Abstract
Alemtuzumab therapy is effective for some refractory chronic lymphocytic leukemia (CLL), but identifying responders requires at least 8 weeks of therapy. Early identification of nonresponders would minimize toxicity and/or facilitate more effective strategies. The aim of this study was to identify a minimally invasive method for early prediction of response and relapse. Flow cytometric monitoring was performed in 887 blood samples and 201 marrow samples from 43 patients undergoing intravenous alemtuzumab therapy. Although the absolute lymphocytosis was resolved in all patients by week 4, significant depletion of bone marrow tumor only occurred if circulating B-lymphocyte counts were persistently less than 0.001 × 109/L, which was rare in nonresponders. The majority of patients (16/28) who did not benefit from a full course of therapy were identified with 100% positive predictive value using the following algorithm: peripheral B-cell count greater than 0.001 × 109/L at week 2 with less than 1 log depletion of circulating B cells between weeks 2 and 4. Monitoring CLL levels after treatment identified patients at risk of early disease progression and could potentially improve patient management. During alemtuzumab therapy, bone marrow CLL depletion only occurs after abrogation of circulating tumor, requiring close monitoring of circulating B-cell levels. If validated in prospective studies, blood monitoring at 2 and 4 weeks may be used to optimize therapy.
Introduction
Alemtuzumab is an effective therapy for a proportion of chronic lymphocytic leukemia (CLL) patients previously treated with purine analogues and/or alkylating agents.1-3 Patients achieving a complete response with no CLL cells detectable by minimal residual disease (MRD) flow cytometry have an extremely good outcome, with a 65% 5-year event-free survival.4 Currently patients receive intravenous alemtuzumab 3 times a week to maximum response as assessed by regular bone marrow examinations throughout alemtuzumab therapy. This usually necessitates patients having bone marrow examinations every 4 weeks for 12 weeks (the median duration of therapy) and nonresponding patients cannot be identified until the eighth week of therapy at the earliest. A simple method to predict which patients are destined to respond and those who are not would have several benefits. These include the potential to reduce toxicity by early cessation of therapy in nonresponders because immunosuppression results in potentially life-threatening cytomegalovirus (CMV) reactivation in approximately 10% of patients. In addition, therapy could be modified to a potentially more effective strategy such as concurrent alemtuzumab and fludarabine.5 There is also the potential to reduce costs. Monitoring of patients after therapy may allow prediction of the rate of relapse and hence more timely introduction of further therapy.
We have used both a minimal and a highly sensitive MRD flow assay to monitor patients undergoing alemtuzumab monotherapy in both peripheral blood and bone marrow in 43 patients. Although the peripheral blood lymphocyte counts decreased to normal levels in all patients, CLL cells were frequently detectable at low levels in peripheral blood and bone marrow even in responding patients. We have therefore performed a retrospective analysis of CLL cell levels in patients undergoing alemtuzumab monotherapy, with the aim of identifying simple and minimally invasive procedures for early prediction of response and rate of relapse.
Patients, materials, and methods
Patients and treatment
Forty-three patients were included in this study, of which 38 were male and 5 were female with a median age of 58 years (range, 32-75 years). All were refractory to purine analogues, defined as progressive or stable disease, or relapse of disease within 6 months after receiving a purine analog–containing regimen. Patients gave written informed consent to treatment in a series of clinical trials ethically approved by the Leeds Teaching Hospitals' Internal Review Board and following the tenets of the Helsinki protocol.
Patients had received a median of 3 prior lines of chemotherapy (range, 1-7). Staging was performed immediately prior to alemtuzumab therapy: 37% (16/43) were Rai stage I/II and 63% (27/43) were Rai stage III/IV. Alemtuzumab was given intravenously at 30 mg 3 times a week after an initial dose escalation starting from 3 mg, increasing to 10 mg, and increasing to the full dose of 30 mg when infusion-related side effects had subsided. All patients were treated with prophylactic cotrimoxazole 480 mg daily and aciclovir 200 mg twice daily during alemtuzumab therapy. Patients received paracetamol and chlorpheniramine prior to each infusion. Therapy was withheld if the neutrophil count fell to less than 0.25 × 109/L, if the platelet count fell to less than 25 × 109/L, or if there were serious infective complications. Therapy was stopped if there was no response or progressive disease on therapy, if the treatment was intolerable, or if an MRD-negative response (CLL cells below 0.05% of leukocytes) was achieved. Treatment was continued for 12 weeks and was given until maximum response as defined by blood, bone marrow, and lymph node assessment with the intention of achieving an MRD-negative remission. Thus responding patients with a morphologically normal bone marrow but detectable CLL by MRD flow continued to receive alemtuzumab until either CLL was not detectable or until the level of MRD was stable. Patients were assessed every 4 weeks by physical examination and bone marrow assessment for response, and peripheral blood samples were assessed weekly. Responses are reported according to the National Cancer Institute (NCI) criteria for response.6 However, complete and partial responders are separated into those with detectable residual disease (ie, CR+ or PR+) and those with no detectable residual disease (ie, CR– or PR–). PR– patients achieve complete depletion of CLL cells but show persistent anemia or thrombocytopenia. Flow cytometric analysis was performed on bone marrow samples prior to therapy; at weeks 4, 8, and 12 of therapy; and at 2 months after therapy to assess response (n = 201). In addition, patients had weekly peripheral blood assessments (n = 887).
Flow cytometric analysis
Leukocytes were prepared by ammonium chloride lysis, labeled with antibody, acquired on a Becton Dickinson FACSort (BD Biosciences, Oxford, United Kingdom), and analyzed as reported previously.7 A minimal panel, applied to weekly blood samples, consisted of CD45 fluorescein isothiocyanate (FITC), CD48 phycoerythrin (PE), CD19 PE-cyanine 5 (Cy5), and CD3 allophycocyanin (APC). Peripheral blood samples before and after therapy and at 4-week intervals and all marrow samples were assessed with an extended MRD flow panel, comprising CD19 PE-Cy5 and CD5 APC, with kappa FITC/lambda PE, CD20 FITC/CD79b PE, CD20 FITC/CD38 PE, and CD38 FITC/CD79b PE, in addition to the minimal panel. All antibody conjugates were prepared in-house except CD20 and CD79b (Beckman Coulter, High Wycombe, United Kingdom) and CD38 PE (BD Biosciences).
Statistical analysis
For statistical analyses, P values were assessed using either Mann-Whitney U or Spearman rank correlation as appropriate (STATA v7; Stata Corporation, College Station, TX).
Results
Disease levels in the peripheral blood during therapy may be adequately monitored using a minimal flow assay
Alemtuzumab therapy resulted in a response by NCI criteria in 16 patients with 27 patients showing no response. Of the responders, 7 showed a partial response, of which 5 had residual disease present (PR+) and 2 had no residual disease but showed prolonged cytopenia (PR–). Nine patients showed a complete response of which 2 had minimal residual disease detectable (CR+) and 7 had no detectable residual disease (CR–).
Independent of the overall response to therapy, alemtuzumab induced profound peripheral lymphocyte depletion, with 34 of 43 patients reaching a lymphocyte count less than 2.0 × 109/L by week 2 and all patients reaching this lymphocyte count by week 4. In many cases, the majority of residual lymphocytes are glycosylphosphatidylinositol (GPI)–deficient T cells that expand during therapy.8 As alemtuzumab is highly effective at depletion of normal B and T cells, it is probable that any residual B cells present during therapy are neoplastic. If this is the case, monitoring CLL cell levels using a full MRD flow approach while patients are on therapy may not be cost effective and a minimal panel may be sufficient. Previous data indicates that at 3 months after therapy normal B cells will be present in most patients and therefore a full panel is required to accurately assess the levels of disease. However, it is not known how soon after completion of therapy normal B-cell regeneration occurs. We therefore assessed the numbers of CLL cells as a proportion of total B cells in patients during and immediately (ie, 0-3 months) after alemtuzumab therapy in blood and marrow.
As expected, CLL cells represented a significantly higher proportion of B lymphocytes in patients during therapy than immediately after therapy. In the peripheral blood, CLL cells represented a median of 96% B cells on therapy (range, 8%-100%; 95% confidence interval, 83%-91%) compared with a median of 57% after therapy (range, 0%-100%; 95% confidence interval, 43%-63%; Mann-Whitney P = .0001). In the bone marrow, CLL cells represented a median of 96% on therapy (range, 0%-100%; 95% confidence interval, 71%-84%) compared with a median of 88% after therapy (range, 0%-100%; 95% confidence interval, 37%-73%; Mann-Whitney P = .0095). The normal B lymphocytes present in the bone marrow during and immediately after therapy were predominantly B-progenitor cells with no expression of CD5, CD48, or surface immunoglobulin but very strong CD38 expression. Normal mature B lymphocytes were extremely rare during treatment in both peripheral blood and bone marrow, but regeneration is rapid after cessation of treatment. Therefore, accurate quantitation of CLL cells requires a full MRD flow panel in the bone marrow at all time points and in the peripheral blood after cessation of therapy. However, there is a high level of confidence that the absolute B-cell numbers in the peripheral blood of patients during therapy will equate closely to the numbers of CLL cells. It may therefore be adequate to use B-cell numbers alone to monitor the effects of therapy in the peripheral blood.
All patients achieve a significant peripheral blood response, but circulating CLL cells remain greater than 0.001 × 109/L in nonresponders
Assessment of response to alemtuzumab is currently monitored by direct analysis of bone marrow CLL levels. It would be preferable if peripheral blood could be used to guide therapy or if fewer bone marrow aspirate samples were necessary for accurate assessment during therapy. The level of CLL in peripheral blood and bone marrow was compared during therapy to determine the optimum method of monitoring. We first determined whether there was a difference in time to maximum response. All nonresponders and the majority (9/15, 60%) of responding patients show their maximum response in the bone marrow at the end of therapy. Therefore, early prediction of response was not possible from bone marrow assessment alone. In contrast, the maximum peripheral blood response occurred early during therapy in all patients, at a median of week 3 (range, weeks 2-7) for responding patients and a median of week 4 (range, weeks 1-12) for nonresponders. The level of B lymphocytes was significantly different between the 2 groups with a median 0.0009 × 109/L (range, < 0.0001 × 109/L to 0.0045 × 109/L) in responders and a median 0.0037 (range, 0.0010 × 109/L to 0.1010 × 109/L) in nonresponders (Mann-Whitney P < .001). This suggests that the assessment of peripheral blood CLL levels prior to week 4 may allow early prediction of response.
Early prediction of response
In order to determine whether peripheral blood B-cell levels could predict response, we have compared outcome with the absolute B-cell count both at week 2 and week 4 (Table 1). Analysis was performed on a cohort of 41 patients (27 nonresponders [NR], 5 PR+, 2 PR–, 2 CR+, 5 CR–), with 2 patients excluded as peripheral blood counts were not available at week 2 or week 4 of therapy, respectively. There was no B-cell level at either time point that completely discriminated between responding and nonresponding patients, apart from a small group of patients (n = 5) who maintained a B-cell count greater than 0.1 × 109/L at week 4 who were always nonresponders. The best differentiation between responding and nonresponding patients was identified by an absolute B-cell count greater than 0.001 × 109/L at week 2 of treatment, although half of the responding patients also had this level of circulating B cells at week 2. Further examination of the kinetics of response in these patients indicated that they had a higher tumor burden prior to therapy and showed continued tumor depletion, while the nonresponders showed little further response in the peripheral blood. We therefore compared the log depletion of circulating B cells between week 2 and week 4 to determine whether this could provide a numeric indicator of continuing response. The data, shown in Table 2, demonstrates that the maximum number of nonresponders could be identified without identifying any responding patients using the following algorithm: week 2 peripheral B-lymphocyte count greater than 0.001 × 109/L and less than 1 log depletion in absolute B-lymphocyte count between weeks 2 and 4 of therapy. The algorithm identified 15 of 27 nonresponders as well as one patient who achieved maximum response at week 4. Thus the algorithm identifies the majority of patients (16/28) who do not benefit from a full course of alemtuzumab without identifying any patients who achieve continued tumor depletion with therapy. All complete responders who had not achieved depletion in circulating B-cell levels to less than 0.001 × 109/L by week 2 showed a decrease in B-cell levels over the next 2 weeks of therapy of at least one order of magnitude.
This algorithm allows detection of patients who will not benefit from a full course of alemtuzumab therapy (ie, nonresponders and patients achieving best response at week 4) with 100% positive predictive value or 94% positive predictive value for identification of nonresponders by NCI criteria. However, the test has only 52% negative predictive value (ie, patients predicted to show further responses after week 4 of therapy are an approximately equal mix of responders and nonresponders).
Peripheral counts must be negligible to achieve significant bone marrow depletion
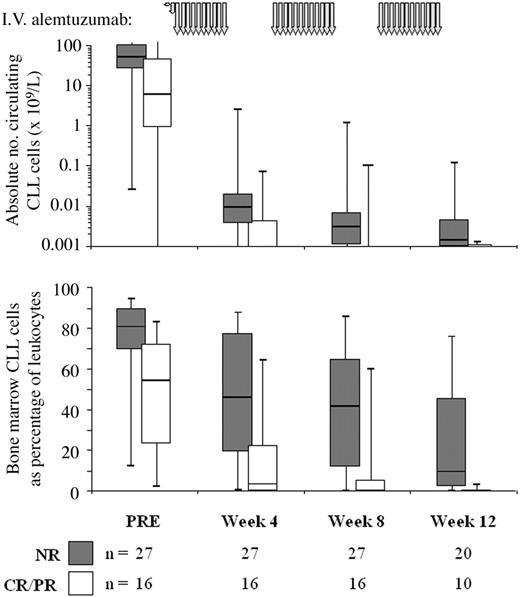
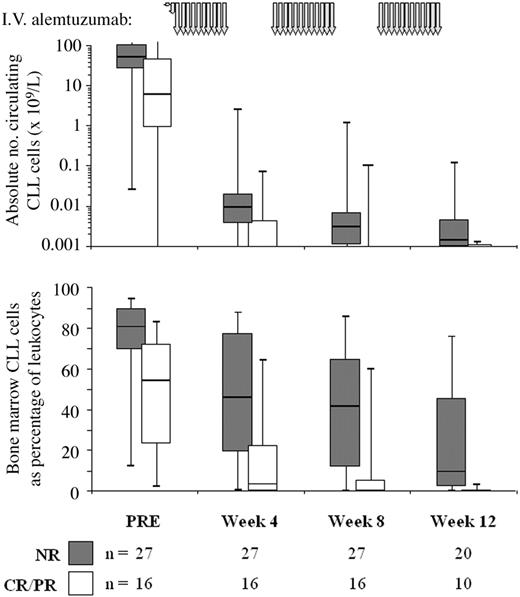
The data above demonstrate a relationship between circulating CLL cells during therapy and eventual outcome. To determine whether circulating B-cell count could predict level of bone marrow disease we compared CLL cell levels in blood and marrow at the same time points (Figure 1).. There was good correlation (Spearman R = 0.7571; P < .0001), but marrow tumor load could not be accurately predicted from individual peripheral counts. However, if the peripheral blood B-cell levels remained less than 0.001 × 109/L over a 4-week treatment course, a significant marrow response (ie, > 1 log depletion or CLL cells maintained less than 0.05% of leukocytes) was seen in 74% (20/27) of these courses. Only 11% (3/27) of courses with continued circulating B-cell levels less than 0.001 × 109/L resulted in a poor marrow response (ie, increasing marrow CLL cells, or < 0.1 log depletion). However, if circulating B-lymphocyte levels were greater than 0.01 × 109/L at most intermediate time points, 41% (9/22) showed a poor marrow response and none showed a significant response. Thus, bone marrow depletion of CLL only occurs to a significant extent after circulating disease has been depleted to a very low level.
Treatment rapidly reduces circulating CLL cell levels in responding patients, while nonresponders show more gradual depletion. The figure shows the absolute peripheral blood CLL cell count on a logarithmic scale (top panel) and the percentage of CLL cells in the bone marrow (bottom panel) in nonresponding (▦, n = 27) and responding patients (□, n = 16). The schema for alemtuzumab therapy is indicated by the gray arrows at the top of the graph. PRE indicates pretreatment. The line at the top of each box denotes the 75th percentile; the line at the bottom of each box, the 25th percentile; the line in the middle of each box, the 50th percentile or median; the upper bar, the 95th percentile; and the lower bar, the 5th percentile.
Treatment rapidly reduces circulating CLL cell levels in responding patients, while nonresponders show more gradual depletion. The figure shows the absolute peripheral blood CLL cell count on a logarithmic scale (top panel) and the percentage of CLL cells in the bone marrow (bottom panel) in nonresponding (▦, n = 27) and responding patients (□, n = 16). The schema for alemtuzumab therapy is indicated by the gray arrows at the top of the graph. PRE indicates pretreatment. The line at the top of each box denotes the 75th percentile; the line at the bottom of each box, the 25th percentile; the line in the middle of each box, the 50th percentile or median; the upper bar, the 95th percentile; and the lower bar, the 5th percentile.
Predicting time to relapse
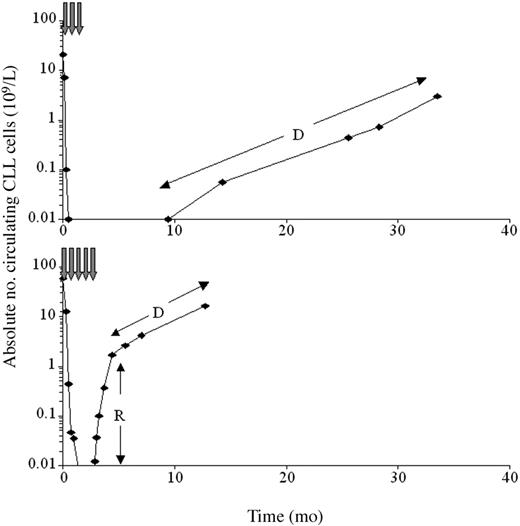
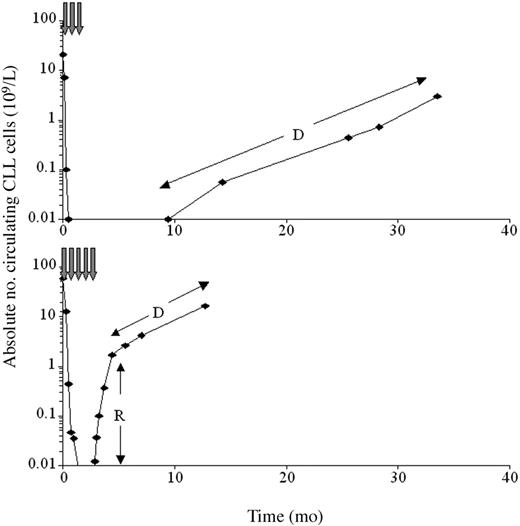
The CLL doubling time immediately after therapy may identify patients who will progress rapidly, requiring closer follow-up and early reintroduction of therapy. The doubling time was difficult to calculate, as the kinetics of disease progression were biphasic in most (24/34) patients. Initially, blood CLL cells increased rapidly, presumably due to redistribution of CLL cells from marrow or nodes. This was followed by a more gradual increase in CLL cells representing the actual rate of proliferation (Figure 2). In 19 patients the doubling time could be calculated (median, 30 days; range, 5-289 days), and the time that would elapse until a patient reaches a certain CLL level (eg, 5 × 109/L) could be predicted. The prediction identified all 5 patients who reached this level within 6 months from end of therapy, despite calculations performed on peripheral counts ranging from 0.001 × 109/L to only 0.025 × 109/L. These patients had not shown nodal responses to therapy and therefore relapse was inevitable; however, the rate of relapse could not be predicted from clinical features alone. Early identification of patients with very rapid relapse should allow more accurate management and application of further treatment. In addition, sequential monitoring in responding patients can identify relapse at a very early clinical stage, allowing improved patient management in this group.
The kinetics of peripheral disease progression after treatment are biphasic in many patients. The figure shows the absolute peripheral blood CLL cell count (logarithmic scale) for a responding patient (top) and a nonresponding patient (bottom). In many nonresponders, there was an initial period of rapidly increasing peripheral CLL cell levels (R), presumably reflecting redistribution of tumor from bone marrow or lymph nodes. This stabilized to show the true rate of progression (D) approximately 2 months after cessation of therapy. In the responding patient, disease levels could be monitored prior to clinical relapse for a period of approximately 2 years from first detection.
The kinetics of peripheral disease progression after treatment are biphasic in many patients. The figure shows the absolute peripheral blood CLL cell count (logarithmic scale) for a responding patient (top) and a nonresponding patient (bottom). In many nonresponders, there was an initial period of rapidly increasing peripheral CLL cell levels (R), presumably reflecting redistribution of tumor from bone marrow or lymph nodes. This stabilized to show the true rate of progression (D) approximately 2 months after cessation of therapy. In the responding patient, disease levels could be monitored prior to clinical relapse for a period of approximately 2 years from first detection.
Discussion
The data presented here demonstrates 2 key points about alemtuzumab monotherapy: (1) bone marrow depletion will not take place until there is almost complete depletion of circulating tumor; and (2) patients respond to alemtuzumab at very different rates, and optimal therapy requires close monitoring of tumor levels during treatment.
The lymphocyte count, as measured by routine hematology analyzers, is not sensitive enough to help predict the outcome of alemtuzumab therapy as all patients respond at this relatively insensitive level of detection in the peripheral blood. However, it is possible to guide therapy with alemtuzumab using a relatively straightforward flow cytometry assay. The vast majority of B cells present in the peripheral blood during therapy are neoplastic, and therefore analysis of B-cell levels provides a sufficient degree of accuracy for monitoring peripheral tumor load. The flow cytometry assay for measuring B-cell numbers is simple, requiring only CD19, CD3, and CD45 to identify B cells, T cells, and total leukocytes, respectively. Identification of cells that are CD19+, with the morphologic (ie, light scatter) characteristics of lymphocytes, is straightforward; exclusion of CD3+ events effectively removes all contaminants from the B-cell gate, allowing an extremely high sensitivity. As such, it is possible to measure extremely low B-cell numbers with high reproducibility as long as sufficient total leukocytes are acquired for analysis on the flow cytometer.
Using this assay, in combination with the more accurate MRD flow panel for measurement of bone marrow CLL cells reported previously,7 it is possible to predict response to therapy by week 4 of treatment according to the following algorithm: week 2 circulating B-cell count greater than 0.001 × 109/L and less than 1 log depletion in circulating B-cell count from week 2 to week 4 of therapy.
This algorithm identified patients who did not respond, or who showed their maximum response to therapy at week 4, with 100% positive predictive value. It is necessary to measure the relative depletion because responders may take several weeks to completely deplete circulating tumor but continue to show depletion week after week until peripheral levels are less than 0.001 × 109/L. In contrast, nonresponders may achieve low levels, between 0.001 × 109/L and 0.01 × 109/L, but there is little or no further depletion with continued therapy. If the assay is validated in a prospective analysis, it would be possible in future studies to remove patients who are unlikely to benefit from alemtuzumab immunotherapy at week 4 of treatment by assessment of peripheral B-lymphocyte counts alone. This would reduce the risk of potentially life-threatening complications, particularly CMV reactivation. A simple B-cell count is adequate for monitoring patients during alemtuzumab therapy because normal B lymphocytes are almost completely depleted by the treatment. However, all bone marrow samples and any peripheral blood samples after completion of alemtuzumab therapy require an MRD flow approach because of the presence of normal B cells and B-cell progenitors that cannot be differentiated from CLL cells by simple flow cytometric analyses.
It is important that CLL levels are monitored during therapy, as the time to complete remission is highly variable among responding patients: some show maximum response at week 4, whereas others take up to 12 weeks to achieve complete remission. This is because bone marrow depletion does not begin until there is almost complete abrogation of circulating tumor, and therefore potentially responsive patients with a heavy peripheral tumor load prior to therapy will require prolonged treatment before a bone marrow response starts.
Accurate and sensitive quantification of the level of CLL in the peripheral blood by multi-parameter flow cytometry during alemtuzumab therapy allows early identification of responders and nonresponders. This permits an informed assessment of the timing of bone marrow examination to assess response, optimally 4 weeks after the circulating CLL levels drop to less than 0.001 × 109/L. This approach has the potential to reduce the number of bone marrow examinations required during alemtuzumab therapy and for the possibility of modifying therapy at an early stage in order to overcome resistance to therapy. Accurate sequential monitoring of CLL levels in the peripheral blood after therapy may allow prediction of clinical relapse at an early time point, therefore improving the assessment of prognosis and allowing early reintroduction of therapy prior to frank relapse.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2002-10-3270.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.