Abstract
The response of the arterial vascular wall to injury is characterized by vascular smooth muscle cell (VSMC) migration, a process requiring metalloproteinase production. This migration is induced by cytokines, however the agonists involved are not fully defined. The CC chemokine receptor 8 (CCR8) is expressed on monocytes and T lymphocytes and is the sole receptor for the human CC chemokine 1 (CCL1, I-309) and for the viral chemokine, vCCL1 (viral macrophage inflammatory protein 1 [vMIP-1]). We have reported that CCR8 is expressed on human umbilical vein endothelial cells (HUVECs) and mediates chemotaxis induced by CCL1. The conditioned medium from incubation mixtures of lipoprotein(a) (Lp(a)) and HUVECs (LCM) contained CCL1 and stimulated both monocyte and HUVEC chemotaxis, providing novel mechanisms for the atherogenicity of Lp(a). We now report that CCL1, vCCL1, and LCM stimulate chemotaxis of human VSMCs that is blocked by murine monoclonal antibody against CCR8 and by the G-protein inhibitor pertussis toxin. The effect of anti-CCR8 was specific, as this antibody failed to effect the chemotaxis of VSMCs in response to CCL3 or by platelet-derived growth factor BB (PDGF-BB). VSMCs contained CCR8 mRNA and CCR8 antigen coprecipitated with VSMC membranes. Antibodies against metalloproteinase-2 (MMP-2) inhibited the CCL1-induced chemotaxis of VSMCs, whereas anti–MMP-9 was less effective. CCL1 induced VSMC pro–MMP-2 mRNA and protein secretion. Poxvirus MC148 inhibited the increase in MMP-2 induced by CCL1, documenting that CCR8 was the receptor responsible. In mouse femoral arteries, CCR8 and TCA3 antigen colocalized with VSMCs and were up-regulated after injury. The induction of CCR8 and CCL1/TCA3 under conditions associated with VSMC proliferation and migration raises the possibility that CCR8 may play an important role in vessel wall pathology.
Introduction
Vascular smooth muscle cell (VSMC) migration, proliferation, and extracellular matrix remodeling are hallmarks of the response of the arterial wall to injury.1,2 Several different cytokines identified in the atherosclerotic plaque induce VSMC migration, including vascular endothelial growth factor (VEGF),3 tumor necrosis factor α (TNF-α),4 and platelet-derived growth factor (PDGF).5 Recent data suggest that CC chemokines may also stimulate VSMCs.6 CC chemokines are a family of closely related proteins characterized by 2 adjacent cysteine residues that function as leukocyte activators and chemoattractants in inflammatory reactions.7,8 CC chemokines bind to G-protein–coupled receptors that comprise a family of 7 transmembrane spanning domains.9 These receptors were first described on leukocytes, where they function to induce chemotaxis, proliferation, and cell activation.7,10,11 VSMCs have been shown to possess CC chemokine receptor 5 (CCR5) mRNA and protein.6 CCR5 was functional as the smooth muscle cells (SMCs) responded to its ligand, CC chemokine ligand 4 (CCL4, macrophage inflammatory protein 1β [MIP-1β]), with increases in intracellular calcium concentration [Ca2+] and in tissue factor activity.6 This study was unable to identify mRNA for CCR1, CCR2, CCR3, CCR4, and TARC (CCL17) in human VSMCs. Luo et al12 have shown that TCA3, CCL3 (MIP-1α), and CCL2 (MCP-1) induce chemotaxis in rat aortic smooth muscle cells. TCA3 is a murine protein that has partial structural homology with CCL1 (I-309),13 however CCL1 is a monocyte/macrophage and T-cell chemoattractant,14,15 whereas TCA3 is chemotactic for neutrophils.16
CC chemokine receptor 8 (CCR8) has been characterized and is expressed on monocytes and T lymphocytes.15,17-20 CCR8 is a G-protein–coupled 7-transmembrane receptor that is the unique receptor for the human CC chemokine CCL1 and for viral monocyte inflammatory protein-1, vCCL1 (vMIP-1) a human chemokine homolog induced by human herpesvirus-8 (HHV8) that has been directly linked to Kaposi sarcoma.21-23 We have reported CCR8 mRNA in human umbilical vein endothelial cells (HUVECs).24 CCR8 was found to be a functional endothelial receptor as it mediated endothelial chemotaxis in response to CCL1 and vCCL1, and this response was inhibited by monoclonal antibody directed against the extracellular portion of CCR8 as well as by the Gi-protein inhibitor pertussis toxin.24 CCR8 has also been identified on HUVECs by Bernardini et al25 and has been shown to induce angiogenesis when stimulated by CCL1. Immunohistochemical studies demonstrated CCR8 antigen on the luminal endothelial surface of human atherosclerotic plaques as well as associated with endothelial-derived spindle cells in biopsy samples of human Kaposi sarcoma.24
CCL1, the CCR8 ligand, is a CC chemokine secreted by activated monocytes and lymphocytes13,26 and is a potent chemoattractant for both of these cell types.14,27 We have shown that the atherogenic lipoprotein, lipoprotein(a) (Lp(a)), induced HUVECs to secrete a monocyte chemoattractant activity.28 CCL1 has been identified as the principal monocyte chemoattractant secreted by HUVECs when incubated with Lp(a) or with the apolipoprotein(a) (apo(a)) portion of Lp(a).29 We have reported that the conditioned medium from HUVECs incubated with Lp(a), apo(a), or a 6 kringle-containing homolog from the C-terminal portion of apo(a) is chemotactic for HUVECs and that CCL1 is the chemoattractant responsible.24
We now report that human aortic VSMCs contain CCR8 mRNA and express CCR8 antigen on their membranes. CCR8 was functional since CCL1, vCCL1, and the conditioned medium from Lp(a)-HUVEC incubation mixtures induced VSMC chemotaxis that was inhibited by a neutralizing monoclonal antibody against CCR8. CCL1 induced an inflammatory phenotype in VSMCs as evidenced by its capacity to increase the concentration of metalloproteinase-2 (MMP-2) but not of metalloproteinase-9 (MMP-9) in the conditioned medium of VSMCs. CCL1 also induced MMP-2 mRNA. MC148, a specific CCR8 antagonist, inhibited the induction of MMP-2 by CCL1. The first description of MC148 reported that it antagonized chemotaxis induced by a large number of CC and CXC chemokines with diverse receptor specificities.30 Subsequent studies found MC148 to be specific for human CCR8 (“Discussion”). CCR8 and TCA3 were up-regulated and colocalized with VSMCs at day 5 after injury, a time when VSMCs are proliferating and migrating. These studies have expanded the role of the CCR8-CCL1 system in inflammatory vessel wall disease. They also suggest that the capacity of Lp(a) to induce endothelial CCL1 may provide a link between this lipoprotein and the activation and migration of VSMCs.
The expression of CCR8 by cells that participate in the response of the vessel wall to injury including endothelial and vascular SMCs, monocyte/macrophages, and T helper 2 lymphocytes suggests that this receptor may play an important role in vessel wall pathology.
Materials and methods
Proteins and antibodies
Recombinant CCL1 (I-309), viral vCCL1 (vMIP-1), CCL3 (MIP-1α), platelet-derived growth factor–BB (PDGF-BB), goat polyclonal antibody against CCL1, as well as immunoglobulin controls were obtained from R&D Systems (Minneapolis, MN). Poxvirus MC148 was obtained from Eurogenetic North America (Philadelphia, PA). Polyclonal antibodies against MMP-2 and MMP-9 were from Santa Cruz Biotechnology (Santa Cruz, CA), and murine monoclonal antibodies against MMP-2 (clone 42-5011) or MMP-9 (clone 6-613) and metalloproteinase standards were from Oncogene Research Products (Cambridge, MA). Polyclonal anti-CCR8 and monoclonal antibody against CCR5, the CCL3 receptor, were from Alexis Biochemicals (San Diego, CA). Antibodies used in immunohistochemical studies included the following: rat antimouse macrophages/monocytes (MOMA-2; Biosource International, Camarillo CA); alkaline phosphatase-conjugated monoclonal anti–smooth muscle α-actin (Sigma-Aldrich, St Louis, MO), TCA3 (hamster antimouse monoclonal antibody; Pharmingen, San Diego, CA), and CCR8 (goat polyclonal antimouse CCR8; Novus Biologicals, Littleton, CO). A murine monoclonal antibody against a 26–amino acid–containing peptide from the extracellular N-terminal portion of CCR8 (VTDYYYPDIFSSPCDALEIQTNGKLC)31,32 has been produced as described.24 Lp(a) was purified as detailed previously.28
Cell culture
Human umbilical vein endothelial cells (HUVECs) were cultured and incubated with Dulbecco modified Eagle medium (DMEM) or with Lp(a) as described.28 Human aortic smooth muscle cells (VSMCs) were obtained from American Type Culture Collection (Manassas, VA) and grown according to the manufacturer's instructions.
VSMC chemotaxis activity
VSMC chemotaxis was measured as described for HUVECs.24 The filter was treated with fibronectin (10 μg/mL; Sigma-Aldrich) for 30 minutes at 37°C prior to the assay. All samples were tested in triplicate.
Murine monoclonal antibody against CCR8 or its immunoglobulin G1 (IgG1) isotypic control was incubated with VSMCs (1 μg/mL) in 6-well tissue culture plates and incubated for 30 minutes at 37°C prior to harvesting the cells to study whether CCR8 mediated VSMC chemotaxis. In other studies, goat polyclonal antibody against CCL1 or a nonspecific IgG control was incubated with CCL1, HUVECs (CM), or the conditioned medium from the Lp(a)-HUVEC incubation (LCM) for 30 minutes at 37°C prior to adding to the bottom wells of the chemotaxis chamber to establish whether CCL1 was the responsible VSMC chemoattractant.
VSMCs were cultured in the presence of 0.1 μg/mL pertussis toxin (Biological Laboratories, Campbell, CA) or 0.4 μg/mL cholera toxin (Sigma-Aldrich) for 16 hours prior to the chemotaxis assay to determine whether CCL1-induced chemotaxis of VSMC was mediated by a Gi-protein–coupled receptor.33
Antibodies against MMP-2 or MMP-9 (500 ng/mL) were added to the lower wells of the chemotaxis chamber containing CCL1 or LCM to determine the role of these metalloproteinases in CCL1-induced chemotaxis.
Northern blot analysis
Northern blot analysis was performed as described earlier.24 The CCR8 probe was a 1118-kb reverse transcriptase–polymerase chain reaction (RT-PCR) fragment amplified from stimulated HUVECs. As a control, filters were hybridized with cDNA encoding the constitutively expressed glyceraldehyde-3-phosphate-dehydrogenase (G3PDH).
Sequence analysis of VSMC CCR8 cDNA
The CCR8 cDNA was amplified by RT-PCR as described in detail.24 The identity of the fragment isolated from human VSMCs as CCR8 mRNA was confirmed by sequence analysis (GenBank accession number, U62556). Superscript II was excluded from the control RT-PCR reaction to ensure that no DNA contamination was present.
Reverse transcriptase–polymerase chain reaction (RT-PCR) and real-time quantitative PCR
Total RNA was isolated using Rneasy mini kit from Qiagen (Chatworth, CA) and RT-PCR reaction was performed as described earlier.29 The MMP-2 oligonucleotide primers (Qiagen Operon, Alameda, CA) were designed as described.34 The primers were 5′GTGCTGAAGGACACACTAAAGAAGA-3′ and 5′-TTGCCATCCTTCTCAAAGTTGTAG-3′, and the expected size of the PCR product was 580 bp. Glyceraldehyde-3 phosphate dehydrogenase (G3PDH) was used as an internal control.
Real-time quantitative PCR was performed by monitoring the increase in fluorescence of the SYBR Green dye on an ABI Prism 7000 Sequence Detection system (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. The relative expression level of MMP2 in stimulated cells was plotted as fold change compared with unstimulated cells. All measurements were done in triplicate. The number of mRNA copies in real-time RT-PCR was adjusted with G3PDH.
Gelatin zymography
Human vascular smooth muscle cells were cultured in 6-well tissue culture plates to 90% confluence. Cells were maintained in 0.5% bovine serum albumin (BSA)/DMEM overnight. Cells were then washed twice in phosphate-buffered saline (PBS), and incubated with DMEM or with recombinant CCL1 (100 ng/mL) for 3 hours. To test the effect of MC148 in inhibiting CCL1-induced production of MMP-2, VSMCs were incubated with MC148 (100 ng/mL DMEM) for 30 minutes prior to the addition of CCL1. Supernatants were then collected and their gelatinolytic activity was measured by gelatin zymography as described.35,36 In studies assessing the production of MMP-9, the cell culture supernatants were concentrated with gelatin-agarose beads (Sigma-Aldrich).36 Cell culture supernatants (0.5 mL from 250 000 viable cells, as determined by trypan blue exclusion) were treated with 25 μL gelatin-agarose beads overnight at 4°C and eluted with 0.03 mL sodium dodecyl sulfate (SDS)–containing buffer for gelatin zymography. These samples were electrophoresed on SDS-acrylamide gels containing 1% gelatin and processed as detailed.36 MMP-2 and MMP-9 standards (Oncogene Research Products) were used as positive controls. The zymograms were scanned with an Epson Expression 800 scanner using Adobe Photoshop 6.0 and SilverFast software (Adobe Systems, San Jose, CA and LaserSoft Imaging, Longboat Key, FL, respectively), and the intensity of the gelatinolytic bands was quantified using the National Institutes of Health (NIH, Rockville, MD) Image 1.61f (http://rsb.info.nih.gov/nih-image).
Purification of VSMC membranes and identification of CCR8 by immunoblotting
Human vascular smooth muscle cells were washed with 1 × Hanks buffered saline (HBS)/phenylmethylsulfonyl fluoride (PMSF) and membranes prepared as described.37 The cells were centrifuged for 10 minutes at 1000 rpm. The pellet was resuspended in 2 mL HBS/PMSF, incubated with 2.5 mM diisopropylfluorophosphate (Sigma-Aldrich) for 5 minutes, and then centrifuged for 10 minutes at 2000 rpm. The pellet was resuspended in 0.25 M sucrose containing the proteinase inhibitors pepstatin and leupeptin (Sigma-Aldrich) and was sonicated at 0°C for 5 10-second cycles (out control set between 5-7 and duty cycle at 60-70). Sucrose with inhibitors was added to increase the volume to 30 mL/tube. Centrifugation at 1500 rpm for 10 minutes pelleted nuclei and intact cells. The supernatant-containing VSMC membranes were centrifuged at 4°C for one hour at 45 000 rpm. The resulting pellet was resuspended in CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 15 mM) containing pepstatin and leupeptin and incubated for one hour at room temperature. The solubilized membranes were centrifuged at 70 000 rpm (Beckman 100, rotor TLA100 for one hour at 4°C; Beckman Coulter, Hialeah, FL). The solubilized membranes were assayed by Western blot as described.24 After blocking in 1% BSA/1% Tween-20 in PBS, murine monoclonal antibody against CCR8 was added (0.4 μg/mL) and detected by sheep anti–mouse IgG horseradish peroxidase (1:5000; Sigma-Aldrich). Detection employed the enhanced chemiluminescence (ECL) detection system from Amersham Pharmacia Biotech (Piscataway, NJ).
Arterial injury
Mice were housed at the Center for Laboratory Animal Sciences at the Mount Sinai Medical Center (New York, NY). Procedures and animal care were approved by the Institutional Animal Care and Use Committee and were in accordance with the “Guide for Care and Use of Laboratory Animals.” Mice underwent femoral artery transluminal injury by passage of a 0.25-mm diameter angioplasty guidewire, as previously described.38 Mice were killed at the times indicated by pentobarbital overdose.
Immunohistochemistry
Femoral arteries were perfusion fixed with 4% paraformaldehyde at 100 mmHg and sections obtained as described.38,39 We have previously demonstrated that this injury technique results in complete endothelial denundation.38 Serial sections (10) were stained for CCR8, TCA3, SMC, and macrophages (MOMA-2). The primary antibody was substituted with an irrelevant antibody of the same isotype as negative controls. The tissue sections were processed and immunostained for using an avidin-biotin technique.40 Tissue sections were blocked with ovalbumin, or in some instances with normal rabbit or horse serum prior to the addition of the specific detecting antibody. Antigen retrieval was used on some tissues.41 A positive control, a nonimmune negative control, and processing controls were performed for each antigen stain. Preabsorption of the CCR8 and TCA3 antibody yielded negative staining.
Statistical analysis
Results of data are reported as the mean ± standard deviation. Levels of significance were determined by 2-tailed Studentt test.
Results
CCL1 (I-309) and vCCL1 (vMIP-1) induce chemotaxis of humanvascular smooth muscle cells (VSMCs)
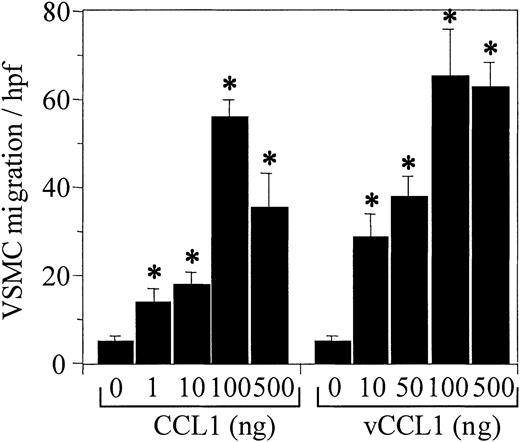
We have studied VSMC chemotaxis in response to the specific CCR8 ligands, human CC chemokine CCL1 (I-309), and the Kaposi sarcoma–associated viral CC chemokine vCCL1 (vMIP-1) to establish that functional CCR8 was expressed on VSMCs. Both ligands induced chemotaxis of VSMCs (Figure 1). CCL1 and vCCL1 stimulated a 14-fold and 21-fold increase in VSMC migration, respectively, compared with the control (55 ± 4 and 85 ± 11 versus 4 ± 1.4 VSMCs per high-power field). The maximum response occurred at a chemokine concentration of 100 ng/mL (∼ 10 nM), a concentration that also induced maximum chemotactic response in cell lines transfected with CCR8.17 The chemotactic response of VSMCs to increasing concentrations of CCL1 and vCCL1 was bimodal, a typical finding for a chemotactic response to CC chemokines.
CCL1 (I-309) and vCCL1 (vMIP-1) induce chemotaxis of human vascular smooth muscle cells (VSMCs). Varying concentrations of either CCL1 or vCCL1 were placed in the lower wells, and cultured VSMCs were added to the upper wells of the Neuroprobe chemotaxis chambers. The Neuroprobe membrane (5-μm pores) was coated with fibronectin. Incubation, staining, and counting of the cells was performed as described in “Materials and methods.” Both CCL1 and vCCL1 induced chemotaxis of VSMCs in a bell-shaped distribution, characteristic of a chemotactic response. *P < .001 comparing VSMCs stimulated with either CCL1 or vCCL1 against cells stimulated with buffer. (hpf indicates high-power field.) Error bars represent SD.
CCL1 (I-309) and vCCL1 (vMIP-1) induce chemotaxis of human vascular smooth muscle cells (VSMCs). Varying concentrations of either CCL1 or vCCL1 were placed in the lower wells, and cultured VSMCs were added to the upper wells of the Neuroprobe chemotaxis chambers. The Neuroprobe membrane (5-μm pores) was coated with fibronectin. Incubation, staining, and counting of the cells was performed as described in “Materials and methods.” Both CCL1 and vCCL1 induced chemotaxis of VSMCs in a bell-shaped distribution, characteristic of a chemotactic response. *P < .001 comparing VSMCs stimulated with either CCL1 or vCCL1 against cells stimulated with buffer. (hpf indicates high-power field.) Error bars represent SD.
Checkerboard analysis was performed to document that the migration of VSMCs in response to CCL1 was chemotactic (directed), rather than chemokinetic (nondirected) (Figure 2). Maximum cell chemotaxis occurred with CCL1 in the bottom wells, and no significant migration compared with the control media was observed when the ligand was in both top and bottom wells, indicating that migration of VSMCs was due to chemotaxis rather than to chemokinesis. CCL1 also induced significant transmembrane migration when added with VSMCs in the top wells of the chemotaxis chamber. This response may be due to reverse chemotaxis, as recently described for T lymphocytes.42
Checkerboard analysis documents that CCL1 induces chemotaxis of VSMCs. CCL1 (100 ng/mL) was placed in both top and bottom wells, only in the top well (also containing VSMCs), or only in the bottom wells of the chemotaxis chamber. CCL1 in the bottom chamber induced the greatest number of migrating VSMCs compared with the buffer control (*P = .00 003); however, the activity induced by CCL1 in the top chamber was also significantly increased (**P = .003). Comparison of the migration of VSMCs showed a significant difference between having the agonist in the top versus the bottom chamber (P = .001). The migration of cells when the agonist was in both top and bottom wells was not different than the control (not significant [NS]). These findings document that the chemotaxis of VSMCs in response to CCL1 is mainly directed or chemotactic. Error bars represent SD.
Checkerboard analysis documents that CCL1 induces chemotaxis of VSMCs. CCL1 (100 ng/mL) was placed in both top and bottom wells, only in the top well (also containing VSMCs), or only in the bottom wells of the chemotaxis chamber. CCL1 in the bottom chamber induced the greatest number of migrating VSMCs compared with the buffer control (*P = .00 003); however, the activity induced by CCL1 in the top chamber was also significantly increased (**P = .003). Comparison of the migration of VSMCs showed a significant difference between having the agonist in the top versus the bottom chamber (P = .001). The migration of cells when the agonist was in both top and bottom wells was not different than the control (not significant [NS]). These findings document that the chemotaxis of VSMCs in response to CCL1 is mainly directed or chemotactic. Error bars represent SD.
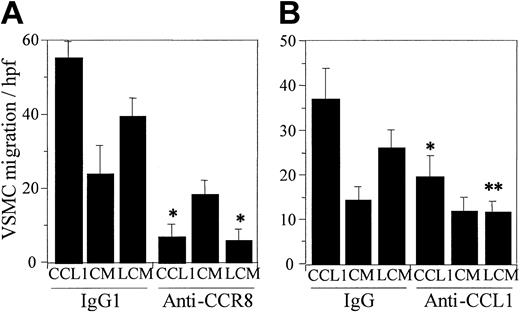
The conditioned medium from incubation mixtures of Lp(a) and HUVECs induces CCR8-dependent migration of VSMCs
Our previous study documented that the CM from HUVECs incubated with Lp(a) or with the apolipoprotein(a) portion of Lp(a) induced chemotaxis of both monocytes and HUVECs.24,29 The chemoattractant activity was shown to be due to the secretion of CCL1 by endothelial cells and the subsequent interaction of CCL1 with CCR8. We have now examined the chemotactic response of VSMCs to CCL1, to HUVEC CM, or to Lp(a)-HUVEC CM (LCM). Both CCL1 and the LCM produced a vigorous chemotactic response (Figure 3A). HUVEC CM demonstrated constitutive chemotactic activity that was significantly less than the LCM (P = .006) (Figure 3A). Lp(a) added directly to the chemotactic chamber did not induce VSMC chemotaxis. The murine monoclonal antibody against a synthetic peptide from the N-terminal extracellular domain of CCR8 inhibited VSMC chemotaxis in response to CCL1 or LCM. This antibody, however, did not inhibit the VSMCs' migration induced by HUVEC CM.
CCL1 and the conditioned medium resulting from incubation of Lp(a) with human umbilical vein endothelial cells induce chemotaxis of VSMCs that is inhibited by anti-CCR8 and anti-CCL1. CCL1 (100 ng/mL) or the CM obtained following incubation of DMEM (CM) or Lp(a) (150 μg/mL) (LCM) with HUVECs for 6 hours at 37°C was tested for VSMC chemotaxis as described in “Materials and methods.” Murine monoclonal antibody against CCR8, the isotypic IgG1 control (A), or polyclonal goat anti-CCL1 and the goat IgG control (B), all at a concentration of 1 μg/mL, were also added to the bottom wells of the chemotaxis chamber. (A) Anti-CCR8 significantly inhibited chemotaxis of VSMCs induced by CCL1 and LCM (*P < .00 001). The cell-stimulating activity of LCM was significantly greater than that of CM (P = .005). Inhibition of CM by anti-CCR8 was of borderline significance (P = .05). Neither DMEM nor Lp(a) added to the lower wells of the chemotaxis chamber induced significant VSMC chemotaxis (4.3 ± 1.4 and 7.1 ± 2.2 cells/high power field (hpf), respectively). These findings indicate that CCR8 mediates chemotaxis of VSMCs. (B) Anti-CCL1 significantly inhibited chemotaxis of VSMCs induced by CCL1 and LCM (*P = .007 and **P = .002, respectively). CM was not significantly inhibited by anti-CCL1. The cell-stimulating activity of LCM was significantly greater than that of CM (P = .01). DMEM and Lp(a) alone induced 7.0 ± 3.3 and 11.7 ± 2.6 cells/high power field, respectively. These data demonstrate that the chemotaxis of VSMCs induced by LCM is due to the presence of CCL1. Error bars represent SD.
CCL1 and the conditioned medium resulting from incubation of Lp(a) with human umbilical vein endothelial cells induce chemotaxis of VSMCs that is inhibited by anti-CCR8 and anti-CCL1. CCL1 (100 ng/mL) or the CM obtained following incubation of DMEM (CM) or Lp(a) (150 μg/mL) (LCM) with HUVECs for 6 hours at 37°C was tested for VSMC chemotaxis as described in “Materials and methods.” Murine monoclonal antibody against CCR8, the isotypic IgG1 control (A), or polyclonal goat anti-CCL1 and the goat IgG control (B), all at a concentration of 1 μg/mL, were also added to the bottom wells of the chemotaxis chamber. (A) Anti-CCR8 significantly inhibited chemotaxis of VSMCs induced by CCL1 and LCM (*P < .00 001). The cell-stimulating activity of LCM was significantly greater than that of CM (P = .005). Inhibition of CM by anti-CCR8 was of borderline significance (P = .05). Neither DMEM nor Lp(a) added to the lower wells of the chemotaxis chamber induced significant VSMC chemotaxis (4.3 ± 1.4 and 7.1 ± 2.2 cells/high power field (hpf), respectively). These findings indicate that CCR8 mediates chemotaxis of VSMCs. (B) Anti-CCL1 significantly inhibited chemotaxis of VSMCs induced by CCL1 and LCM (*P = .007 and **P = .002, respectively). CM was not significantly inhibited by anti-CCL1. The cell-stimulating activity of LCM was significantly greater than that of CM (P = .01). DMEM and Lp(a) alone induced 7.0 ± 3.3 and 11.7 ± 2.6 cells/high power field, respectively. These data demonstrate that the chemotaxis of VSMCs induced by LCM is due to the presence of CCL1. Error bars represent SD.
We also examined whether CCL1 was responsible for the chemotactic effect of the LCM. CCL1, CM, or LCM was preincubated for 30 minutes at 37°C with an immunospecific polyclonal antibody against CCL1 or a nonspecific IgG control (Figure 3B). The chemotactic response of the VSMCs in this experiment was somewhat less than those used in the anti-CCR8 study (Figure 3A). The antibody against CCL1 significantly inhibited the chemotactic activity of both CCL1 and the LCM compared with the immunoglobulin control, but did not inhibit the chemotaxis of VSMCs in response to CM. These data confirm that CCL1 was the chemoattractant for VSMCs in the CM of Lp(a)-stimulated HUVECs.
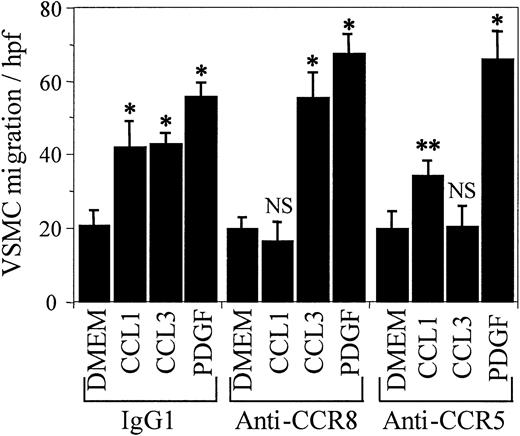
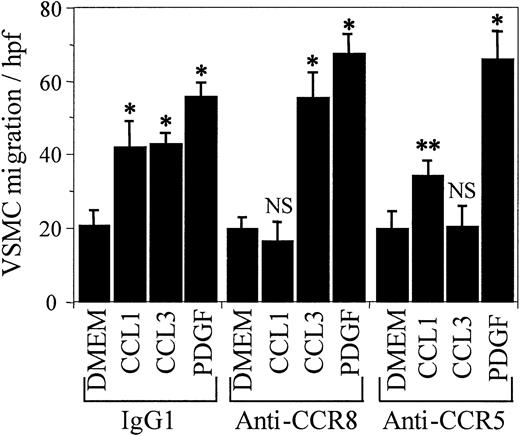
As an additional confirmation of the specificity of anti-CCR8 in inhibiting VSMC chemotaxis by blocking CCL1 interaction with its receptor, other VSMC chemoattractants were examined (Figure 4). Anti-CCR8 that inhibited chemotaxis of VSMCs in response to CCL1 did not inhibit chemotaxis to either CCL3 (MIP-1α) or to platelet-derived growth factor–BB (PDGF-BB). In addition, antibody against CCR5, a receptor for CCL3, did not inhibit the chemotactic response of VSMCs to either CCL1 or to PDGF-BB, but did inhibit CCL3-induced chemotaxis.
The inhibition of CCL1-induced chemotaxis of VSMCs by anti-CCR8 is specific. Media, CCL1 (100 ng/mL), CCL3 (MIP-1α; 100 ng/mL), or PDGF-BB (10 ng/mL) was added to the bottom wells of the chemotaxis chamber. VSMCs, in the presence of IgG1, anti-CCR8, or anti-CCR5 (1 μg/mL), were added to the top wells and the chemotaxis assay was performed as detailed in “Materials and methods.” IgG1, the isotypic antibody control, did not inhibit the chemotactic response of human VSMCs to CCL1 (C1), CCL3, or PDGF-BB (*P < .005). Anti-CCR8 completely inhibited CCL1-induced VSMC chemotaxis but had no inhibitory effect on the chemotaxis induced by CCL3 or by PDGF (P < .005). Antibody directed against the CCL3 receptor CCR5 inhibited the chemotactic response of VSMCs to CCL3 but not to either CCL1 (**P < .003) or to PDGF-BB (*P < .005). This study shows that the inhibition of chemotaxis by anti-CCR8 is specific and not due to a hindrance of chemotaxis due to the attachment of an antibody to a cell-surface receptor. Error bars represent SD.
The inhibition of CCL1-induced chemotaxis of VSMCs by anti-CCR8 is specific. Media, CCL1 (100 ng/mL), CCL3 (MIP-1α; 100 ng/mL), or PDGF-BB (10 ng/mL) was added to the bottom wells of the chemotaxis chamber. VSMCs, in the presence of IgG1, anti-CCR8, or anti-CCR5 (1 μg/mL), were added to the top wells and the chemotaxis assay was performed as detailed in “Materials and methods.” IgG1, the isotypic antibody control, did not inhibit the chemotactic response of human VSMCs to CCL1 (C1), CCL3, or PDGF-BB (*P < .005). Anti-CCR8 completely inhibited CCL1-induced VSMC chemotaxis but had no inhibitory effect on the chemotaxis induced by CCL3 or by PDGF (P < .005). Antibody directed against the CCL3 receptor CCR5 inhibited the chemotactic response of VSMCs to CCL3 but not to either CCL1 (**P < .003) or to PDGF-BB (*P < .005). This study shows that the inhibition of chemotaxis by anti-CCR8 is specific and not due to a hindrance of chemotaxis due to the attachment of an antibody to a cell-surface receptor. Error bars represent SD.
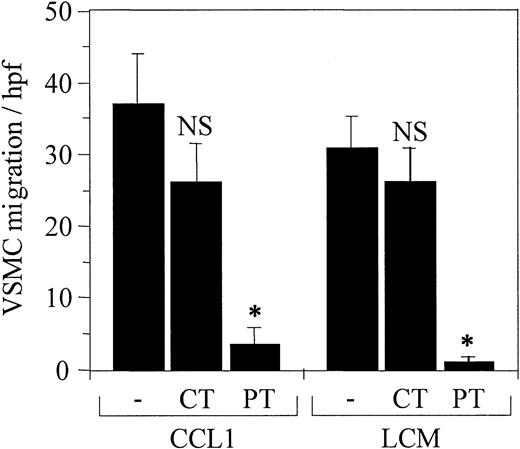
Pertussis toxin inhibits VSMC chemotaxis induced by CCL1 and LCM
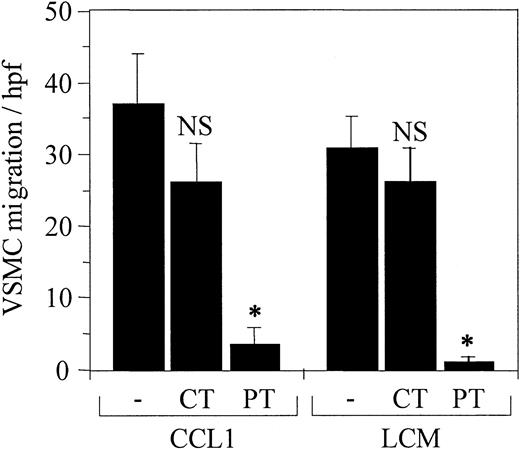
To provide further evidence that the induction of VSMC chemotaxis by CCL1 and LCM was associated with a G-protein–coupled receptor, the VSMCs were treated with the Gi-protein inhibitor pertussis toxin (PT) or with cholera toxin (CT) that does not inhibit Gi-protein–coupled reactions (Figure 5). Cholera toxin did not significantly inhibit the migration of VSMCs in response to either CCL1 or LCM. In contrast, pertussis toxin reduced migration induced by both ligands 90% and 97% compared with the controls. These studies indicate the VSMC chemotaxis observed was dependent upon a Gi-protein–linked cell-surface receptor.
Pertussis toxin inhibits VSMC chemotaxis induced by CCL1 and by Lp(a) conditioned medium (LCM). CCL1 and LCM prepared as detailed in the legend for Figure 3 were tested for chemotactic activity using VSMCs pretreated with either pertussis or cholera toxin as described in “Materials and methods.” Pertussis toxin (PT) inhibited VSMC chemotaxis in response to both agonists (*P < .00001), whereas cholera toxin (CT) did not inhibit chemotaxis (NS). These data confirm that Gi-coupled protein receptors are involved in the chemotactic response of VSMCs to CCL1 and LCM. Error bars represent SD.
Pertussis toxin inhibits VSMC chemotaxis induced by CCL1 and by Lp(a) conditioned medium (LCM). CCL1 and LCM prepared as detailed in the legend for Figure 3 were tested for chemotactic activity using VSMCs pretreated with either pertussis or cholera toxin as described in “Materials and methods.” Pertussis toxin (PT) inhibited VSMC chemotaxis in response to both agonists (*P < .00001), whereas cholera toxin (CT) did not inhibit chemotaxis (NS). These data confirm that Gi-coupled protein receptors are involved in the chemotactic response of VSMCs to CCL1 and LCM. Error bars represent SD.
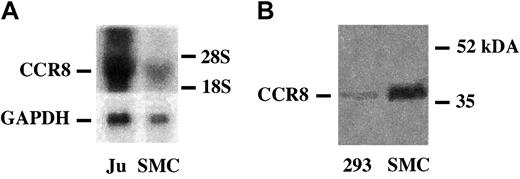
CCR8 mRNA and antigen are expressed in VSMCs
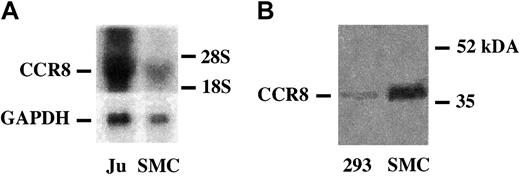
RNA blot analysis with CCR8 cDNA demonstrated a single band in confluent VSMCs corresponding to a band observed in Jurkat cells (Figure 6A). The identity of the DNA transcript as CCR8 was confirmed by sequence analysis as detailed in “Materials and methods” (GenBank accession number U62556). To identify CCR8 antigen in VSMCs, cell membranes were purified and examined by Western blotting. A polyclonal anti-CCR8 antibody demonstrated a single band at approximately 40 kDa (Figure 6B), which corresponds to the size predicted by the putative coding sequence.
VSMCs contain CCR8 mRNA and membrane-associated CCR8 antigen. (A) RNA blot analysis of CCR8 mRNA in VSMCs. Aliquots (10 μg) of total RNA from Jurkat cells (Ju) or from confluent human VSMCs (SMC) were size-fractionated on agarose gels and hybridized to 32P-labeled human CCR8 cDNA as described in “Materials and methods.” GAPDH is shown as a control for loading of samples. The location of 28 and 19S markers is indicated. The cDNA was generated as detailed in “Materials and methods,” and the identity of the fragment as CCR8 mRNA was confirmed by sequence analysis. (B) Immunoblot analysis of CCR8 in VSMCs. Human VSMC membranes were prepared as detailed in “Materials and methods,” and the solubilized membrane protein and CCR8 expression was analyzed by immunoblot analysis using a polyclonal anti-CCR8, which identified an approximately 40-kDa band.
VSMCs contain CCR8 mRNA and membrane-associated CCR8 antigen. (A) RNA blot analysis of CCR8 mRNA in VSMCs. Aliquots (10 μg) of total RNA from Jurkat cells (Ju) or from confluent human VSMCs (SMC) were size-fractionated on agarose gels and hybridized to 32P-labeled human CCR8 cDNA as described in “Materials and methods.” GAPDH is shown as a control for loading of samples. The location of 28 and 19S markers is indicated. The cDNA was generated as detailed in “Materials and methods,” and the identity of the fragment as CCR8 mRNA was confirmed by sequence analysis. (B) Immunoblot analysis of CCR8 in VSMCs. Human VSMC membranes were prepared as detailed in “Materials and methods,” and the solubilized membrane protein and CCR8 expression was analyzed by immunoblot analysis using a polyclonal anti-CCR8, which identified an approximately 40-kDa band.
Blocking antibodies directed against metalloproteinase-2(MMP-2) inhibit CCL1 and LCM-induced chemotaxis of VSMCs
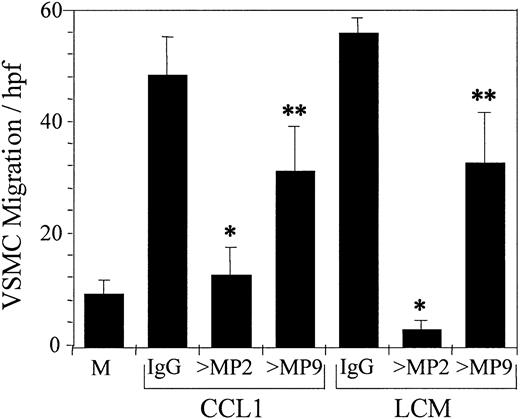
Since MMP-2 and MMP-9 are implicated in the migration of VSMCs following vessel wall injury,43 we have examined the potential role of these metalloproteinases in the chemotaxis of VSMCs induced by CCL1 or by LCM. Anti–MMP-2 and anti–MMP-9 inhibited VSMC chemotaxis induced by CCL1 74% and 33%, respectively (Figure 7). Anti–MMP-2 caused a 95% inhibition of VSMC chemotaxis induced by LCM, and anti–MMP-9 a 42% inhibition of chemotaxis. Monoclonal antibodies against MMP-2 and MMP-9 produced similar results (data not shown).
Antibody against metalloproteinase-2 (MMP-2) inhibits CCL1- and LCM-induced chemotaxis of VSMCs. Mixtures of CCL1 (100 ng/mL) and goat IgG or goat anti–MMP-2 (> MP2) or anti–MMP-9 (> MP9) (1 μg/mL) were added to the bottom wells of the chemotaxis chamber. Similarly, LCM, produced as detailed in the legend to Figure 3, was added in the presence of the same immunoglobulin preparations. The chemotactic activity of these mixtures was tested against VSMCs as detailed in “Materials and methods.” Anti–MMP-2 inhibited chemotaxis induced by CCL1 74.5% and by LCM 95% (*P < .0001), whereas anti–MMP-9 was less effective, inhibiting CCL1- and LCM-induced chemotaxis 32.6% and 41.6%, respectively (**P < .03). Inhibition of VSMC chemotaxis by antibody against MMP-2 was significantly greater than by anti–MMP-9 (P < .007). These studies suggest that MMP-2 is the principal metalloproteinase involved in the chemotaxis of VSMCs in response to either CCL1 or LCM on a fibronectin-coated surface. M indicates DMEM. Error bars represent SD.
Antibody against metalloproteinase-2 (MMP-2) inhibits CCL1- and LCM-induced chemotaxis of VSMCs. Mixtures of CCL1 (100 ng/mL) and goat IgG or goat anti–MMP-2 (> MP2) or anti–MMP-9 (> MP9) (1 μg/mL) were added to the bottom wells of the chemotaxis chamber. Similarly, LCM, produced as detailed in the legend to Figure 3, was added in the presence of the same immunoglobulin preparations. The chemotactic activity of these mixtures was tested against VSMCs as detailed in “Materials and methods.” Anti–MMP-2 inhibited chemotaxis induced by CCL1 74.5% and by LCM 95% (*P < .0001), whereas anti–MMP-9 was less effective, inhibiting CCL1- and LCM-induced chemotaxis 32.6% and 41.6%, respectively (**P < .03). Inhibition of VSMC chemotaxis by antibody against MMP-2 was significantly greater than by anti–MMP-9 (P < .007). These studies suggest that MMP-2 is the principal metalloproteinase involved in the chemotaxis of VSMCs in response to either CCL1 or LCM on a fibronectin-coated surface. M indicates DMEM. Error bars represent SD.
CCL1 stimulates VSMCs to secrete pro-MMP2 and induces MMP-2 mRNA
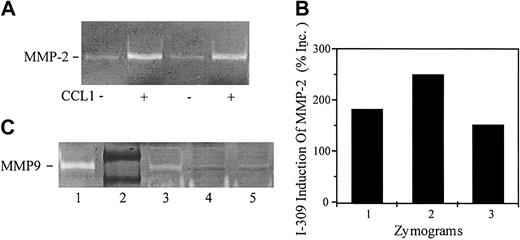
The results of Figure 7 raised the possibility that CCL1 might directly modulate production of MMP2. To quantify metalloproteinase production, CCL1 was incubated with VSMCs for 3 hours and assayed for gelatinolytic activity. As shown in Figure 8A, CM from VSMCs incubated with DMEM demonstrated constitutive gelatinolytic activity with an electrophoretic mobility identical to that of the positive pro–MMP-2 control. Treatment of VSMCs with CCL1 increased pro–MMP-2 gelatinolytic activity in 3 separate experiments 187%, 250%, and 152% (mean = 196%) compared with the constitutive activity (Figure 8B). Immunoblots confirmed the identity of MMP-2 in the VSMC supernatants (data not shown).
CCL1 induces pro-MMP2 in human vascular smooth muscle cells. VSMCs were incubated with DMEM or with CCL1 (150 ng/mL) for 6 hours. The samples were analyzed by gelatinolytic zymography as detailed in “Materials and methods.” (A) CM from VSMCs incubated with DMEM (lanes 1,3) or with CCL1 (lanes 2,4) was tested in duplicate for gelatinolytic activity. CM from CCL1-treated VSMCs showed enhanced gelatinolytic activity. (B) Gelatinolytic zymograms from 3 separate experiments were quantified using the NIH Image 1.61f program. CCL1 increased VSMC gelatinolytic activity by 187%, 250% (samples of panel A), and 152%, respectively, over the untreated controls. (C) CM was concentrated approximately 16-fold using heparin-agarose beads as detailed in “Materials and methods.” Samples containing equal amounts of protein were analyzed by gelatinolytic zymography. MMP-9 standard (1); protein standards phosphorolase-B (107 kDa), albumen (90 kDa) (2); VSMCs stimulated with TNF-α (20 ng/mL) (3), DMEM (4) or CCL1 (5) (150 ng/mL) for 6 hours. TNF-α increased MMP-9 gelatinolytic activity in VSMCs compared with the media control but CCL1 did not. These studies show that CCL1 increases the secretion of MMP-2 by VSMCs but not the secretion of MMP-9.
CCL1 induces pro-MMP2 in human vascular smooth muscle cells. VSMCs were incubated with DMEM or with CCL1 (150 ng/mL) for 6 hours. The samples were analyzed by gelatinolytic zymography as detailed in “Materials and methods.” (A) CM from VSMCs incubated with DMEM (lanes 1,3) or with CCL1 (lanes 2,4) was tested in duplicate for gelatinolytic activity. CM from CCL1-treated VSMCs showed enhanced gelatinolytic activity. (B) Gelatinolytic zymograms from 3 separate experiments were quantified using the NIH Image 1.61f program. CCL1 increased VSMC gelatinolytic activity by 187%, 250% (samples of panel A), and 152%, respectively, over the untreated controls. (C) CM was concentrated approximately 16-fold using heparin-agarose beads as detailed in “Materials and methods.” Samples containing equal amounts of protein were analyzed by gelatinolytic zymography. MMP-9 standard (1); protein standards phosphorolase-B (107 kDa), albumen (90 kDa) (2); VSMCs stimulated with TNF-α (20 ng/mL) (3), DMEM (4) or CCL1 (5) (150 ng/mL) for 6 hours. TNF-α increased MMP-9 gelatinolytic activity in VSMCs compared with the media control but CCL1 did not. These studies show that CCL1 increases the secretion of MMP-2 by VSMCs but not the secretion of MMP-9.
MMP-9 gelatinolytic activity was not observed in unconcentrated CM. Therefore, the effect of CCL1 on MMP-9 production was studied using CM concentrated approximately 16-fold. A band corresponding to pro–MMP-9 was observed in the concentrated CM of VSMCs stimulated with tumor necrosis factor-α (Figure 8C). A faint band of gelatinolytic activity was identified in the concentrated CM from VSMCs incubated with CCL1, and this low level of gelatinolytic activity appeared to be similar to that of the CM from VSMCs incubated with DMEM (Figure 8C). These studies show that CCL1 modulates VSMC MMP-2 activity, but has no appreciable effect on MMP-9 activity.
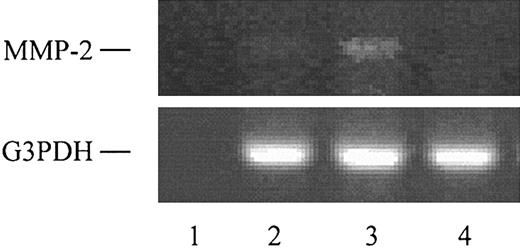
CCL1 was found to induce MMP-2 mRNA in VSMCs (Figure 9). The VSMCs stimulated with CCL1 showed an increase in MMP-2 mRNA that was approximately 2-fold increased over the DMEM control. Human embryonic kidney cells did not demonstrate a corresponding MMP-2 mRNA band. These results were substantiated by real-time PCR analysis, which showed a 3.03-fold increase in MMP-2 mRNA in cells stimulated with CCL1 when compared with unstimulated cells.
CCL1 induces MMP-2 mRNA in VSMCs. Total RNA was obtained from VSMCs or from human embryonic kidney cells, and the reverse transcriptase–polymerase chain reaction (RT-PCR) was performed using MMP-2 and glyceraldehyde-3 phosphate dehydrogenase (G3PDH) oligonucleotide primers as detailed in “Materials and methods.” Lane 1 shows no RNA control; lane 2, RNA from VSMCs incubated with DMEM; lane 3, RNA from VSMCs incubated 3 hours with 150 ng/mL CCL1; and lane 4, RNA from human embryonic kidney cells. G3PDH was used as an internal control.
CCL1 induces MMP-2 mRNA in VSMCs. Total RNA was obtained from VSMCs or from human embryonic kidney cells, and the reverse transcriptase–polymerase chain reaction (RT-PCR) was performed using MMP-2 and glyceraldehyde-3 phosphate dehydrogenase (G3PDH) oligonucleotide primers as detailed in “Materials and methods.” Lane 1 shows no RNA control; lane 2, RNA from VSMCs incubated with DMEM; lane 3, RNA from VSMCs incubated 3 hours with 150 ng/mL CCL1; and lane 4, RNA from human embryonic kidney cells. G3PDH was used as an internal control.
Poxvirus MC148, a CCR8 antagonist, inhibits CCL1-induced pro–MMP-2 secretion in VSMCs
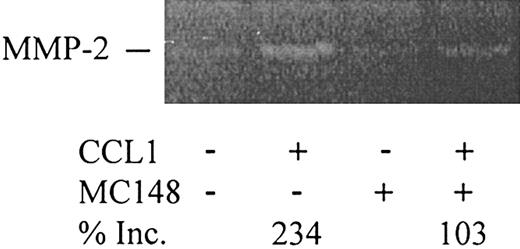
To determine whether CCL1 induction of MMP-2 was mediated by CCR8 signaling, the effect of monoclonal anti-CCR8 antibodies was tested. Our preliminary studies showed that the antibody as well as the IgG control increased baseline secretion of MMP-2 (data not shown). We therefore used the novel CCR8 antagonist poxvirus MC148. CCL1 stimulated a 234% increase in MMP-2 gelatinolytic activity compared with the control cells. In the presence of MC148, this increase was substantially inhibited (Figure 10). MC148 inhibited VSMC chemotaxis induced by CCL1 but not by PDGF-BB (study not shown), suggesting that the effect of MC148 was not due to direct cell toxicity. These findings strongly support the role of CCR8 in the induction of pro–MMP-2 by CCL1.
Poxvirus MC148 inhibits CCL1-induced MMP-2 production in VSMCs. VSMCs were preincubated with DMEM or with poxvirus MC148 chemokine (100 ng/mL) for 30 minutes at 37°C. After washing, DMEM or CCL1 (100 ng/mL) with or without MC148 was incubated with the VSMCs for 3 hours at 37°C in 5% CO2. The supernatants were collected and analyzed by gelatinolytic zymography. Gelatinolytic zymograms were quantified using the NIH Image 1.62f program. CCL1 increased VSMC gelatinolytic activity by 234% over the control. In the presence of MC148 chemokine, however, production of MMP-2 was substantially inhibited. Inc. indicates increase.
Poxvirus MC148 inhibits CCL1-induced MMP-2 production in VSMCs. VSMCs were preincubated with DMEM or with poxvirus MC148 chemokine (100 ng/mL) for 30 minutes at 37°C. After washing, DMEM or CCL1 (100 ng/mL) with or without MC148 was incubated with the VSMCs for 3 hours at 37°C in 5% CO2. The supernatants were collected and analyzed by gelatinolytic zymography. Gelatinolytic zymograms were quantified using the NIH Image 1.62f program. CCL1 increased VSMC gelatinolytic activity by 234% over the control. In the presence of MC148 chemokine, however, production of MMP-2 was substantially inhibited. Inc. indicates increase.
CCR8 and TCA3 are induced and colocalize with vascular smooth muscle cells in arterial injury models
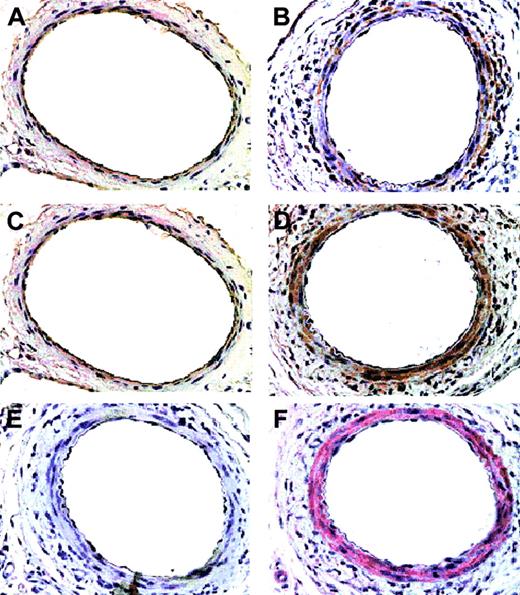
At 5 days after femoral arterial injury (Figure 11), the media from mice stained more intensely for CCR8 and TCA3 (B,D) compared with the uninjured mice (A,C). The CCR8- and TCA3-positive cells stained for α-actin, indicating that most of the cells were vascular smooth muscle cells (Figure 11F). Only rare cells stained for monocytes/macrophages (Figure 11E).
Immunohistochemical analysis of CCR8 and TCA3 up-regulation in injured femoral arteries. Serial sections from femoral arteries from mice 5 days after injury (B,D-F) and from uninjured mice (A,C) were stained for macrophages, MOMA-2 (E, 1:400 dilution), smooth muscle α-actin (F, 1:100 dilution), CCR8 (C-D, 1:5000 dilution), or TCA3 (A-B, 1:10 dilution). Magnification shown is × 40. Cells that stain for CCR8 and TCA3 stain for smooth muscle cell α-actin and both CCR8 and TCA3 are induced in injured vessel. Only rare cells stain for Moma-2.
Immunohistochemical analysis of CCR8 and TCA3 up-regulation in injured femoral arteries. Serial sections from femoral arteries from mice 5 days after injury (B,D-F) and from uninjured mice (A,C) were stained for macrophages, MOMA-2 (E, 1:400 dilution), smooth muscle α-actin (F, 1:100 dilution), CCR8 (C-D, 1:5000 dilution), or TCA3 (A-B, 1:10 dilution). Magnification shown is × 40. Cells that stain for CCR8 and TCA3 stain for smooth muscle cell α-actin and both CCR8 and TCA3 are induced in injured vessel. Only rare cells stain for Moma-2.
Discussion
Injury to the arterial wall that occurs during the development of atherosclerosis or following mechanical injury is characterized by smooth muscle migration and accumulation in the neointima accompanied by extracellular matrix remodeling.1 The migration of VSMCs is mediated by a number of cytokines, however the full spectrum of agonists that stimulate VSMCs has not been determined. VSMC production of matrix-degrading metalloproteinases appears to be essential in the formation of the neointima following vascular injury.44 Migration of VSMCs is dependent upon metalloproteinase-2 (MMP-2) and metalloproteinase-9 (MMP-9) production, however MMP-2 appears to be the most critical.45 We now report that the CC chemokine CCL1 induces both chemotaxis and MMP-2 production in human aortic smooth muscle cells. The unique receptor for CCL1, CCR8, was expressed on VSMC membranes, and Northern blot analysis identified the presence of cytoplasmic CCR8 mRNA.
Evidence that CCR8 was the receptor for CCL1 and vCCL1 was shown by the capacity of a monoclonal antibody against CCR8 to inhibit CCL1- and vCCL1-induced VSMC chemotaxis. This antibody was shown previously to inhibit the chemotaxis of human monocytes and HUVECs when stimulated by CCL1.24 The inhibition of CCL1-induced VSMC chemotaxis by anti-CCR8 was specific since this monoclonal antibody did not inhibit chemotaxis induced by either CCL3 or by PDGF-BB. Additional confirmation that a G-protein–coupled transmembrane receptor was responsible was shown by the inhibition of chemotaxis produced by pertussis toxin, a specific Gi-protein inhibitor, and not by cholera toxin. Human arterial wall injury caused by percutaneous transluminal balloon angioplasty is associated with neointimal formation due to VSMC migration and proliferation. We have previously identified CCR8 and CCL1 antigens in plaque in atherectomy tissues.24,29 In the present study we used a model of arterial injury to further study the expression of CCR8 and CCL1 in vivo. We chose to examine chemokine/receptor expression at 5 days after injury because that time point is associated with widespread SMC migration and proliferation. The presence of CCR8 and CCL1 at this time point raises the possibility that they may participate in the response to VSMCs to arterial injury.
Elevated blood levels of Lp(a) are associated with an increased risk for atherosclerosis (reviewed in Seman et al46 ). Structurally, Lp(a) consists of a low-density lipoprotein particle disulfide linked to apolipoprotein(a),47,48 a glycoprotein of variable size that shares a partial homology with plasminogen.49,50 The mechanisms responsible for the atherogenicity of Lp(a) are only partially characterized. In the present study we document that the conditioned media from HUVECs incubated with Lp(a) induce chemotaxis of VSMCs. The monoclonal antibody against CCR8 significantly inhibited chemotaxis of VSMCs to both CCL1 and the Lp(a)-conditioned media (LCM) to an equivalent extent, indicating that CCR8 was the responsible receptor. That CCL1 was the responsible agonist that induced VSMC chemotaxis was documented by the inhibition of activity using an antibody directed against CCL1. Anti-CCR8 or anti-CCL1 failed to inhibit the activity of HUVEC CM in inducing chemotaxis, indicating that CCR8 was not responsible for this constitutive activity. These studies show a potential new mechanism for the interaction between Lp(a) and VSMCs. The induction of VSMC chemotaxis by CCL1-containing conditioned medium from Lp(a)-stimulated endothelial cells suggests a novel pathway whereby Lp(a) may induce VSMC activation and migration.
The matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that degrade extracellular matrix proteins. They are synthesized as prepro-enzymes and are secreted primarily as latent proenzymes.43 MMPs include the interstitial collagenases, the stromelysins, the gelatinases, and the membrane-type MMPs.43 Administration of a gelatinase inhibitor following vascular injury in the rat was associated with a 97% reduction in the number of VSMCs migrating into the intima, thus directly implicating gelatinases in VSMC activation.51 Gelatinases have been identified in human atherosclerotic plaques52-54 and are up-regulated following arterial wall injury in animal models.51,55-57
The present study has documented that CCL1 induces human VSMCs to secrete pro–MMP-2 (gelatinase A) but does not stimulate MMP-9 (gelatinase B) production. CCL1 also induces an increase in pro–MMP-2 mRNA, indicating that this chemokine modulates MMP-2 translation. The MC148 derived from the human poxvirus molluscum contagiosum (MCV) has been shown to be a highly selective CCR8 antagonist.58,59 In competition binding assays, examining 16 chemokine receptors encompassing all 4 chemokine receptor classes (XCR, CCR, CXCR, and CX(3)CR), MC148 bound only to CCR8.58 Further, MC148 specifically blocked CCL1-induced chemotaxis and did not stimulate calcium mobilization. In the present study, incubation of VSMCs with MC148 effectively inhibited the increase in MMP-2 induced by CCL1, a finding indicating that CCR8 was the receptor responsible. In a parallel study (not shown), MC148 blocked CCL1-induced chemotaxis but had no effect upon chemotaxis induced by PDGF-BB. Thus the inhibitory effect of MC148 on MMP-2 production was not due to cell toxicity. These results indicate that the increase in pro–MMP-2 stimulated by CCL1 was mediated by CCR8. Using selective antibodies against MMPs, we found that anti–MMP-2 substantially inhibited chemotaxis across a fibronectin-coated membrane, whereas anti–MMP-9 had a lesser inhibitory effect. Prior studies have shown that chemotaxis of rat VSMCs across a basement membrane barrier induced by PDGF-BB (platelet-derived growth factor BB) was inhibited by blocking antisera against MMP-2. Pro–MMP-2 was the principal MMP secreted, however increased MMP-2 production was not observed.45 PDGF-BB was shown in a recent study to increase MMP-2 protein and gelatinase activity in rat VSMCs.60
Several studies support the concept proposed by Pauly et al45 that VSMC migration in vivo is dependent on MMP-2 activity. Adenovirus-mediated transfer of the cDNA for human tissue inhibitor of MMP-2 (TIMP-2) inhibited VSMC invasiveness in vitro and delayed neointimal development in a rat carotid injury model.61 Jenkins et al quantified gelatinase activity in the rat carotid artery following injury.44 MMP-2 increased to 4-fold the initial protein concentration, and remained elevated at one month following injury. Gelatin zymography showed that the main gelatinolytic bands were due to MMP-2, as only small amounts of MMP-9 appeared early and transiently.44 MMP-2 activity was confined mainly to the neointima. As active MMP-2 increased between 1 and 6 days after injury, there was a concomitant increase in pro–MMP-2. The ratio of activated to latent MMP-2 did not exceed 0.8, suggesting that the concentration of secreted pro–MMP-2 may regulate MMP-2 activation.
Dexamethasone inhibited PDGF-BB–induced chemotaxis of rat aortic smooth muscle cells and simultaneously inhibited pro–MMP-2 secretion in a dose-dependent manner.62 A 10% inhibition in the secretion of pro–MMP-2 resulted in an approximate 40% decrease in VSMC chemotaxis. Thus a relatively modest decrease in MMP-2 secretion was associated with a relatively large decrease in VSMC migration, suggesting that there is a quantitative relationship between secretion of MMP-2 and VSMC migration. These findings, in addition to those of Jenkins44 that activation of MMP-2 is linked to pro–MMP-2 secretion, suggest that the CCL1-induced increase in pro–MMP-2 secretion reported in the present study may be significant in modulating VSMC invasiveness and migration in vivo.
Our data that CCL1 increased the secretion of MMP-2 but not MMP-9 extend observations that these proteinases are regulated differently. MMP-2 production was found to be constitutive and was not induced in VSMCs by interleukin-1 (IL-1) or TNF-α, whereas these cytokines induced MMP-9.63 In rabbit aorta smooth muscle cells MMP-9 but not MMP-2 expression was increased by phorbol myristic acetate, fetal calf serum, thrombin, and IL-1α.64 Nerve growth factor (NGF) induced MMP-9 expression in primary cultured rat aortic smooth muscle cells through the activation of the mitogen-activated protein (MAP) kinases, extracellular signal-related kinase 1 (Erk-1) and Erk-2, without altering the expression of MMP-2 or MMP-3.65 VEGF was found to up-regulate MMP-1, MMP-3, and MMP-9 through the flt-1 receptor but did not up-regulate MMP-2.66 Pro–MMP-2 was significantly increased, however, by exposing human VSMCs to chronic cyclical mechanical strain.67 MMP-2 and MMP-9 also differ as to their substrate specificity. Although both degrade types IV and V collagen and elastin,68,69 MMP-2 specifically degrades fibronectin and laminin,70 whereas MMP-9 degrades types I and III collagens.68
In this report we show that human VSMCs express CCR8 and that this receptor modulates CCL1-induced VSMC chemotaxis and the secretion of pro–MMP-2. We have previously found that CCL1 antigen is widely distributed in human atherosclerotic plaques.24,29 The source for this chemoattractant was not established, however mononuclear cells that participate in atherogenesis,1 including monocytes/macrophages and T lymphocytes, possess functional CCR8 and secrete CCL1. Endothelial cells also possess functional CCR8 and can be induced to produce CCL1 by Lp(a).24 Since VSMC migration and the formation of the neointima are critical components of the arterial response to injury, this study strongly implicates CCL1 in vessel wall biology.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2002-05-1480.
Supported in part by National Institutes of Health grant HL-544469 (P.C.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 2. Checkerboard analysis documents that CCL1 induces chemotaxis of VSMCs. CCL1 (100 ng/mL) was placed in both top and bottom wells, only in the top well (also containing VSMCs), or only in the bottom wells of the chemotaxis chamber. CCL1 in the bottom chamber induced the greatest number of migrating VSMCs compared with the buffer control (*P = .00 003); however, the activity induced by CCL1 in the top chamber was also significantly increased (**P = .003). Comparison of the migration of VSMCs showed a significant difference between having the agonist in the top versus the bottom chamber (P = .001). The migration of cells when the agonist was in both top and bottom wells was not different than the control (not significant [NS]). These findings document that the chemotaxis of VSMCs in response to CCL1 is mainly directed or chemotactic. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/4/10.1182_blood-2002-05-1480/6/m_zh80040457070002.jpeg?Expires=1769111849&Signature=OyK8ti8tfzEvl7csx-QFJtOcLwZAOho3HOmyA8CObXI7tA4zBQiogDjMXB3u288WUxkDbse2N6uxnZ2BXBnHUDhZqZLi63rK2M3wif8t0zf~kOVUik9RM3jdCwvpf5TwllCGu5hjlbcYHdC9HgidMU4gzv0CeJ81P9zi1eRlNk3CeyEKefD6~fSXtZhAvYZOkCZCNk4XxrrG-NqmX-dqVvKdFzJ7fqmN2eGTqux4WuA4KeRkxiMoyuUUt3Dzby59G~btiZOIIj5m2IaLEu~sQ5fwi6AAqiMucI4Hb18PH65HW6Q86MnKUCCDiUb9uW3cUeacSQkY7c96-AVPwRHj7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 2. Checkerboard analysis documents that CCL1 induces chemotaxis of VSMCs. CCL1 (100 ng/mL) was placed in both top and bottom wells, only in the top well (also containing VSMCs), or only in the bottom wells of the chemotaxis chamber. CCL1 in the bottom chamber induced the greatest number of migrating VSMCs compared with the buffer control (*P = .00 003); however, the activity induced by CCL1 in the top chamber was also significantly increased (**P = .003). Comparison of the migration of VSMCs showed a significant difference between having the agonist in the top versus the bottom chamber (P = .001). The migration of cells when the agonist was in both top and bottom wells was not different than the control (not significant [NS]). These findings document that the chemotaxis of VSMCs in response to CCL1 is mainly directed or chemotactic. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/4/10.1182_blood-2002-05-1480/6/m_zh80040457070002.jpeg?Expires=1769111850&Signature=gTj1gzeoZtH9LMrLuje7etPEhCjuDr1VV32g3A~5kSItIuW0i7wcG2YvcoAQ5cRg07bYGu20wZnTClwESlATpF~Zd2LW8Q87s4u2qTFGDy92ANlj~G3zmTXW5C03r5ZsFuseIrKhRquvR--S~6gsQM626oRvMfig9yNiVyD7MMy3TfJaeK~o-WyjfO70evhLQYtifaBRxy3Rgt-E5-8J~0RuT~pOuQelVPXXRXxXJvm9Kc597Q~F0k9VDE5SGFKXrMcuT7LzqAqW4qzp34IDhSDL9lBkMMuR-Ep4s8sYbOEt~IlRfsEwIHCPdS-bPlOc1O4D6ny~f1MWtZ42DJKAog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)