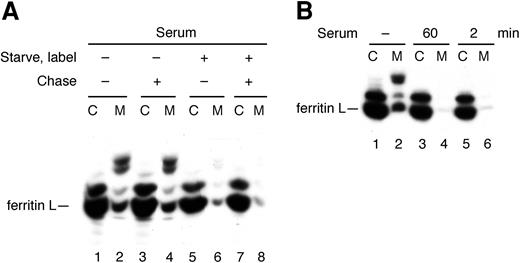
In Figure 6, serum was added throughout the entire experiment so the inhibitory effect on secretion of ferritin L could occur before, during, or after its synthesis. We next delineated whether the inhibitory effect of serum on ferritin secretion occurs co- or posttranslationally. Serum was added to the media of AdX-FL–infected hepatocytes during the periods of methionine starvation and labeling or just the chase period (Figure 7A). As expected, infected hepatocytes secreted ferritin L in the absence of serum throughout the experiment (Figure 7A, lanes 1-2), and this secretion was strongly inhibited by the presence of serum in the medium throughout the entire experiment (Figure 7A, lanes 7-8). When serum is added just during the chase period, secretion of ferritin L appears unaffected (Figure 7A, lanes 3-4). When serum is present during the time of methionine starvation and labeling but not during the chase period, however, secretion of ferritin L is blocked to about the same extent as when serum is present throughout the entire experiment (Figure 7A, lanes 5-6). From these data, we draw 2 important conclusions. First, ferritins and antibodies in bovine serum do not interfere with immunoprecipitation of labeled ferritin in the chase media. Equivalent amounts of ferritin L are recovered from the chase media in the absence (Figure 7A, lane 2) or presence (Figure 7A, lane 4) of serum in the immunoprecipitated sample. Instead, serum decreases the amount of ferritin secreted into the chase media (compare lanes 6 and 4 in Figure 7A). Second, these data indicate that a factor in serum modulates secretion of ferritin from hepatocytes by acting before or during translation (labeling) of ferritin but not posttranslationally (during the chase period).
Inhibition of ferritin L secretion by serum is rapid and cotranslational. (A) Serum acts during the starvation or labeling periods but not the chase period. AdX-FL–infected HepG2 cells were treated in 4 different conditions as follows: starved, labeled, and chased in medium lacking serum (lanes 1-2); starved and labeled in medium lacking serum but chased in medium with 30% fetal bovine serum (lanes 3-4); starved and labeled in the presence of 30% fetal bovine serum but chased in medium lacking serum (lanes 5-6); or starved, labeled, and chased in medium with 30% fetal bovine serum (lanes 7-8). The presence of serum during labeling—and not during the chase period—results in inhibition of ferritin L secretion. This result also indicates that serum in the chase medium does not affect the immunoprecipitation of ferritin L. (B) Serum inhibit the secretion of ferritin L rapidly and cotranslationally. Some cells were incubated in media lacking serum throughout the experiment (lanes 1-2). Fetal bovine serum was added at a 30% concentration to AdX-FL–infected HepG2 cells either 60 minutes prior to labeling with 35S-methionine (at the outset of the methionine starvation period) (lanes 3-4) or 2 minutes prior to labeling (lanes 5-6) and continued through the 5-minute labeling period in both cases. At the onset of the chase period, all cells were washed in phosphate-buffered saline and chased in serum-free complete medium for 2 hours. The cell lysates (labeled C) and media (M) were immunoprecipitated with polyclonal antibodies against ferritins.
Inhibition of ferritin L secretion by serum is rapid and cotranslational. (A) Serum acts during the starvation or labeling periods but not the chase period. AdX-FL–infected HepG2 cells were treated in 4 different conditions as follows: starved, labeled, and chased in medium lacking serum (lanes 1-2); starved and labeled in medium lacking serum but chased in medium with 30% fetal bovine serum (lanes 3-4); starved and labeled in the presence of 30% fetal bovine serum but chased in medium lacking serum (lanes 5-6); or starved, labeled, and chased in medium with 30% fetal bovine serum (lanes 7-8). The presence of serum during labeling—and not during the chase period—results in inhibition of ferritin L secretion. This result also indicates that serum in the chase medium does not affect the immunoprecipitation of ferritin L. (B) Serum inhibit the secretion of ferritin L rapidly and cotranslationally. Some cells were incubated in media lacking serum throughout the experiment (lanes 1-2). Fetal bovine serum was added at a 30% concentration to AdX-FL–infected HepG2 cells either 60 minutes prior to labeling with 35S-methionine (at the outset of the methionine starvation period) (lanes 3-4) or 2 minutes prior to labeling (lanes 5-6) and continued through the 5-minute labeling period in both cases. At the onset of the chase period, all cells were washed in phosphate-buffered saline and chased in serum-free complete medium for 2 hours. The cell lysates (labeled C) and media (M) were immunoprecipitated with polyclonal antibodies against ferritins.
To delineate the onset of this inhibitory effect, we added serum to the media of AdX-FL–infected HepG2 cells at different times prior to labeling. As expected, ferritin L is secreted in the absence of serum (Figure 7B, lanes 1-2), and when serum is present beginning 60 minutes prior to labeling until the onset of the chase period, the secretion of ferritin L is strongly inhibited (Figure 7B, lanes 3-4). Surprisingly, when serum is added to the media just 2 minutes prior to labeling and removed at the beginning of the chase period (hence, serum is present for only 7 minutes total), secretion of ferritin L also is markedly inhibited (Figure 7B, lanes 5-6). Therefore, the serum-induced block of ferritin L secretion is rapid in onset and cotranslational.
Discussion
Our study provides several novel observations about the secretion of ferritin L from human hepatocytes. First, we demonstrate that the same ferritin L gene product that resides in the cytosol can be secreted actively from HepG2 cells. This result supports the idea that serum and cytosolic ferritins are the products of the same35-42 rather than different26,28 transcripts. Second, enhanced expression of individual ferritin chains via adenovirus transduction results in marked secretion of ferritin L but not ferritin H over a relatively short period of time and permits investigation of co- and posttranslational effects. Third, we show for the first time that some of the ferritin L secreted from hepatocytes is N-glycosylated. Fourth, our experimental system permits analysis of the secretion of ferritin L mutants. Using this system, we find that assembly of ferritin L into oligomers is not necessary for secretion. Fifth, we demonstrate that secretion of ferritin L can be regulated rapidly and specifically. Enhanced ferritin secretion over 24 hours in response to cytokines and iron has been shown.26 In contrast, our study is the first demonstration that the secretion of ferritin can be regulated quickly—within minutes—and specifically. Sixth, we also provide the first evidence for a factor in serum that inhibits the secretion of ferritin L. Seventh, our data indicate that targeting of ferritin L to the secretory pathway occurs cotranslationally. These observations provide new insights into the genesis and regulation of serum ferritin.
Ferritin L is secreted best when its levels appear to exceed that needed for iron storage. When ferritin levels are stimulated by treatment with iron, some ferritin L is secreted; however, the amount secreted is less than that secreted by L1 cells in which ferritin L expression is increased independent of iron status. The marked secretion of ferritin L, when expressed at high levels by a noniron stimulus (ie, adenovirus transduction), mimics the release of iron-poor ferritin as an acute phase reactant in inflammatory conditions. Alternatively, ferritin L is secreted better from hepatocytes when its expression is increased out of proportion to ferritin H synthesis. Ferritin H, which is normally synthesized in smaller amounts than ferritin L in hepatocytes, is secreted in small amounts when expressed at high levels. If even greater expression of ferritin H is achieved, then perhaps increased secretion would also be seen. In humans, serum ferritin consists largely of forms derived from ferritin L and only small amounts of ferritin H. This balance may reflect differences in expression or the ease with which the 2 ferritins enter the secretory pathway.
In agreement with a previous study,26 we find that ferritin L is actively secreted through the classical secretory pathway rather than being released via cell lysis. The evidence for active secretion rather than cell lysis is that concomitant release of cytosolic LDH is very low compared with the amount of ferritin L, some of the secreted ferritin L is N-glycosylated, and export of ferritin L is blocked by BFA or a factor in serum. The BFA-induced block of ferritin L secretion indicates that this protein passes through the Golgi apparatus just as other conventional secretory proteins. Modification of the high-mannose oligosaccharide core so that its cleavage is resistant to endoH occurs in the Golgi apparatus. Hence, the endoH resistance of N-glycosylated ferritin L is additional evidence that the protein traverses the Golgi apparatus. Since proteins typically enter the secretory pathway through a proteinaceous channel in the ER membrane and polysomes for ferritins have been isolated on this compartment,48-50 it is likely that ferritin L enters the secretory pathway via the ER. Furthermore, the initial step of asparagine-linked glycosylation occurs in the ER lumen, so N-glycosylation of some ferritin L chains indicates their entry into this compartment. Taken together, these data suggest that ferritin L traverses the entire classical secretory pathway.
Ferritin G, so named because it is glycosylated, was demonstrated in human serum more than 20 years ago.32,33 This glycosylated ferritin is thought to be derived from ferritin L because of their shared immunoreactivity.32,33 We provide the first demonstration that overexpression of ferritin L in cultured hepatocytes leads to secretion of N-glycosylated forms from these cells that are of similar mobility to ferritin G. Only some of the ferritin L is glycosylated, and the factors that determine the balance between glycosylated and nonglycosylated forms remain to be explored. In humans, the ratio between ferritins G and L also varies in different conditions.51-54
We also demonstrate that the secretion of ferritin L can be specifically, effectively, and rapidly regulated by a factor in serum. This regulation raises the possibility that other stimuli, such as IL-1β or iron,26 could act on the secretion of ferritin directly rather than just by increasing its synthesis. At present, we do not know the identity of the inhibitory factor in serum, although it does not appear to be transferrin or ferritin itself (S.G., S.H., and S.L.C., unpublished observations, 2003). The rapid onset of inhibition of secretion by this serum factor suggests that it either enters hepatocytes quickly or binds to a cellular receptor and transduces a signal within just a few minutes to divert ferritin L from the secretory pathway. Understanding the mechanism by which the secretion of ferritin L is inhibited may elucidate the unconventional process by which the protein is secreted. The effect of serum on the secretion of ferritin L indicates that its release is a governed rather than a stochastic process. The physiologic reasons for this regulation remain to be explored.
In mammalian cells, proteins with signal sequences are targeted cotranslationally to the classical ER-Golgi pathway for secretion.55 The effect of serum indicates that newly synthesized ferritin L is directed either to the secretory pathway or to the cytosol cotranslationally. The rapidity of this process suggests that amino acid determinants in nascent ferritin L are specifically recognized by a cellular constituent to target the normally cytosolic iron storage protein to the secretory pathway like the binding of signal sequences by signal recognition particle. Ferritin L, however, lacks a typical amino-terminal, hydrophobic signal sequence, and does not appear to enter the ER in vitro48 ; how the protein enters the secretory pathway is not known. Future studies aimed at defining the sequence in ferritin L and its cognate cellular constituent that together direct export of this normally cytosolic protein could establish a new paradigm for protein secretion.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-09-3050.
Supported by HL62545 from National Heart, Lung, and Blood Institute, National Institutes of Health (S.L.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
S.G. and S.H. contributed equally to this work.
Sharmistha Ghosh and Sarah Hevi contributed equally to this work.