Abstract
Rarely, myelodysplastic syndrome (MDS) is complicated by an acquired form of α-thalassemia (α-thalassemia in myelodysplastic syndrome [ATMDS]) characterized by hypochromic, microcytic, anisopoikilocytic red blood cells with hemoglobin H (HbH) inclusions. Acquired mutations in ATRX, a chromatin remodeling gene, have recently been found in 12 patients with typical features of ATMDS, though they have not been detected in MDS patients with similar red blood cell findings but little HbH. The α-globin genes themselves have appeared normal in all ATMDS patients studied to date. Here we characterize the molecular defect in a unique MDS patient with rare HbH inclusions in which an abnormal clone lost a greater than 1.9-Mb segment of the telomeric region of the short arm of one allele of chromosome 16, including both α-globin genes. Red blood cell changes associated with this acquired somatic genotype (––/αα) are surprisingly severe, demonstrating that a minor globin chain imbalance may be unexpectedly deleterious during the abnormal erythropoiesis that occurs in the context of MDS.
Introduction
The myelodysplastic syndromes (MDS) are a heterogeneous group of bone marrow disorders characterized by ineffective clonal hematopoiesis, acquired genomic instability, and variable risk for transformation to acute leukemia.1 Most patients with MDS have macrocytic or normocytic anemia, but occasional patients have microcytic red blood cell indices.2 Rarely, MDS patients with striking hypochromia, microcytosis, and anisopoikilocytosis are found to have acquired hemoglobin H (HbH) disease (α-thalassemia); this constellation of findings has been called α-thalassemia myelodysplastic syndrome (ATMDS).3 In most patients with ATMDS, a substantial proportion of red blood cells contain HbH inclusions after supravital staining, and severely decreased α-globin chain synthesis is paralleled by diminished α-globin cytoplasmic and nuclear mRNA levels.3,4
The common inherited forms of α-thalassemia are frequently a consequence of deletions or point mutations affecting the duplicated α-globin genes (αα/αα) on chromosome 16 or, less commonly, deletions of the remote regulatory elements that control α-globin expression.5,6 Germline mutations of ATRX, an X-linked gene encoding a chromatin remodeling protein that regulates the expression of diverse genes, cause mild α-thalassemia associated with developmental abnormalities (ATR-X syndrome).7 ATRX mutations down-regulate the expression of all 4 α-globin genes, but the α-globin cluster itself is normal.
Recently, we have shown that acquired, somatic mutations in the ATRX gene can also underlie ATMDS syndrome.8,9 To date, 12 unique pathologic ATRX mutations have been found in patients with typical features of ATMDS, including striking “thalassemic” blood pictures, substantial amounts (more than 10%) of HbH, and severely reduced α/β globin chain biosynthesis ratios.8,9 However, we have also studied several patients with MDS who have hypochromic, microcytic, red blood cell morphology, but in whom HbH inclusions are only present in a small proportion of erythrocytes or cannot be detected at all. To date, none of these patients have been shown to have mutations in the ATRX gene; the underlying molecular defect remains unknown.
Here we report acquired α-thalassemia and rare HbH inclusions in an MDS patient. In contrast to previously reported cases of ATMDS, an abnormal hematopoietic clone in this patient had an acquired deletion involving the telomeric region of one allele of chromosome 16, removing 2 of the 4 α-genes (ie, genotype ––/αα). This case illustrates a second, less severe mechanism by which α-thalassemia may occur as an acquired abnormality in the context of hematologic malignancy.
Study design
A 72-year-old white British man had microcytic, hypochromic anemia (hemoglobin count, 10.4 g/dL; mean corpuscular volume, 64 fL; mean corpuscular hemoglobin level, 21.7 pg) and neutropenia (leukocyte count, 4.8 × 109/L with 14.7% neutrophils). Two years earlier, he had undergone partial pneumonectomy for localized lung carcinoma; at that time complete blood count findings were normal. The patient had no Mediterranean or Asian ancestors, there was no family history of a hematologic disorder, and iron study results were unremarkable.
Peripheral blood smears demonstrated a dimorphic red blood cell picture with striking anisopoikilocytosis, microcytosis, and severely hypochromic “ghost” erythrocytes (Figure 1A). A bone marrow aspirate was hypercellular, with marked erythroid hyperplasia, trilineage dysplasia, rare ringed sideroblasts, and no excess of myeloblasts (MDS subtype, refractory cytopenia with multilineage dysplasia.) The patient consented to detailed hematologic and genetic analyses of his condition.
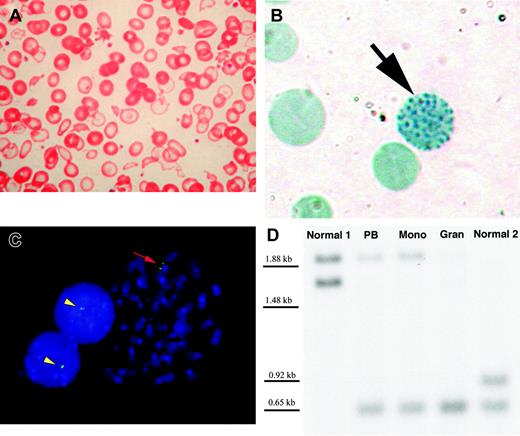
Peripheral blood findings and molecular evidence for loss of the telomeric region of chromosome 16p in a patient with acquired hemoglobin H and myelodysplastic syndrome. (A) Wright-Giemsa–stained peripheral blood smear demonstrates severe anisopoikilocytosis with hypochromic “ghost cells” almost completely devoid of hemoglobin (original magnification × 400). (B) Brilliant cresyl blue stain of peripheral blood reveals a “golf ball” cell (black arrow) with classical HbH inclusions (original magnification × 1000). Such cells represented only 0.11% of anucleate erythrocytes in the patient but were observed on multiple occasions. HbH-containing cells are not present in healthy persons. (C) Metaphase spread and 2 interphase nuclei from the patient's bone marrow hybridized with probe GG1 (green signal) reveals GG1 signal on the normal chromosome 16 only (original magnification, × 1000). Yellow arrowheads indicate the single GG1 signal in interphase, corresponding to the normal chromosome 16 short arm. Red arrow denotes the normal chromosome 16 homologue at metaphase, with paired GG1 signals on sister chromatids. (D) Southern blot of the 3′ hypervariable region in the alpha-globin cluster shows 2 bands for the 2 healthy controls (1 from each allele, corresponding to the normal diploid chromosome 16 short arm). In contrast, the second (upper) band from the patient's sample shows reduced probe hybridization. PB indicates DNA derived from unfractionated peripheral blood; Mono, DNA from the mononuclear cell fraction (predominantly lymphocytes); and Gran, the granulocyte-enriched fraction. The superior band is virtually absent from the patient's granulocyteenriched DNA, corresponding to the loss of an alpha-globin cluster in these cells. However, both bands are present in the mononuclear fraction, which is likely to be enriched for normal cells. In the patient's unfractionated peripheral blood, the superior band is less intense than the corresponding band in the mononuclear fragment, consistent with the expected admixture of clonal and nonclonal cells in unfractionated blood.
Peripheral blood findings and molecular evidence for loss of the telomeric region of chromosome 16p in a patient with acquired hemoglobin H and myelodysplastic syndrome. (A) Wright-Giemsa–stained peripheral blood smear demonstrates severe anisopoikilocytosis with hypochromic “ghost cells” almost completely devoid of hemoglobin (original magnification × 400). (B) Brilliant cresyl blue stain of peripheral blood reveals a “golf ball” cell (black arrow) with classical HbH inclusions (original magnification × 1000). Such cells represented only 0.11% of anucleate erythrocytes in the patient but were observed on multiple occasions. HbH-containing cells are not present in healthy persons. (C) Metaphase spread and 2 interphase nuclei from the patient's bone marrow hybridized with probe GG1 (green signal) reveals GG1 signal on the normal chromosome 16 only (original magnification, × 1000). Yellow arrowheads indicate the single GG1 signal in interphase, corresponding to the normal chromosome 16 short arm. Red arrow denotes the normal chromosome 16 homologue at metaphase, with paired GG1 signals on sister chromatids. (D) Southern blot of the 3′ hypervariable region in the alpha-globin cluster shows 2 bands for the 2 healthy controls (1 from each allele, corresponding to the normal diploid chromosome 16 short arm). In contrast, the second (upper) band from the patient's sample shows reduced probe hybridization. PB indicates DNA derived from unfractionated peripheral blood; Mono, DNA from the mononuclear cell fraction (predominantly lymphocytes); and Gran, the granulocyte-enriched fraction. The superior band is virtually absent from the patient's granulocyteenriched DNA, corresponding to the loss of an alpha-globin cluster in these cells. However, both bands are present in the mononuclear fraction, which is likely to be enriched for normal cells. In the patient's unfractionated peripheral blood, the superior band is less intense than the corresponding band in the mononuclear fragment, consistent with the expected admixture of clonal and nonclonal cells in unfractionated blood.
Fresh peripheral blood was incubated for 4 hours with 1% brilliant cresyl blue in 0.9% NaCl and was examined for intraerythrocytic HbH inclusions. Hemoglobin subtype analysis was performed by an automated high-performance liquid chromatography unit (Bio-Rad, Hemel Hempstead, United Kingdom) using a manufacturer-recommended protocol.
Globin chain synthesis was measured by labeling an enriched reticulocyte preparation derived from fresh heparinized marrow with 3H leucine, precipitating globin chains, and measuring radioactivity on a liquid scintillation counter, as previously described.10
Conventional G-banded cytogenetic analysis of unstimulated bone marrow was followed by fluorescence in situ hybridization (FISH) studies of metaphase spreads and interphase nuclei using standard hybridization protocols with cosmid probes specific for the telomeric region of the short arm of chromosome 16, including CRA36, GG1,11 415C1, and 439A6 (the most proximal of the 4 cosmids, mapping 1.9 Mb from the telomere).
Peripheral blood was separated into granulocyte-enriched and mononuclear cell–enriched fractions by double-density Ficoll-Hypaque (Sigma, St Louis, MO) centrifugation, and DNA was extracted from each fraction using the phenol-chloroform method. For analysis of the α-globin 3′ hypervariable region (an array of 17-bp tandem repeats with high interallelic variability located 8 kb downstream of the α-globin gene complex12 ), DNA was digested with HinfI restriction endonuclease, and the fragments were electrophoresed through a 1% agarose gel and identified by blot hybridization using a 32P-labeled 4.0-kb HinfI fragment as a probe and autoradiographed. The 5′ hypervariable region (100 kb upstream of the α-globin genes13 ) was analyzed in similar fashion after DNA digestion with AluI. To probe the α and ζ genes directly, DNA was digested with BamHI and BglII and was hybridized on separate blots with radiolabeled 0.5-kb HindIII PstI α-globin probe and ζ globin probe and then autoradiographed.
Haplotype analysis of the β-globin gene cluster was performed on unfractionated blood and granulocyte and mononuclear cell fractions as previously described,14 with the addition of the XmnI restriction site at position –158. The remaining α-globin genes were sequenced to exclude point mutations15 using the BigDye (ABI, Foster City, CA) technique.
Results and discussion
Severe microcytosis and hypochromia is unusual in MDS in the absence of iron deficiency but is typical of ATMDS.3 In the present case, a hypochromic microcytic blood film (Figure 1A) prompted further study to elucidate the underlying molecular defect. In contrast to most previously reported cases of ATMDS in which patients who have not undergone transfusion have had more than 10% HbH-containing red blood cells, here supravital staining demonstrated only 0.11% erythrocytes containing HbH inclusions (Figure 1B). Hemoglobin electrophoresis showed 2.0% hemoglobin A2 and 1.4% hemoglobin F; no clear peak corresponding to HbH was observed. The patient's α/β globin chain synthesis ratio was reduced to 0.81 (normal, 0.90-1.20), a value typical of heterozygous α-thalassemia (genotypes –α/αα or ––/αα).
The patient's marrow karyotype was complex and included a rearrangement of the telomeric region of the short arm of chromosome 16: 45, X, –Y, add(3)(q?25), der(3)add(3)(?q25)del(3)(q?25), del(4)(p?15), der(5)add(5)(q?31)del(5)(p?14), del(6)(p?21), –7, der(11)add(p?11)add(q?23), der(13)t(13;21)(p11;q11), der(16)add (p?13)add(q?22), –18, –20, –21, +4amr [cp10]/46, XY[1]. FISH studies with the 4 cosmid probes demonstrated signals on the normal chromosome 16 only, indicating that the deletion extended at least 1.9 Mb from the 16p telomere (Figure 1C).
Southern blot analysis of DNA from the patient's unfractionated blood with16p-specific polymorphic markers12 demonstrated moderately reduced intensity of 1 of the 2 bands corresponding to the closely linked α-globin alleles when compared with healthy controls (Figure 1D). Using DNA isolated from the patient's purified granulocytes, the band corresponding to the abnormal allele was almost absent, consistent with deletion of 1 of 2 α-globin clusters, but the band was preserved in the mononuclear cells (Figure 1D). In MDS, involvement of myeloid lineages, including granulocytes, in the neoplastic clone is more common than involvement of lymphocytes,16 and it is likely that the granulocyte fraction in this patient was enriched for the chromosomal abnormality compared with unfractionated blood and the mononuclear cell fraction. Southern blots using probes for the α and ζ genes also demonstrated reduced band intensity compared with healthy controls (data not shown), consistent with the loss of 1 copy of the entire α-globin cluster in myeloid cells. Therefore, the MDS clone exhibited a thalassemic (––/αα) genotype despite a normal (αα/αα) constitutional genotype. Haplotype analysis of the β globin gene cluster was unremarkable, demonstrating no loss of heterozygosity, and the sequence of the remaining α1 and α2 genes in granulocyte DNA was also normal (data not shown).
Genomic instability in MDS is well recognized, though the underlying etiology remains unclear.17,18 It is probable that the clonal chromosomal loss in the present MDS patient was a random genetic event, manifest as a dramatic red blood cell phenotype as a consequence of loss of 2 α-globin genes; 16p13 is not a common breakpoint in MDS.19 Other examples of acquired red blood cell abnormalities associated with MDS include loss of glycosylphosphatidylinositol (GPI)–anchored proteins including CD55 and CD59,20 enzymopathies (especially pyruvate kinase deficiency),21 membrane defects including elliptocytosis,22 and blood group isotype changes including exposure of cryptantigens.23,24
To our knowledge this is the first report of a patient with ATMDS in whom an acquired α-globin deletion was demonstrable. This case illustrates that the loss of a single α-globin cluster can cause a dramatic acquired thalassemic phenotype in MDS. It is unclear why the red blood cell changes in this patient are more severe than one would normally see associated with the loss of only 2 of 4 α genes (––/αα); this may reflect an interaction between minor globin chain imbalance and the already markedly disordered hematopoiesis in MDS. Further molecular characterization of ATMDS patients will help define the mechanisms responsible for this interesting association and may explain the unexpectedly severe red blood cell phenotype.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-09-3222.
V.V., J.L., R.J.G., and D.R.H. are supported by the Medical Research Council.
D.P.S. is a Mayo Foundation Research Scholar supported by the Mayo Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Helena Ayyub for technical assistance and Veronica J. Buckle for comments on the manuscript.