Abstract
Invasive aspergillosis causes significant mortality among patients with hematologic malignancies. This infection is characterized by vascular invasion and thrombosis. To study the pathogenesis of invasive aspergillosis, we investigated the interactions of Aspergillus fumigatus conidia and hyphae with endothelial cells in vitro. We found that both forms of the organism induced endothelial cell microfilament rearrangement and subsequent endocytosis. Conidia were endocytosed 2-fold more avidly than hyphae, and endocytosis was independent of fungal viability. Endocytosed conidia and hyphae caused progressive endothelial cell injury after 4 hours of infection. Live conidia induced more endothelial cell injury than did live hyphae. However, endothelial cell injury caused by conidia was dependent on fungal viability, whereas injury caused by hyphae was not, indicating that conidia and hyphae injure endothelial cells by different mechanisms. Neither live nor killed conidia increased tissue factor activity of endothelial cells. In contrast, both live and killed hyphae stimulated significant endothelial cell tissue factor activity, as well as the expression of tissue factor antigen on the endothelial cell surface. These results suggest that angioinvasion and thrombosis caused by A fumigatus hyphae in vivo may be due in part to endothelial cell invasion, induction of injury, and stimulation of tissue factor activity.
Introduction
Invasive aspergillosis has become one of the leading causes of death among bone marrow transplantation and leukemia patients. Even with current therapy, the mortality rate of invasive aspergillosis is 80% to 90% in immunocompromised patients.1 Aspergillus fumigatus is responsible for 90% of invasive Aspergillus infections.1 This ubiquitous mould releases numerous conidia into the atmosphere, which are small enough (2 to 3 μm in diameter) to reach the pulmonary alveoli after they are inhaled.2 Once the conidia reach the alveoli, they swell and germinate, producing hyphae that invade the pulmonary parenchyma. These hyphae have a marked tropism for blood vessels. A key finding in invasive aspergillosis is angioinvasion, which subsequently leads to thrombosis and tissue infarction.3-5 In immunocompromised hosts, invasion of the pulmonary vasculature can result in widespread hematogenous dissemination to organs such the brain, kidneys, heart, and eyes.1,3
During angioinvasion, A fumigatus hyphae interact with vascular endothelium. We hypothesize that this interaction plays an important role in the pathogenesis of invasive aspergillosis. Once the hyphae have entered the bloodstream, they must adhere to and penetrate the endothelial cell lining of the blood vessels to invade the deep tissues of the target organs.
In addition, the prominent thrombosis at sites of A fumigatus angioinvasion suggests that the organism stimulates endothelial cells to become prothrombotic. A major mechanism by which endothelial cells can promote intravascular thrombus formation is by expressing tissue factor, also known as thromboplastin or CD142.6-9 This 45-kDa transmembrane cell surface glycoprotein is expressed by endothelial cells, platelets, and leukocytes. It is also present on endothelial microparticles.10,11 Tissue factor binds to factor VII, and forms a tissue factor–factor VIIa complex, which initiates the extrinsic coagulation cascade.7,9
To study the pathogenesis of angioinvasion and thrombosis during invasive aspergillosis, we investigated the mechanisms by which A fumigatus hyphae invade and injure vascular endothelial cells in vitro. We also examined whether these hyphae were able to stimulate endothelial cells to express tissue factor.
Material and methods
Reagents and antibodies
Lipopolysaccharide (LPS) (Escherichia coli 0111.B4) was from List Biological Laboratories (Campbell, CA). Human factor VII and human factor X were from Enzyme Research Laboratories (South Bend, IN). Pefachrome FXa and Russel viper venom were from Centerchem (Norwalk, CT). Alexa Fluor 488 phalloidin, Alexa Fluor 568 goat anti–rabbit immunoglobulin G (IgG), and Slow Fade mounting medium were from Molecular Probes (Eugene, OR). Mouse anti–tissue factor monoclonal antibody, IgG1 (clone GMA-311) was from Green Mountain Antibodies (Burlington, VT). Fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse IgG, cytochalasin D, thimerosal (2-[ethylmercuriomercapto] benzoic acid sodium salt), and bovine serum albumin (BSA) were from Sigma-Aldrich (St Louis, MO). Fibronectin was from BD Biosciences (Bedford, MA). Medium 199 (M-199), gelatin, l-glutamine, penicillin, and streptomycin were from Gibco (Grand Island, NY). Fetal bovine serum and bovine calf serum were from Gemini Bio-Products (Woodland, CA). Hanks balanced salt solution was from Irvine Scientific (Santa Clara, CA). Sabouraud dextrose agar, Sabouraud dextrose broth, and yeast nitrogen base broth were from Difco Laboratories (Detroit, MI).
Microorganisms
Aspergillus fumigatus strains H237 and AF293 were kind gifts of Dr Judith Rhodes (University of Cincinnati) and Dr Paul T. Magee (University of Minnesota), respectively. Strains AF2, AF7, and AF11 were generously supplied by Dr Nancy Madinger (University of Colorado Health Sciences Center). Candida albicans SC5314 was obtained from Dr William Fonzi (Georgetown University). All fungal strains used in these experiments were clinical isolates.
Growth and preparation of the organisms
The A fumigatus strains were grown on Sabouraud agar for 7 days at 37°C. Their conidia were harvested by gently rinsing the plates with 0.1% Tween 80 in phosphate-buffered saline (PBS), pH 7.4 and then washed twice in PBS without Tween 80. Germ tubes were obtained by incubating the conidia in Sabouraud broth on gelatin-coated Petri dishes for 5 to 6 hours at 37°C. Next, the medium above the germinated conidia was aspirated and the Petri dishes were washed twice with PBS. When necessary, the germ tubes were stored overnight at 4°C. The germ tubes were harvested from the dishes with a cell scraper. By microscopic examination, the majority of the organisms were single germ tubes, with a few small clumps containing 2 to 4 organisms. To obtain an even more homogeneous germ tube preparation, the suspension was gently sonicated for 10 seconds using a Branson Sonifier 450 (output level 2; Branson Ultrsonics, Danbury, CT). The C albicans cells were grown overnight in yeast nitrogen base broth supplemented with 2% glucose (wt/vol) at 20°C on a rotating drum. All organisms were counted in a hemacytometer and adjusted to the desired concentration in tissue culture medium (M-199 medium containing 2 mM l-glutamine, penicillin, and streptomycin, and supplemented with 10% fetal bovine serum and 10% bovine calf serum) prior to use. Conidia or germ tubes were killed by incubating them in 0.02% thimerosal in PBS overnight at 4°C.12 This method of killing was used to preserve cellular integrity and minimize loss of cell wall components. Killing was verified by plating the organisms on Sabouraud agar. The killed organisms were rinsed extensively in PBS before use.
Endothelial cells
Endothelial cells were harvested from human umbilical veins by the method of Jaffe et al.13 The cells were grown in tissue culture medium. For use in the various assays, second- or third-passage endothelial cells were grown to confluency on a fibronectin matrix in 96-well or 24-well tissue culture plates (BD Biosciences) or on 12-mm diameter circular glass coverslips. All incubations were at 37°C in 5% CO2.
Endocytosis assay
The number of A fumigatus conidia and germ tubes internalized by the endothelial cells was determined by a modification of our previously described differential fluorescence assay.14-17 Briefly, endothelial cells grown on glass coverslips were infected with 6 × 104 conidia or germ tubes of A fumigatus H237 in tissue culture medium for 45 minutes. In some experiments, the microfilament inhibitor cytochalasin D was added to the wells just before the organisms to achieve a final concentration of 70 nM. In these experiments, the control wells received an equal volume of the diluent (dimethyl sulfoxide [DMSO]). At the end of the incubation period, the cells were fixed with 3% paraformaldehyde and then blocked overnight with 1% goat serum in PBS. The noninternalized microorganisms were stained by first incubating the infected monolayers with a 1:100 dilution of an anti–A fumigatus polyclonal rabbit serum (kindly supplied by Dr Jean-Philippe Bouchara, Université D'Angers, France), followed by incubation with an Alexa Fluor 568 (which fluoresces red) goat anti–rabbit IgG (1 μg/mL) in PBS with 1% BSA. Next, the endothelial cells were permeabilized in 0.1% (vol/vol) Triton X-100 in PBS, after which both internalized and noninternalized organisms were stained with 1% (vol/vol) Uvitex (which fluoresces blue).18 The coverslips were mounted inverted on a microscope slide with Slow Fade, sealed with nail polish, and viewed under epifluorescence using filters specific for Alexa Fluor 568 and Uvitex. In each high-powered microscopic field, the number of organisms endocytosed by endothelial cells was determined by subtracting the number of noninternalized organisms (which fluoresced red) from the total number of organisms (which fluoresced blue). At least 100 organisms were counted on each coverslip, and all experiments were performed in duplicate on at least 3 separate occasions. The results were expressed as both the number of endocytosed organisms per high-power field (HPF).
Fluorescent microscopy
For all fluorescence microscopy experiments, the endothelial cells were grown to confluency on glass coverslips and incubated with 3 × 105A fumigatus cells as described for the endocytosis assay. To visualize the endothelial cell microfilaments, the endothelial cells were fixed with 3.7% formaldehyde, permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 5 minutes, and then blocked with 1% BSA in PBS for 30 minutes. The microfilaments were stained with Alexa Fluor 488 phalloidin following the manufacturer's instructions.
To detect tissue factor expression, the endothelial cell monolayers were fixed in 3.7% formaldehyde and blocked with 1% goat serum as above. Next, the cells were incubated with 10 μg/mL of a mouse anti–tissue factor monoclonal antibody for one hour, followed by an FITC-conjugated goat anti–mouse IgG at a 1:100 dilution. In all experiments, the coverslips were mounted inverted onto slides and viewed by confocal microscopy with a Leica TCS SP2 microscope (Leica Microsystems, Bannockburn, IL). The confocal images were constructed by stacking 0.5 micron optical sections acquired along the z-axis.
Endothelial cell injury
The amount of endothelial cell injury induced by conidia and hyphae was quantified by the release of 51Cr as previously described.15,19,20 Briefly, endothelial cells were grown to confluence in 96-well plates containing detachable wells. The cells were incubated overnight with 1 μCi (0.037 MBq) Na251CrO4 (ICN Biomedicals, Irvine, CA) per well. The following day, the unincorporated tracer was aspirated and the wells were rinsed twice with prewarmed Hanks balanced salt solution. Next, endothelial cells were incubated with 105 organisms per well in 100 μL tissue culture medium. After incubation, the upper 50% of the medium was aspirated from each well and then the wells were manually detached from one another. The amount of 51Cr in the aspirates and in the wells was determined by gamma counting. To measure the spontaneous release of 51Cr, uninfected endothelial cells exposed to medium alone were processed in parallel. In experiments with killed organisms, the equivalent volume of the supernatant from the last thimerosal wash was added to the endothelial cells to control for the release of any toxic material as a result of the thimerosal treatment.
The results were adjusted for the volume of medium removed from the wells and the percent specific release of 51Cr was calculated using the following formula: (experimental release–spontaneous release)/(total incorporation–spontaneous release) × 100. Each experiment was performed in triplicate at least 3 different times. After 8 hours of incubation in medium alone, the average spontaneous release of 51Cr was 24% ± 4%. To determine the maximal release of 51Cr by the endothelial cells, the cells were lysed by the addition of 0.5% Triton X-100 to the medium. The average specific release of 51Cr in these cells was 85% ± 5%. This specific release of 51Cr was essentially the same when Triton X-100 was added to either endothelial cells incubated with medium alone or infected with A fumigatus for 8 hours.
In preliminary experiments, we incubated A fumigatus hyphae with 51Cr under the same labeling conditions that were used for the endothelial cells. These hyphae incorporated minimal 51Cr (data not shown). Therefore, A fumigatus cells were unlikely to take up a significant amount of 51Cr released by the injured endothelial cells.
For the experiments to determine if soluble factors released by A fumigatus could injure the endothelial cells, a modification of the above injury assay was used. The endothelial cells were seeded on 24-well tissue culture plates and cell culture inserts (pore size, 0.4 μm; BD Biosciences) were placed in each well, suspended approximately 1 mm above endothelial cells. Next, 6 × 105 conidia was added to the endothelial cells, the cell culture inserts, or both. At the end of the incubation, the medium was gently removed and the endothelial cells were lysed by the addition of 0.5 mL of 6 N NaOH. The lysed cells were aspirated and the wells were rinsed with 0.5 mL Radiac Wash (Atomic Products, Shirley, NY). These rinses were added to the cell lysate. The 51Cr activity was determined in the medium and in the cell lysates and the specific release of 51Cr calculated as described above.
Tissue factor assay
The expression of tissue factor by the endothelial cells was measured by a colorimetric assay.21 All reagents and solutions used in these experiments were endotoxin-free. Briefly, confluent endothelial cells in 96-well plates were washed once with warm PBS and incubated with 105A fumigatus or C albicans cells for 2, 4, 8, and 16 hours. Control wells with A fumigatus alone were run in parallel. As a positive control, endothelial cells were stimulated with 1 ng LPS per milliliter. At each time point, the medium above the cells was gently aspirated. Next, the endothelial cells were incubated with 50 μL of 10 nM factor VII in buffer A (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 137 mM NaCl, 4 mM KCl, 11 mM glucose, 25 mM CaCl2, and 5 mg/mL BSA, pH 7.4) for 20 minutes at 37°C under rotation at 200 g to allow formation of the tissue factor–factor VII–Ca++ complex. Next, 10 μL containing 6 U of factor X per milliliter of buffer A was added to each well. After a 5-minute incubation at 37°C, a 25-μL sample was removed from each well and added to 25 μL of 125 mM triethanolamine, 250 mM NaCl, 50 mM EDTA (ethylenediaminetetraacetic acid), 25 μM BSA, pH 8.2, in a 96-well enzyme-linked immunosorbent assay (ELISA) plate, at 4°C to stop the formation of factor Xa. The plate was warmed to 37°C and 10 μL of a 5-mM solution of the substrate Pefachrome FXa in 100 mM triethanolamine, 200 mM NaCl, 5 mM CaCl2, pH 8.2 was added to each well. The reaction was developed for 5 minutes, stopped with 50 μL of 50% acetic acid, and the optical density determined at 405 nm in an ELISA plate reader (Dynex Technologies, Middlesex, United Kingdom). The kinetic conditions and the linearity of FXa formation were determined by time course experiments using factor X that had been fully activated with Russell viper venom. The data were expressed as the stimulation index, which was the fold increase in tissue factor expression relative to the basal level of tissue factor expressed by endothelial cells incubated with tissue culture medium alone. Each experiment was performed in duplicate and repeated at least 3 times.
Statistical analysis
Differences among the experimental groups were assessed using analysis of variance with the Bonferroni correction for multiple comparisons. P values of .05 or less were considered significant.
Results
Interactions of A fumigatus conidia, germ tubes, and hyphae with endothelial cells
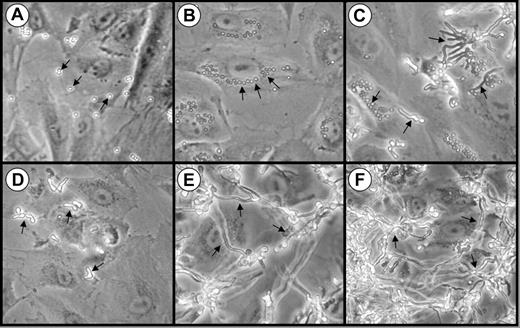
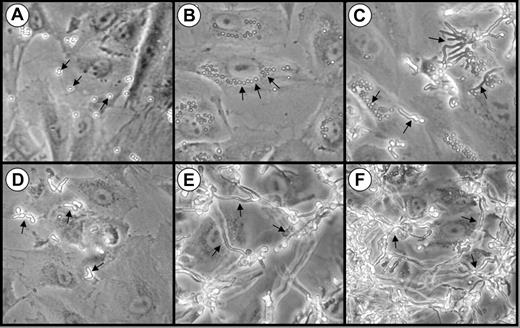
We first examined the interactions of A fumigatus conidia, germ tubes, and hyphae with endothelial cells in vitro using phase contrast microscopy. When the organisms were added to the endothelial cells as resting conidia, they settled onto the cells within 30 minutes (Figure 1A) and began to swell by 3 hours (Figure 1B). Some of these swollen conidia were visible in the perinuclear position of the endothelial cells, suggesting that they had been internalized (Figure 1B). After 8 hours of infection, the swollen conidia became polarized and began to germinate. Both pear-shaped organisms and a few germ tubes were visible at this time point (Figure 1C).
Interactions of A fumigatus conidia and germ tubes with endothelial cells. Photomicrographs of endothelial cell monolayers infected with A fumigatus H237 conidia (A-C) and germ tubes (D-F) after 30 minutes (A,D), 3 hours (B,E), and 8 hours (C,F). Original magnification × 20. Arrows indicate the organisms.
Interactions of A fumigatus conidia and germ tubes with endothelial cells. Photomicrographs of endothelial cell monolayers infected with A fumigatus H237 conidia (A-C) and germ tubes (D-F) after 30 minutes (A,D), 3 hours (B,E), and 8 hours (C,F). Original magnification × 20. Arrows indicate the organisms.
During invasive aspergillosis, it is highly probable that the inhaled A fumigatus conidia germinate and form hyphae before they come in contact with the vascular endothelium. Therefore, we examined the endothelial cell interactions of organisms that had been pregerminated prior to being added to the endothelial cell monolayers. These germ tubes rapidly settled onto the monolayer (Figure 1D) and progressively elongated so that a hyphal mat was formed by 3 hours (Figure 1E). After 8 hours of infection, this hyphal mat had completely covered the endothelial cells (Figure 1F).
Conidia and hyphae induce rearrangement of endothelial cell microfilaments and subsequent endocytosis
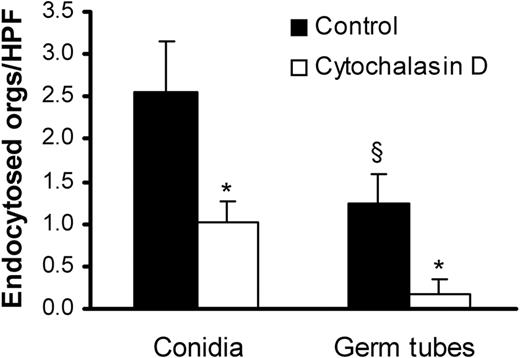
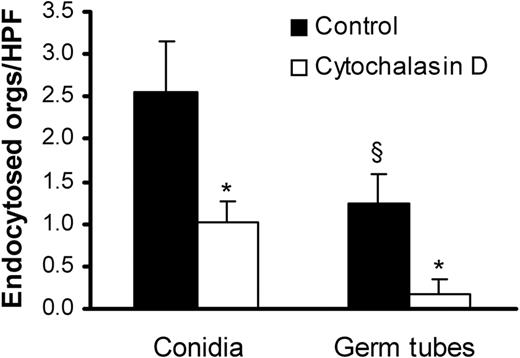
We compared the endothelial cell uptake of A fumigatus conidia and germ tubes using a differential fluorescence assay. After 45 minutes, 2-fold more conidia than germ tubes were internalized by the endothelial cells (Figure 2). Addition of cytochalasin D, a microfilament inhibitor, reduced the uptake of conidia and germ tubes by 60% and 80%, respectively. The inhibitory effect of cytochalasin D on the internalization of the organisms indicates that both forms of A fumigatus invade endothelial cells by inducing their own endocytosis.
Endocytosis of A fumigatus by endothelial cells.A fumigatus H237 conidia and germ tubes were incubated with endothelial cells for 45 minutes in the presence (□) or absence (▪) of the microfilament inhibitor, cytochalasin D. The number of organisms (orgs) endocytosed by the endothelial cells was determined by a differential fluorescence assay. Results are mean ± standard deviation of 3 experiments. *P < .001 compared with cells without cytochalasin D; §P < .05 compared with conidia.
Endocytosis of A fumigatus by endothelial cells.A fumigatus H237 conidia and germ tubes were incubated with endothelial cells for 45 minutes in the presence (□) or absence (▪) of the microfilament inhibitor, cytochalasin D. The number of organisms (orgs) endocytosed by the endothelial cells was determined by a differential fluorescence assay. Results are mean ± standard deviation of 3 experiments. *P < .001 compared with cells without cytochalasin D; §P < .05 compared with conidia.
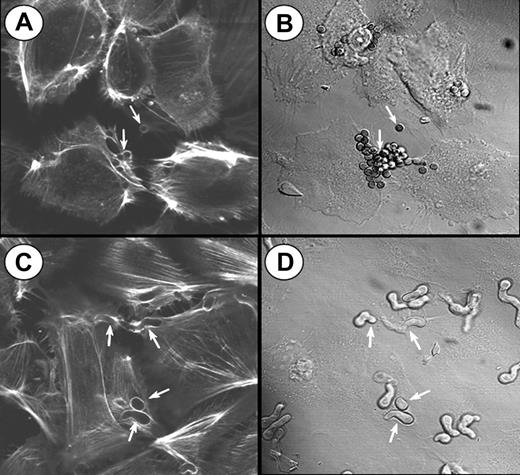
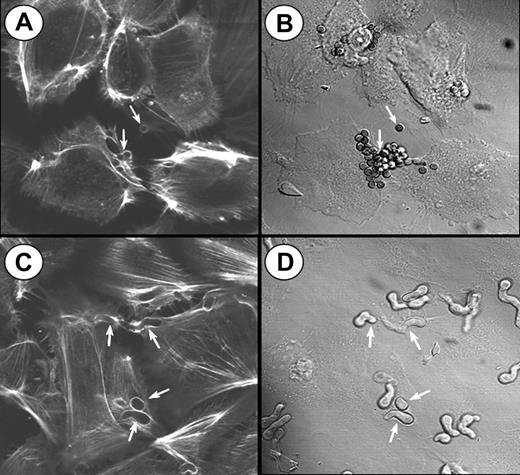
To visualize the involvement of microfilaments in the endocytosis of A fumigatus, the infected endothelial cells were stained with Alexa Fluor 488 phalloidin and viewed by confocal microscopy. Endocytosis of both conidia and hyphae was accompanied by the polymerization of endothelial cell actin around the organisms (Figure 3). Some endothelial cells endocytosed multiple organisms, particularly conidia. In some cases, 2 endothelial cells could be observed endocytosing different portions of the same hyphus. After endocytosis was complete, the internalized organisms were no longer surrounded by polymerized actin. A similar pattern of actin polymerization was observed when the endothelial cells were incubated with killed organisms (data not shown), indicating that the endocytosis of A fumigatus is independent of the viability of the organism.
Confocal microscopy of actin filaments surrounding endocytosed organisms. Endothelial cells were incubated for 45 minutes with A fumigatus H237 conidia (A-B) or germ tubes (C-D), and then stained for filamentous actin. (A,C) Confocal images of the filamentous actin. (B,D) Differential interference contrast images of the same microscopic fields shown in panels A and C, respectively. Arrows indicate endothelial cell actin polymerizing around the organisms (A,C) and the organisms themselves (B,D). Original magnification × 1000.
Confocal microscopy of actin filaments surrounding endocytosed organisms. Endothelial cells were incubated for 45 minutes with A fumigatus H237 conidia (A-B) or germ tubes (C-D), and then stained for filamentous actin. (A,C) Confocal images of the filamentous actin. (B,D) Differential interference contrast images of the same microscopic fields shown in panels A and C, respectively. Arrows indicate endothelial cell actin polymerizing around the organisms (A,C) and the organisms themselves (B,D). Original magnification × 1000.
A fumigatus conidia and germ tubes injure endothelial cells by different mechanisms
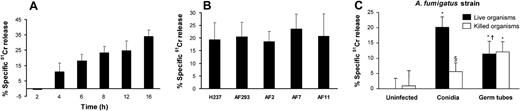
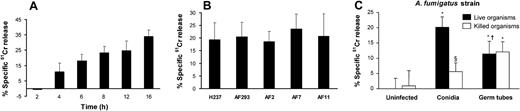
It is known that C albicans and Cryptococcus neoformans, which interact with endothelial cells during hematogenous dissemination, cause significant endothelial cell injury in vitro.19,22 We therefore evaluated the extent of endothelial cell injury caused by A fumigatus conidia and germ tubes. When the endothelial cells were infected with resting conidia, cellular injury became detectable after 4 hours (Figure 4A). At this time, the conidia had become swollen, but had not yet germinated. The extent of endothelial cell injury caused by A fumigatus increased progressively with time as the swollen conidia subsequently germinated (Figure 1 and Figure 4A).
Endothelial cell injury induced by A fumigatus. (A) Time course of injury. Endothelial cells were incubated with conidia of A fumigatus H237 for the indicated times, and the extent of endothelial cell injury was determined by the specific release of 51Cr as described in “Materials and methods.” (B) Extent of injury caused by different clinical isolates. Endothelial cells were infected with conidia of the indicated clinical isolates of A fumigatus for 8 hours, after which endothelial cell injury was measured. (C) Comparison of endothelial cell injury caused by live and thimerosal-killed conidia and hyphae of A fumigatus H237 after 8 hours. Results are means ± standard deviation of at least 3 independent experiments. Error bars indicate standard deviation. *P < .001 versus uninfected control wells; §P < .05 versus live organisms; †P < .001 versus conidia.
Endothelial cell injury induced by A fumigatus. (A) Time course of injury. Endothelial cells were incubated with conidia of A fumigatus H237 for the indicated times, and the extent of endothelial cell injury was determined by the specific release of 51Cr as described in “Materials and methods.” (B) Extent of injury caused by different clinical isolates. Endothelial cells were infected with conidia of the indicated clinical isolates of A fumigatus for 8 hours, after which endothelial cell injury was measured. (C) Comparison of endothelial cell injury caused by live and thimerosal-killed conidia and hyphae of A fumigatus H237 after 8 hours. Results are means ± standard deviation of at least 3 independent experiments. Error bars indicate standard deviation. *P < .001 versus uninfected control wells; §P < .05 versus live organisms; †P < .001 versus conidia.
To investigate whether different strains of A fumigatus varied in their capacity to injure endothelial cells, we tested 5 clinical isolates of A fumigatus in the endothelial cell injury assay. All 5 isolates caused a similar amount of endothelial cell injury after 8 hours of infection (Figure 4B).
Next, we compared the extent of endothelial cell injury caused by live and killed conidia and germ tubes. Live conidia of A fumigatus caused greater endothelial cell injury than did live germ tubes after 8 hours of infection (Figure 4C). Fungal viability was important for endothelial cell injury by conidia, as killed conidia induced minimal injury after 8 hours (Figure 4C) or even after 16 hours of incubation (data not shown). Surprisingly, endothelial cell injury caused by germ tubes was independent of fungal viability, because killed germ tubes induced the same extent of endothelial cell injury as did live germ tubes (Figure 4C). These results suggest that A fumigatus conidia and germ tubes injure endothelial cells by different mechanisms.
Endothelial cell injury by A fumigatus requires endocytosis of the organism
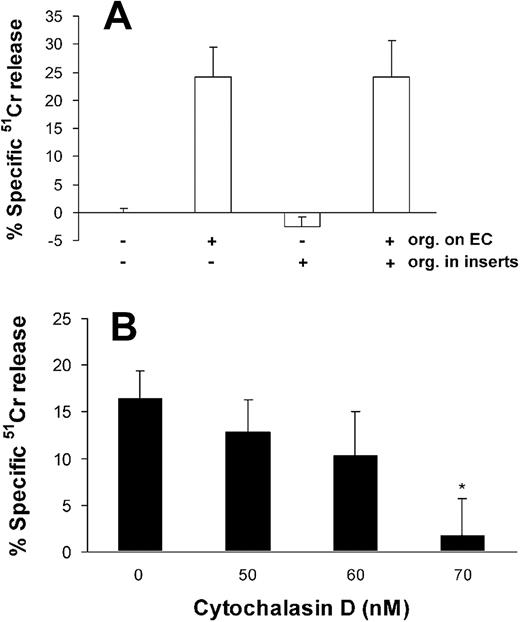
To determine if direct contact is required for A fumigatus to injure endothelial cells, we interposed cell culture inserts between the endothelial cells and live conidia. This procedure completely abolished endothelial cell injury after 8 hours (Figure 5A) and 16 hours (data not shown) of infection.
Effects of cell contact and endocytosis on endothelial cell injury caused by A fumigatus. (A) Extent of endothelial cell injury caused by conidia of A fumigatus H237 that were added directly onto endothelial cell monolayers in a 24-well plate and/or into 0.4 μm cell culture inserts suspended above the monolayers. (B) Extent of endothelial cell injury caused by A fumigatus H237 conidia in the presence of the indicated concentrations of the microfilament inhibitor, cytochalasin D. Endothelial cell injury was measured after 8 hours of infection and the results are the mean ± standard deviation of at least 3 independent experiments. Error bars indicate standard deviation. *P < .001 compared with control.
Effects of cell contact and endocytosis on endothelial cell injury caused by A fumigatus. (A) Extent of endothelial cell injury caused by conidia of A fumigatus H237 that were added directly onto endothelial cell monolayers in a 24-well plate and/or into 0.4 μm cell culture inserts suspended above the monolayers. (B) Extent of endothelial cell injury caused by A fumigatus H237 conidia in the presence of the indicated concentrations of the microfilament inhibitor, cytochalasin D. Endothelial cell injury was measured after 8 hours of infection and the results are the mean ± standard deviation of at least 3 independent experiments. Error bars indicate standard deviation. *P < .001 compared with control.
Next, we infected the endothelial cells in the presence of cytochalasin D to determine whether endocytosis of A fumigatus is required for endothelial injury to occur. We found that 70 nM cytochalasin D caused an 89% reduction in endothelial cell injury caused by A fumigatus (Figure 5B). This concentration of cytochalasin D had no visible effect on the A fumigatus cells and caused less than 5% specific release of 51Cr when added to uninfected endothelial cells (data not shown).
A fumigatus hyphae induces tissue factor expression
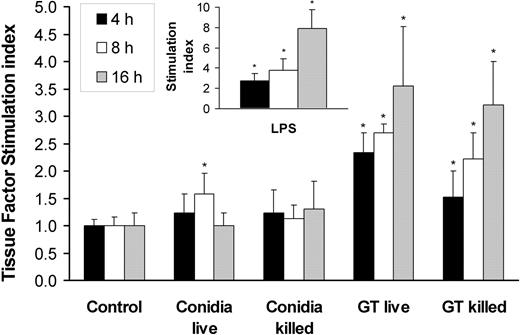
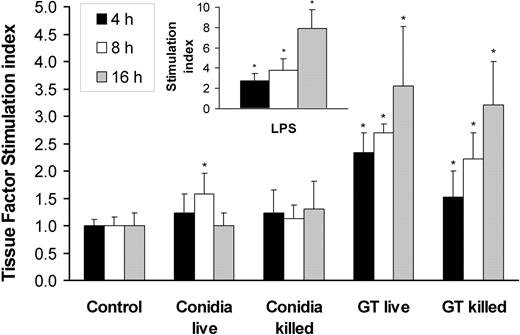
In invasive aspergillosis, angioinvasion is accompanied by thrombus formation.3-5,23 One mechanism by which A fumigatus can induce intravascular thrombosis is by inducing tissue factor expression by endothelial cells. We therefore investigated whether A fumigatus conidia and hyphae stimulate endothelial cell tissue factor activity. We found that the basal level of tissue factor activity expressed by uninfected endothelial cells was low, although this level varied with the endothelial cell donor (range, 0.78 to 2.34 factor Xa units). Incubation of endothelial cells with live or killed conidia for up to 16 hours resulted in minimal increase in tissue factor activity (Figure 6). In contrast, germ tubes stimulated a progressive increase in tissue factor activity, starting at 4 hours of incubation. Both live and killed germ tubes induced similar levels of tissue factor activity.
Tissue factor activity of endothelial cells incubated with A fumigatus. Time course of tissue factor activity of endothelial cells incubated for 4, 8, and 16 hours with viable and nonviable conidia or germ tubes of A fumigatus. Endothelial cells were also stimulated with LPS as a positive control (insert). Tissue factor activity was expressed as fold increase (stimulation index) relative to the basal level of activity of unstimulated cells. The results are means ± standard deviation of at least 3 independent experiments. Error bars indicate standard deviation. *P < .05 compared with control.
Tissue factor activity of endothelial cells incubated with A fumigatus. Time course of tissue factor activity of endothelial cells incubated for 4, 8, and 16 hours with viable and nonviable conidia or germ tubes of A fumigatus. Endothelial cells were also stimulated with LPS as a positive control (insert). Tissue factor activity was expressed as fold increase (stimulation index) relative to the basal level of activity of unstimulated cells. The results are means ± standard deviation of at least 3 independent experiments. Error bars indicate standard deviation. *P < .05 compared with control.
In these experiments, the endothelial cells were stimulated with LPS as a positive control. The time course of LPS stimulation of endothelial cell tissue factor activity was similar to that induced by A fumigatus germ tubes, although LPS induced a greater response than did the germ tubes at 16 hours (Figure 6, inset).
To determine if the induction of tissue factor activity by A fumigatus represents a generalized response to endothelial cell injury, we tested whether C albicans could induce endothelial cell tissue factor activity. We have shown previously that this organism causes extensive endothelial cell injury.15,19,20 Interestingly, endothelial cells infected with C albicans expressed only basal levels of tissue factor activity at all time points tested (data not shown).
We also examined whether A fumigatus germ tubes themselves express tissue factor activity by testing wells containing organisms in the absence of endothelial cells. These wells had no activity (data not shown). Finally, we omitted factor VII from the assay for tissue factor activity to determine if substances such as proteases released by A fumigatus could mimic the activity of the tissue factor–factor VII complex and convert factor X to factor Xa. We found that in the absence of factor VII, no factor Xa was formed by endothelial cells infected with A fumigatus (data not shown). Collectively, these results indicate that the increased tissue factor activity associated with endothelial cells infected with A fumigatus is due to the endothelial cell response to the organism, rather than factors produced by the organism itself.
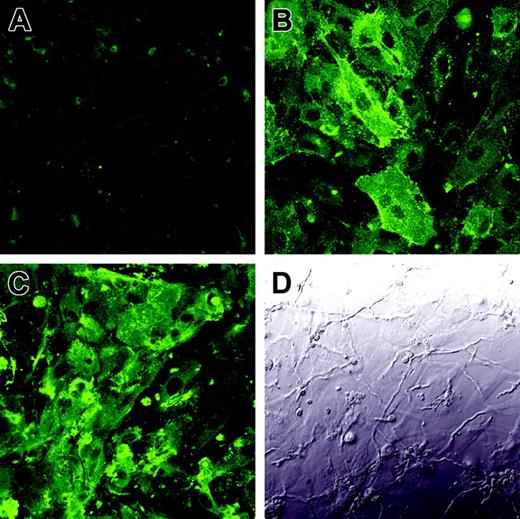
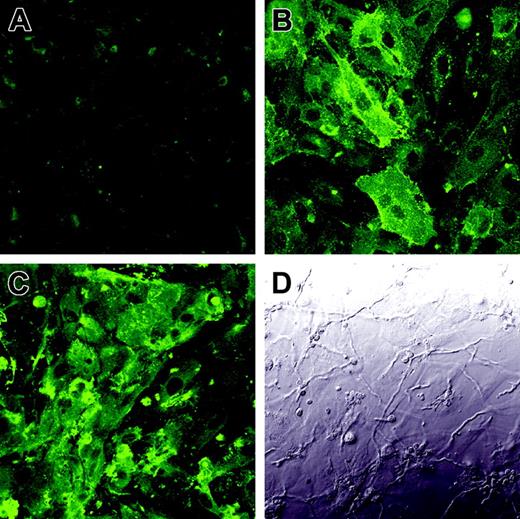
To verify that the enhanced tissue factor activity was due to the increased expression of tissue factor on the endothelial cell surface, we infected endothelial cells with A fumigatus germ tubes for 8 hours and then detected tissue factor expression by indirect immunofluorescence. As predicted by the results of the tissue factor activity assay, there was low expression of tissue factor on the surface of uninfected endothelial cells (Figure 7A), whereas endothelial cells infected with A fumigatus expressed high levels of tissue factor on their surface (Figure 7C). Interestingly, tissue factor was expressed over the entire luminal surface of the infected endothelial cells, not just at points of hyphal contact.
Expression of tissue factor on the surface of infected endothelial cells. Endothelial cell monolayers grown on glass coverslips were incubated for 8 hours with tissue culture medium (A), LPS (B), or germ tubes of A fumigatus (C-D). The expression of tissue factor on the endothelial cell surface was detected by confocal microscopy using an anti–tissue factor monoclonal antibody (A,C). Panel D shows the differential interference contrast image corresponding to the same field as panel C. Results are representative of 3 independent experiments. Original magnification × 400.
Expression of tissue factor on the surface of infected endothelial cells. Endothelial cell monolayers grown on glass coverslips were incubated for 8 hours with tissue culture medium (A), LPS (B), or germ tubes of A fumigatus (C-D). The expression of tissue factor on the endothelial cell surface was detected by confocal microscopy using an anti–tissue factor monoclonal antibody (A,C). Panel D shows the differential interference contrast image corresponding to the same field as panel C. Results are representative of 3 independent experiments. Original magnification × 400.
Discussion
During angioinvasion by A fumigatus in both experimental animal models and humans, only hyphal phase organisms are visible in the tissues.2,4,5,24 This finding indicates that the hyphus is the principal form of the organism that interacts with endothelial cells and suggests that hyphae have a unique capacity to survive and proliferate within the host. We therefore compared the interactions of A fumigatus conidia and hyphae with endothelial cells.
We found that while both forms of A fumigatus were internalized by endothelial cells via an actin-dependent mechanism, conidia were endocytosed more avidly than were hyphae. This difference in the rate of endocytosis between the 2 morphologies may be due to the difference in their size. In addition, it is possible that conidia and hyphae possess different numbers and/or types of endothelial cell binding proteins on their surface. Although the endocytosis of A fumigatus conidia by endothelial cells in vitro has been described by others,25,26 the endocytosis of A fumigatus hyphae has not been reported previously. In autopsy studies, A fumigatus hyphae can clearly be seen to cross the wall of the blood vessels.4,5,23,24 However, to our knowledge, the electron microscopy studies necessary to observe endothelial cell invasion by the hyphae have not been performed.
A fumigatus conidia and hyphae also differed in their capacity to injure endothelial cells. Organisms that were added to endothelial cells as conidia caused significantly more endothelial cell injury after 8 hours than did organisms that were added to endothelial cells as germ tubes. This finding was surprising because the conidia had just begun to germinate after 8 hours of incubation with the endothelial cells (Figure 1C). In contrast, the pregerminated organisms had almost completely covered the endothelial cells with a hyphal mat by this time point (Figure 1F). Thus, there was much greater contact between the endothelial cells and the A fumigatus cells when the organisms were pregerminated before being added to the monolayers. It is possible that conidia caused greater injury because more of them were endocytosed by the endothelial cells. It is also possible that contact with the endothelial cells induced conidia to synthesize greater amounts of injurious factors than were synthesized by organisms that were pregerminated in the absence of endothelial cells.
Another important finding was that although killed conidia caused no detectable endothelial cell injury, killed germ tubes caused as much injury as did live germ tubes. One explanation for the lack of endothelial cell injury caused by killed, resting conidia is that they must begin to swell or germinate before they can cause endothelial cell injury. The finding that killed germ tubes injured endothelial cells suggests that a factor associated with the cell surface of the germ tubes causes endothelial cell injury when the germ tubes are endocytosed. In support of this conclusion, we have found that cell wall extracts of A fumigatus hyphae can also injure endothelial cells (L.M.L.B. and S.G.F., unpublished data, October 2001).
By interposing filter inserts between A fumigatus and the endothelial cells, we determined that direct contact between the organisms and endothelial cells appears to be necessary for endothelial cell damage to be induced under these culture conditions. Moreover, the finding that inhibiting endocytosis of the organism with cytochalasin D blocked endothelial cell injury indicates that endocytosis is an additional prerequisite for the induction of endothelial cell injury. A fumigatus is known to secrete a variety of lytic enzymes including proteases and phospholipases.27,28 It is possible that these secreted enzymes are most active when they achieve high concentrations in the endocytic vacuole of the endothelial cell. As mentioned previously, it is also likely that the hyphal cell wall itself is toxic to endothelial cells.
To investigate potential mechanisms by which A fumigatus causes thrombosis at sites of angioinvasion, we examined whether this organism could induce endothelial cells to express tissue factor activity. We found that A fumigatus hyphae, but not conidia, stimulated endothelial cells to express tissue factor activity. This finding again indicates that A fumigatus conidia and hyphae interact differently with endothelial cells. There are 2 lines of evidence that demonstrate that the increase in tissue factor activity was due to the endothelial cells rather than A fumigatus. First, neither live nor killed A fumigatus hyphae had any detectable tissue factor activity in the absence of endothelial cells. Furthermore, endothelial cells exposed to killed A fumigatus hyphae expressed similar amounts of tissue factor activity as did endothelial cells exposed to live hyphae. Second, tissue factor antigen was detectable on the surface of the infected endothelial cells, but not on A fumigatus hyphae.
C albicans is another angioinvasive fungus and its interactions with endothelial cells in vitro have been investigated extensively. This organism is similar to A fumigatus in that both live and killed hyphae are endocytosed by endothelial cells and this process can be inhibited by cytochalasin D.14,16,19 However, a major difference between the 2 organisms is that A fumigatus conidia are endocytosed by endothelial cells more avidly than hyphae, whereas C albicans hyphae are endocytosed more avidly than blastoconidia.15 There are also significant differences in endothelial cell injury caused by A fumigatus and C albicans. Unlike A fumigatus conidia, C albicans blastoconidia do not cause detectable endothelial cell injury.15 Furthermore, although killed A fumigatus hyphae cause significant endothelial cell injury, killed C albicans hyphae do not.19 Therefore, A fumigatus and C albicans likely injure endothelial cells by different mechanisms.
We also found that C albicans did not stimulate endothelial cell tissue factor activity. This result was particularly interesting because C albicans is known to stimulate multiple endothelial cell responses including the expression of leukocyte adhesion molecules, and secretion of proinflammatory cytokines and prostaglandins.20,29-31 The difference in the ability of A fumigatus and C albicans to induce a prothrombotic state in endothelial cells in vitro parallels the histopathology of infections caused by these organisms in vivo. Invasive aspergillosis is characterized by extensive intravascular thrombosis at foci of infection,3-5,23 whereas thrombosis is not a prominent feature of disseminated candidiasis.32,33
Other microorganisms, such as Staphylococcus aureus and Neisseria meningitidis are known to stimulate endothelial cells to express tissue factor in vitro.34-36 It is likely that stimulation of endothelial cell tissue factor expression contributes to formation of vegetations during staphylococcal endocarditis and to disseminated intravascular coagulation during meningococcemia.34,37 Other microbial pathogens, such as cytomegalovirus, Chlamydia pneumoniae, Streptococcus sanguis, and Plasmodium falciparum may also induce tissue factor expression at sites of infection.38-40 However, these organisms induce tissue factor expression by activating monocytes, which can express tissue factor by themselves, as well as stimulate endothelial cells to express tissue factor. Whether monocytes augment A fumigatus induction of endothelial cell tissue factor expression has not yet been determined.
In summary, A fumigatus hyphae invade endothelial cells in vitro by inducing their own endocytosis. The internalized organisms then injure the endothelial cells and stimulate them to express tissue factor. This process provides a potential mechanism for the vascular invasion and thrombosis that characterize invasive aspergillosis. Studies to examine the mechanism by which A fumigatus hyphae induce tissue factor expression and to confirm tissue factor expression at foci of invasive aspergillosis in vivo are currently in progress.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-06-2186.
Supported in part by the Public Health Service grant MO1 RR00425 and contract N01-AI-30041 from the National Institutes of Health. L.M.L.-B. is supported by Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) of Brazil, grant BEX 0165/01-8. S.G.F. is supported by the Burroughs Welcome Fund New Investigator Award in Molecular Pathogenic Mycology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the perinatal nurses at Harbor-UCLA Medical Center for collecting the umbilical cords, Quynh Phan for help with tissue culture, and Dr Matthew J. Schibler at the Brain Research Institute at UCLA for expert assistance with confocal microscopy.