Abstract
We hypothesized that the allergen-induced increased number of airway eosinophils results from increased recruitment of eosinophils from bone marrow (BM) and local development of CD34+ cells into eosinophils. We also assumed that the phenotype of airway eosinophils depends on whether these cells have differentiated within BM or airway. C57BL/6 mice were sensitized and subsequently exposed to ovalbumin (OVA) on 5 consecutive days. Newly produced cells were labeled with a thymidine analog. Clonogenic activity and interleukin 5 (IL-5) release from bronchoalveolar lavage fluid (BALf) CD34+ cells were evaluated by using cell-culture techniques. Allergen exposure induces increase in CD135+ primitive myeloid progenitors within the BM CD34+ cell population, without significant changes in total number of CD34+ cells or newly produced CD34+ cells. CD34+/IL-5Rα+ cells in the first stage of cell differentiation were found only in BM, arguing that early commitment of CD34+ cells into the eosinophil lineage is restricted to the BM compartment. Allergen exposure induces a shift in differentiation of BM, blood, and BALf eosinophillineage–committed CD34+ cells toward mature eosinophils and recruitment of these cells via blood into airway. We further demonstrate in vitro that ability to multiply persists in BALf CD34+ cells but not CD34– cells, likely via autocrine IL-5 release and IL-5–induced up-regulation of IL-5Rα.
Introduction
The most important pathologic feature of allergic airway (AW) inflammation is associated with T-lymphocyte activation and increase in eosinophil number in the AW wall and lumen.1,2 Accumulation of inflammatory cells is considered to be the result of increased production and traffic of cells from bone marrow (BM) into AW via the circulation.3-8
Eosinophils develop from CD34+ hematopoietic progenitor cells. CD34 is a stage-specific rather than a lineage-specific leukocyte-differentiation antigen. Its expression appears at the highest density on the earlier progenitors, decreases progressively with cell maturation, and is lost on terminally differentiated cells.9 To date, the functional significance of CD34 antigen expression on hematopoietic cells remains to be established.10 Nevertheless, CD34-deficient mice have been shown to have reduced eosinophil number in AW after allergen exposure, despite normal leukocyte development and distribution.11 These data, therefore, suggest that the presence of a sufficient number of CD34+ cells is required for development of eosinophilic inflammation in response to allergen exposure.
Recent data from patients with allergies suggest that eosinophil differentiation can also occur within sites of allergic inflammation. Presence of this additional eosinophil-differentiation pathway is supported by reports showing that allergic subjects have increased numbers of the eosinophil-lineage–committed CD34+ cells in BM12 and AW13 and that AW CD34+ cells can complete their differentiation following ex vivo stimulation with interleukin 5 (IL-5) or allergen.14 However, whether these cells retain their ability to multiply within AW and subsequently maturate into eosinophils in vivo is not known.
We hypothesized that allergen exposure can induce not only the differentiation of CD34+ cells toward eosinophils but also the multiplication of CD34+ cells within AW. Also, we assumed that the phenotype of AW eosinophils depends on whether these cells were differentiated within the BM or AW compartment. To assess our hypothesis, we used a mouse model of eosinophilic inflammation induced by ovalbumin (OVA),7,15 in which newly produced cells were labeled with 5′-bromo-2′-deoxyuridine (BrdU). Also, we used in vitro cell-culture techniques to assess eosinophil colony-forming activity and IL-5 release from AW CD34+ cells.
Materials and methods
In vivo experiments
Animals. This study was approved by the Ethical Committee for Animal Studies at Göteborg University, Sweden. C57BL/6 mice were purchased from B&K Universal AB (Sollentuna, Sweden). The mice were 5 to 6 weeks old and were maintained under conventional animal-housing conditions and provided with food and water ad libitum.
Allergen sensitization and exposure protocol. C57BL/6 mice were sensitized by intraperitoneal injections of 0.5 mL aluminum-precipitated antigen containing 8 μg OVA (Sigma Aldrich Sweden AB, Tyresö, Sweden) bound to 4 mg aluminum hydroxide (Sigma Aldrich Sweden AB) in phosphate-buffered saline (PBS) twice, 5 days apart. Eight days after the second sensitization, the animals were briefly anesthetized by using CO2 gas and exposed intranasally to 10 μg OVA or PBS (n = 9 in each exposure group). These exposures were performed on 5 consecutive days.
Labeling of newly produced cells. BrdU, a thymidine analog that is incorporated in DNA during the S-phase of the cell cycle, was used to label newly produced inflammatory cells.16 BrdU (Roche Diagnostics, Mannheim, Germany) was given at dose of 1 mg in 0.25 mL PBS intravenously twice (6 hours apart) on exposure days 1 and 3.
Cell collection and sample processing. All samples were collected 24 hours after the last exposure. The animals were anesthetized with a mixture of xylazine (130 mg/kg; Rompun; Bayer, Gothenburg, Sweden) and ketamine (670 mg/kg, Ketalar; Pfizer, Täby, Sweden). First, blood was obtained by puncture of the heart right ventricle. Second, bronchoalveolar lavage was performed through the tracheal cannula by instillation of 0.25 and 0.20 mL PBS. Finally, BM was harvested by excising one femur, which was cut at the epiphyses and flushed with 2 mL PBS.
Blood. A mixture was made of 200 μL blood with 800 μL 2 mM ethylenediaminetetraacetic acid (Sigma Aldrich Sweden AB) in PBS. Red blood cells were lysed using 0.1% potassium bicarbonate and 0.83% ammonium chloride solution for 15 minutes at 4°C. The white blood cells were resuspended in PBS containing 0.03% bovine serum albumin (BSA; Sigma Aldrich Sweden AB).
Bronchoalveolar lavage fluid (BALf) and bone marrow. BALf and BM samples were centrifuged at 300g for 10 minutes at 4°C, and cell pellet was resuspended in 0.03% BSA in PBS.
The total cell numbers in blood, BALf, and BM were determined by using standard hematologic procedures. Cytospins of blood, BALf, and BM cells were prepared.
Immunocytochemistry (ICC). All double stainings were performed as a sequential method, based on 2 individually performed complete staining procedures. All steps were performed at room temperature unless otherwise stated. As controls, isotype-matched immunoglobulins (purified rat IgG2a or biotinylated rat IgG2a) were used. Cells were determined by counting 400 cells using a light microscope (magnification × 1000; Zeiss Axioplan 2; Carl Zeiss, Jena, Germany).
Double ICC for CD34 antigen and nuclear BrdU. On day 1, cytospin preparations were fixed with 2% formaldehyde for 10 minutes and incubated with 5% rabbit serum (DAKO, Glostrup, Denmark) to avoid unspecific binding. Slides were incubated with a purified rat antimouse CD34 monoclonal antibody (mAb) (clone RAM34; BD Biosciences Europe, Erembodegem, Belgium) for 2 hours followed by a 45-minute incubation with either alkaline phosphatase–conjugated rabbit F(ab′)2 anti-rat IgG secondary antibody (Ab) (Southern Biotechnology Associates, Birmingham, AL) or rabbit anti-rat Ig Ab followed by rat alkaline phosphatase anti-alkaline phosphatase mAb (APAAP; DAKO). Bound Abs were visualized with the Vector Red alkaline phosphatase substrate kit (Vector Laboratories, Burlingame, CA). Samples were fixed a second time overnight in 4% paraformaldehyde. On day 2, samples were treated with 0.1% trypsin (Sigma Aldrich Sweden AB) at 37°C for 15 minutes, followed by 4 M HCl for 15 minutes and Holmes Borate-Borax buffer (pH 8.5) for 10 minutes. Endogenous peroxidase was blocked by immersion of the slides for 45 minutes in a glucose oxidase solution, preheated to 37°C, containing tris(hydroxymethyl)aminomethane-buffered saline 0.0064% sodium azide, 0.1% saponin, 0.18% glucose, and 1:1000 glucose oxidase. Endogenous biotin was blocked with Biotin Blocking System (DAKO). BrdU-labeled cells were detected with a biotinylated rat anti-BrdU mAb (clone BU1/75; Harlan-Sera Lab, Loughborough, United Kingdom), followed by ExtrAvidin-Peroxidase (Sigma Aldrich Sweden AB) and visualized with 3,3′-diaminobenzidine (DAB) Substrate Chromogen System (DAKO). Mayer Hematoxylin (Sigma Aldrich Sweden AB) was used for counterstaining.
Double ICC for CD34 and CD135 antigen. Cytospin preparations were fixed for 2 minutes in a 4% zinc formaldehyde solution (Histolab Products AB, Göteborg, Sweden). Detection of CD34 antigen was performed by using a purified rat anti-mouse CD34 mAb, rabbit anti-rat Ig Ab, and rat APAAP mAb as described in “Double ICC for CD34 antigen and nuclear BrdU,” followed by blocking of endogenous biotin. To detect CD135 antigen, the slides were then incubated with a biotinylated rat anti-mouse CD135 mAb (clone A2F10.1; BD Biosciences Europe) for 1 hour followed by streptavidin-β-galactosidase (Roche Diagnostics) for 45 minutes. For visualization of CD135 antigen, samples were incubated in X-Gal solution (β-Gal Staining set; Roche Diagnostics) at 37°C.
Double ICC for CD34 antigen and stem cell antigen-1 (Sca-1). Cytospin preparations were fixed with 2% formaldehyde for 10 minutes and incubated with 10% rabbit serum (DAKO). Immunostaining for CD34 antigen was performed by using purified rat anti-mouse CD34 mAb, rabbit anti-rat Ig Ab, and APAAP mAb as described in “Double ICC for CD34 antigen and nuclear BrdU.” Slides were then treated with Biotin Blocking System (DAKO) and incubated overnight at 4°C with biotinylated rat antimouse Sca-1/Ly6 mAb (clone 177228; R&D Systems, Abingdon, United Kingdom). Incubation with streptavidin-β-galactosidase (Roche Diagnostics) and X-Gal solution (β-Gal Staining set; Roche Diagnostics) and counterstaining with Mayer hematoxylin were performed as described previously.
Double ICC for CD34 antigen and IL-5 receptor α chain (IL-5Rα). The formaldehyde-fixed cytospin preparations were treated with biotin blocking solution as mentioned earlier, followed by incubation with a biotinylated rat anti-mouse CD34 mAb (clone RAM34; BD Biosciences Europe) for 1 hour. Then slides were treated with streptavidin-β-galactosidase (Roche Diagnostics) followed by incubation in X-Gal solution as mentioned earlier. Slides were kept overnight at 4°C in 10% donkey serum (Jackson Immuno Research Laboratories, West Grove, PA). The next day, samples were incubated with a rabbit anti-mouse IL-5Rα polyclonal Ab (Research Diagnostics, Flanders, NJ) for 1 hour. As secondary Ab, an alkaline phosphatase–conjugated donkey F(ab′)2 antirabbit IgG (Jackson Immuno Research Laboratories) was used. Positive staining was visualized by Vector Red alkaline phosphatase substrate kit and then counterstained with Mayer hematoxylin.
ICC for CD34 antigen with luxol fast blue (LFB) counterstaining. Formaldehyde-fixed samples were treated with glucose oxidase solution to quench endogenous peroxidase and incubated in biotin blocking solution as described previously. Then slides were incubated with a biotinylated rat anti-mouse CD34 mAb (clone RAM34; BD Biosciences Europe) for 2 hours and subsequently incubated for 45 minutes with ExtrAvidin-peroxidase (Sigma Aldrich Sweden AB). Bound Abs were visualized with DAB-chromogen system. Finally, counterstaining with LFB (to detect eosinophilic granulation) was performed, followed by counterstaining with Mayer hematoxylin.
Biotinylation of BrdU and CD135 antibody. The Ab solution was poured into a Slide-A-Lyzer Mini Dialysis unit (Pierce, Rockford, IL) and dialyzed against 0.1 M NaHCO3 for 4 hours at room temperature. Biotin solution was prepared by dissolving biotinamidocaproate N-hydroxysuccinimide ester in ice-cold N,N-dimethylformamide and 0.1 M NaHCO3 up to a concentration of 1 mg/mL (Sigma Aldrich Sweden AB). The biotin solution was added to the antibody solution at a ratio of 1:5 μg and was incubated for 2 hours in a glass vial in the dark. A second dialysis was performed against PBS for 36 hours at 4°C.
Stages of differentiation of CD34+/IL-5Rα+cells, CD34–/IL-5Rα+cells, CD34+eosinophilic cells, and CD34–eosinophils. Cell differentiation was divided into 4 distinct stages (first, second, third, or fourth), according to cell size, nuclear morphology, and cytoplasmic ratio—the criteria described previously by Lee et al.17 Cells in the first, second, and third stages are mitotically active, whereas cells in the fourth stage are terminally differentiated and unable to divide.17
In vitro experiments
For all in vitro experiments, animals were sensitized and exposed to OVA as described earlier. BALf was performed by instillation of 1 mL PBS 4 times. BM was harvested as described previously. For each experiment, BALf and BM cells from 15 to 30 mice were pooled. All separations were performed sequentially by using a magnetic cell-sorting system with indirect microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). First, mononuclear cells were depleted by using a biotinylated Griffonia Simplicifolia lectin 1 Ab (Vector Laboratories).18 Second, CD3+ cells were depleted by using a biotinylated hamster anti-mouse CD3ϵ mAb (clone 145-2C11; BD Biosciences Europe). Third, neutrophils were depleted by using a biotinylated rat anti-mouse Ly-6G and Ly-6C mAb (clone RB6-8C5; BD Biosciences Europe). Finally, CD34+ cells were enriched by using a biotinylated rat anti-mouse CD34 mAb (clone RAM34; BD Biosciences Europe). Purity of the BALf CD34+ fraction was evaluated by using a fluorescein isothiocyanate (FITC)–labeled goat anti-rat polyclonal Ab (BD Biosciences) by fluorescent microscopy; more than 70% of total cells stained positively. Eosinophilic cells comprised 94% and 90% of total cells in BALf CD34+ and CD34– fraction, respectively (May-Grünwald-Giemsa staining).
Semisolid cultures of BALf and BM CD34+cells. CD34+ cells were cultured in a 12-well plate (BD Biosciences Europe) in RPMI 1640 culture medium complemented with 0.9% methylcellulose, 20% fetal calf serum (FCS), 1% penicillin-streptomycin, 2 mM l-glutamine, and 0.0006% β-mercaptoethanol (all obtained from Sigma Aldrich Sweden AB) at 37°C in an atmosphere containing 5% CO2. Cells were seeded in a concentration of 0.25 × 106/1 mL/well, and wells were divided into groups depending on the recombinant mouse cytokines added (all obtained from R&D Systems): (1) none (control); (2) rIL-5 (10 ng/mL); (3) recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF; 10 ng/mL); and (4) rIL-5 and rGM-CSF (IL-5/GM-CSF, both 10 ng/mL). On the sixth and twelfth days in culture, 100 μL RPMI medium complemented with penicillin-streptomycin, l-glutamine, and respective cytokines were added (end concentration as described earlier). Following 8 and 14 days in culture, colonies contained more than 40 cells were counted by using an inverted light microscope as described previously.19 To confirm the identification of eosinophil colonies on day 14, cells were washed with prewarmed RPMI medium supplied with 0.5% BSA and 20% FCS; cytospins were made and stained by the May-Grünwald-Giemsa method for differential cell counting. In addition, immunocytochemistry for CD34 antigen was performed together with LFB counterstaining and immunocytochemistry for IL-5Rα. Results are shown as means from 3 separate experiments, each performed in a single well for BALf CD34+ cells and in triplicate wells for BM CD34+ cells.
Liquid cultures of BALf CD34+eosinophilic cells and CD34–eosinophils. CD34+ eosinophilic cells and CD34– eosinophils were seeded in a 96-well plate (BD Biosciences) in RPMI media supplemented with 10% FCS, 1% penicillin-streptomycin, 2 mM l-glutamine, and 1% sodium pyruvate (all obtained from Sigma Aldrich Sweden AB) in a concentration of 0.15 × 106/200 μL/well (3 to 4 wells per group). Cells were stimulated with phorbol myristate acetate and calcium ionophore (end concentration 2 ng/mL and 1 μg/mL, respectively, both obtained from Sigma Aldrich Sweden AB) for 20 hours, and IL-5 in supernatants was measured using IL-5 enzyme-linked immunosorbent assay (ELISA) commercial kit (R&D Systems).
Statistical analysis
Data are presented as means (SEM). The Mann-Whitney U test was used for comparison of data between groups. P values less than .05 were considered statistically significant.
Results
In vivo experiments
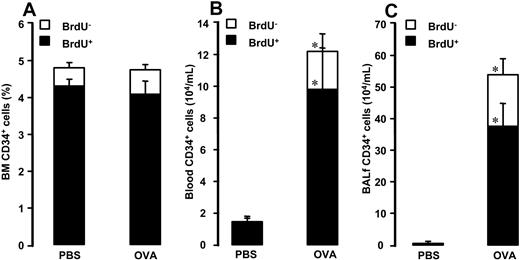
Bone marrow. Allergen exposure did not significantly affect the relative number of BM CD34+/BrdU+ cells or CD34+/BrdU– cells (4.1% ± 0.3% and 0.7% ± 0.1% after allergen versus 4.3% ± 0.2% and 0.5% ± 0.1% after vehicle, respectively; Figure 1A).
Number of (A) BM CD34+ cells, (B) blood CD34+ cells, and (C) BALf CD34+ cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS or OVA on 5 consecutive days. Data are shown as means + SEM. *P < .05.
Number of (A) BM CD34+ cells, (B) blood CD34+ cells, and (C) BALf CD34+ cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS or OVA on 5 consecutive days. Data are shown as means + SEM. *P < .05.
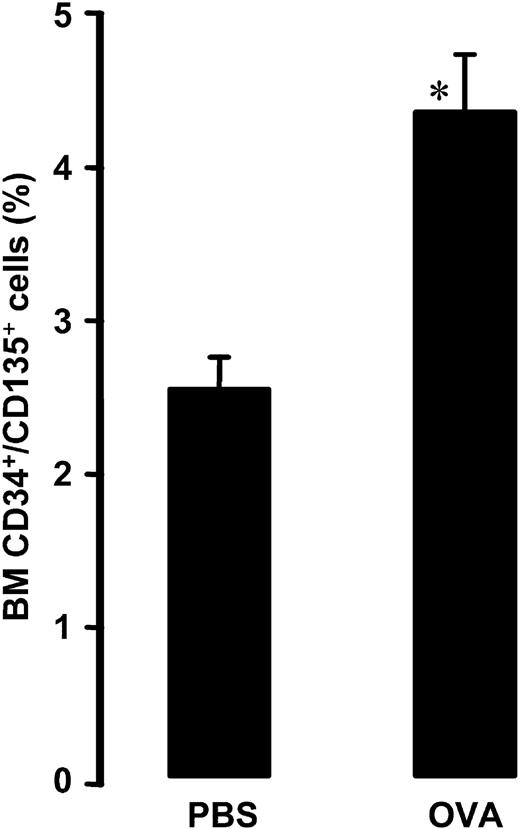
Allergen exposure induced an increase in the relative number of CD34+/CD135+ cells (4.4% ± 0.4% after allergen versus 2.5% ± 0.2% after vehicle, P = .004; Figure 2).
Relative number of bone marrow CD34+/CD135+ cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
Relative number of bone marrow CD34+/CD135+ cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
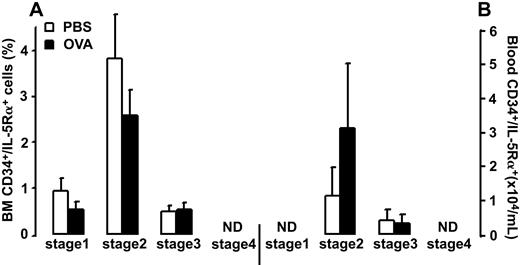
Allergen exposure did not cause significant changes in the relative number of BM CD34+/IL-5Rα+ cells or CD34–/IL-5Rα+ cells (3.7% ± 0.5% and 30.7% ± 1.9% after allergen versus 5.2% ± 1.2% and 31.0% ± 1.7% after vehicle, respectively). The CD34+/IL-5Rα+ cell population was composed of cells in the first, second, and third stages of differentiation (0.5% ± 0.2%, 2.6% ± 0.6%, and 0.5% ± 0.2% after allergen versus 1.0% ± 0.3, 3.4% ± 1.0%, and 0.5% ± 0.1% after vehicle, respectively; Figure 3A). No CD34+/IL-5Rα+ cells were detected in the 4th stage of differentiation.
Number of (A) bone marrow (BM) and (B) blood CD34+/IL-5Rα+ cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM.
Number of (A) bone marrow (BM) and (B) blood CD34+/IL-5Rα+ cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM.
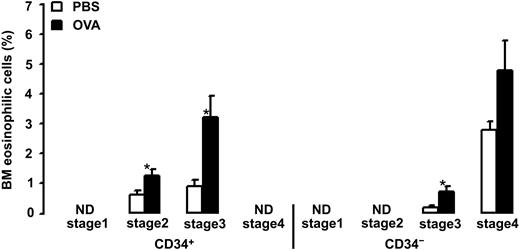
Allergen exposure caused an increase in the relative number of BM CD34+ eosinophilic cells and CD34– eosinophils (4.4% ± 0.8% and 5.5% ± 1.1% after allergen versus 1.5% ± 0.2% and 2.9% ± 0.3% after vehicle, P = .002 and P = .03, respectively). The CD34+ eosinophilic cell population was composed of cells in the second and third stages of differentiation (1.3% ± 0.2% and 3.2% ± 0.7% after allergen versus 0.6% ± 0.1% and 0.9% ± 0.2% after vehicle, P = .01 and P = .003, respectively; Figure 4). No CD34+ eosinophilic cells were detected in the first or fourth stages of differentiation. The CD34– eosinophil population was composed of cells in the third and fourth stages of differentiation (0.7% ± 0.2% and 4.8% ± 1.0% after allergen versus 0.2% ± 0.1% and 2.8% ± 0.3% after vehicle, P = .003 and P = .1, respectively; Figure 4). No CD34– eosinophils were detected in the first or second stages of differentiation.
Relative number of bone marrow (BM) eosinophilic cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
Relative number of bone marrow (BM) eosinophilic cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
Blood. Allergen exposure induced increase in the number of blood CD34+/BrdU+ cells and CD34+/BrdU– cells (9.8 ± 2.6 and 2.4 ± 1.1 × 104/mL after allergen versus 1.4 ± 0.3 and 0.08 ± 0.08 × 104/mL after vehicle, P = .0003 and P = .004, respectively; Figure 1B).
No CD135 immunostaining was detected in blood cytospins.
Allergen exposure did not significantly affect the total number of CD34+/IL-5Rα+ cells or CD34–/IL-5Rα+ cells (3.4 ± 1.8 and 33.4 ± 6.7 × 104/mL after allergen versus 1.5 ± 0.8 and 28.1 ± 2.6 × 104/mL after vehicle, respectively). The CD34+/IL-5Rα+ cell population was composed of cells in the second and third stages of differentiation (3.1 ± 1.9 and 0.3 ± 0.2 × 104/mL after allergen versus 1.1 ± 0.8 and 0.4 ± 0.3 × 104/mL after vehicle, respectively; Figure 3B). No CD34+/IL-5Rα+ cells were detected in the first or fourth stages of differentiation.
In the blood, allergen exposure increased the number of CD34+ eosinophilic cells and CD34– eosinophils (3.1 ± 1.7 and 16.6 ± 3.5 × 104/mL after allergen versus 0.4 ± 0.2 and 3.6 ± 0.6 × 104/mL after vehicle, P = .04 and P = .0009, respectively). The CD34+ eosinophilic cell population was composed of the cells in the second and third stages of differentiation (0.4 ± 0.2 and 2.7 ± 1.5 × 104/mL after allergen versus 0 and 0.4 ± 0.2 × 104/mL after vehicle, P = .07 and P = .1, respectively; Figure 5). No CD34+ eosinophilic cells were detected in the first or fourth stages of differentiation. The CD34– eosinophil population was composed of cells in the third and fourth stages of differentiation (6.9 ± 1.7 and 9.6 ± 2.0 × 104/mL after allergen versus 1.6 ± 0.3 and 2.0 ± 0.6 × 104/mL after vehicle, P = .003 and P = .002, respectively; Figure 5). No CD34– eosinophils were detected in the first or second stages of differentiation.
Number of blood eosinophilic cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
Number of blood eosinophilic cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
Bronchoalveolar lavage fluid. Allergen exposure increased the number CD34+/BrdU+ cells and CD34+/BrdU– in BALf (37.6 ± 7.0 and 16.4 ± 4.8 × 104/mL after allergen versus 0.1 ± 0.05 and 0.1 ± 0.05 × 104/mL after vehicle, P = .0003; Figure 1C).
No CD135 or IL-5Rα immunostaining was detected in BALf cytospins.
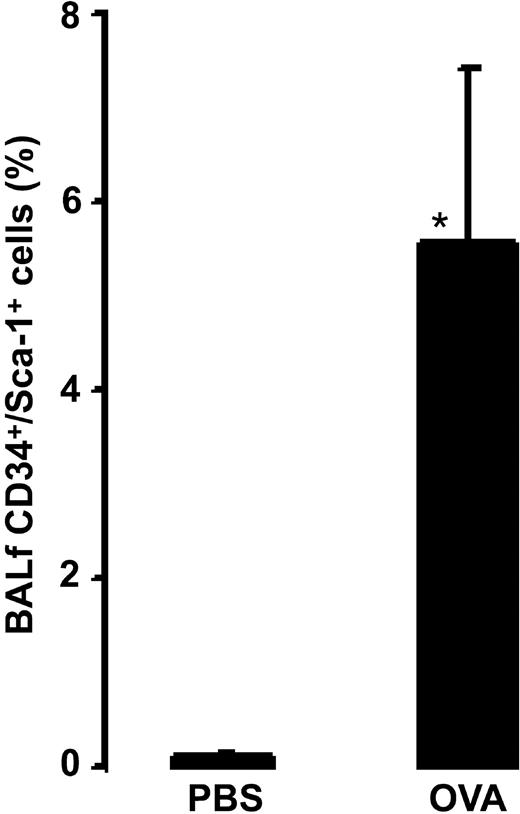
Allergen exposure induced an increase in the relative number of CD34+/Sca-1+ cells (5.6% ± 1.8% after allergen versus 0.08% ± 0.04% after vehicle, P = .0016; Figure 6).
Relative number of BALf CD34+/Sca-1+ cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
Relative number of BALf CD34+/Sca-1+ cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
Immunocytochemical staining for CD34 antigen with LFB counterstaining in cytospins prepared from BALf of allergen-exposed mouse is illustrated in Figure 7.
Photograph of immunocytochemical staining for CD34 antigen together with LFB counterstaining on BALf cytospin (× 1000). Dark brown staining indicates positive staining for CD34 antigen, and the green cytoplasmic staining illustrates eosinophilic granulation. CD34+ terminally differentiated eosinophilic cells are marked i, and the CD34– terminally differentiated eosinophil is marked ii.
Photograph of immunocytochemical staining for CD34 antigen together with LFB counterstaining on BALf cytospin (× 1000). Dark brown staining indicates positive staining for CD34 antigen, and the green cytoplasmic staining illustrates eosinophilic granulation. CD34+ terminally differentiated eosinophilic cells are marked i, and the CD34– terminally differentiated eosinophil is marked ii.
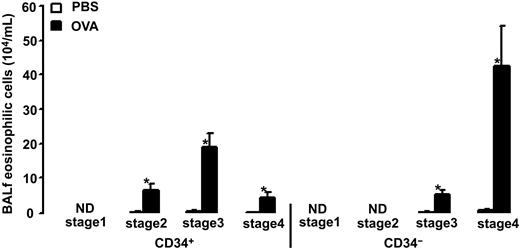
Allergen exposure caused an increase in the number of CD34+ eosinophilic cells and CD34– eosinophils (29.9 ± 6.1 and 48.1 ± 12.5 × 104/mL after allergen versus 0.4 ± 0.2 and 0.6 ± 0.3 × 104/mL after vehicle, P = .0003). The CD34+ eosinophilic cell population was composed of cells in the second, third, and fourth stages of differentiation (6.4 ± 1.8, 1.9 ± 4.0, and 4.4 ± 1.4 × 104/mL after allergen versus 0.1 ± 0.07, 0.3 ± 0.1, and 0.05 ± 0.03 × 104/mL after vehicle, respectively, P = .0003; Figure 8). No CD34+ eosinophilic cells were detected in the first differentiation stage. The CD34– eosinophil population was composed of cells in the third and fourth stages of differentiation (5.3 ± 1.1 and 42.8 ± 11.5 × 104/mL after allergen versus 0.1 ± 0.047 and 0.5 ± 0.2 × 104/mL after vehicle, respectively, P = .0003; Figure 8). No CD34– eosinophils were detected in the first or second stages of differentiation.
Number of BALf eosinophilic cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
Number of BALf eosinophilic cells in OVA-sensitized C57BL/6 mice. Animals were exposed to PBS (PBS) or OVA (OVA) on 5 consecutive days. Data are shown as means + SEM. *P < .05.
In vitro experiments
Colony-forming units (CFU). There was no CFU growth from BALf or BM CD34+ cells observed without cytokine stimulation. After 14 days in culture without cytokine stimulation, more than 82% of recovered cells were mononuclear cells (3.8 ± 0.8 and 4.0 ± 0.3 × 104/well for BALf and BM CD34+ cells, respectively). In both BALf and BM cultures after stimulation with rGM-CSF alone or in combination with rIL-5, only neutrophil-macrophage CFUs were detected; mononuclear cells comprised more than 93% of total cells in respective wells. Eosinophil CFUs (Eo-CFUs) in both BALf and BM cultures were detected only when cells were stimulated with rIL-5 alone. The number of Eo-CFUs grown from BALf CD34+ cells was significantly lower compared with Eo-CFUs grown from BM CD34+ cells (2.3 ± 0.9 and 8.3 ± 4.3 Eo-CFU/well versus 24.8 ± 9.7 and 30.5 ± 6.5 Eo-CFU/well after 8 and 14 days in culture, respectively, P = .049; Figure 9).
Number of Eo-CFUs grown from BALf and BM CD34+ cells after 8 and 14 days in semisolid culture with rIL-5. Data are shown as means + SEM from 3 separate experiments. *P < .05.
Number of Eo-CFUs grown from BALf and BM CD34+ cells after 8 and 14 days in semisolid culture with rIL-5. Data are shown as means + SEM from 3 separate experiments. *P < .05.
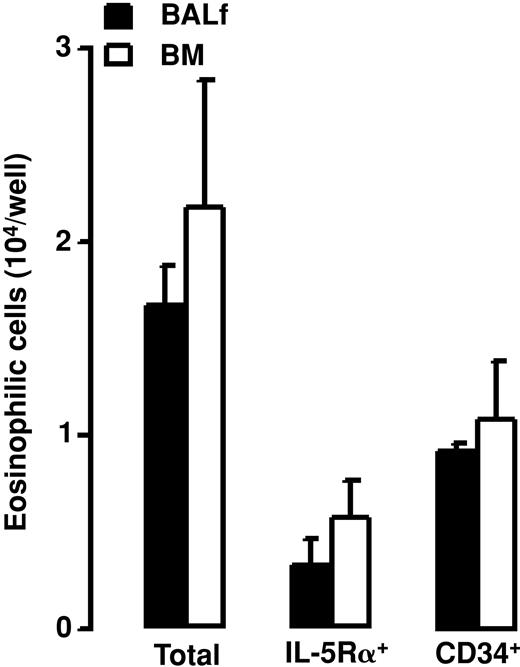
The Eo-CFU phenotype was confirmed by differential cell counting on cytospins prepared from cells recovered after 14 days in culture. Eosinophils were present only in wells in which CD34+ cells were cultured with rIL-5. After a 14-day culture of BALf and BM CD34+ cells with rIL-5, 1.7 ± 0.2 and 2.2 ± 0.6 × 104/well eosinophilic cells, respectively, were recovered (Figure 10). Eosinophilic cells recovered after culture of BALf and BM CD34+ cells with rIL-5 expressed IL-5Rα and CD34 antigen (0.3 ± 0.1 and 0.6 ± 0.2 × 104 eosinophilic cells/well expressed IL-5Rα, and 0.9 ± 0.02 and 1.1 ± 0.3 × 104 eosinophilic cells/well expressed CD34 antigen, respectively; Figure 10).
Number of eosinophilic cells (total), number of IL-5Rα+ eosinophilic cells (IL-5Rα+), and number of CD34+ eosinophilic cells (CD34+) recovered after 14 days in semisolid culture of BALf and BM CD34+ cells with rIL-5. Data are shown as means + SEM from 3 separate experiments.
Number of eosinophilic cells (total), number of IL-5Rα+ eosinophilic cells (IL-5Rα+), and number of CD34+ eosinophilic cells (CD34+) recovered after 14 days in semisolid culture of BALf and BM CD34+ cells with rIL-5. Data are shown as means + SEM from 3 separate experiments.
IL-5 release from BALf CD34+eosinophilic cells and CD34–eosinophils. BALf CD34+ eosinophilic cells released markedly more IL-5 protein compared with CD34– eosinophils after 20 hours in culture with unspecific stimulation (476.2 ± 3.7 versus 28.9 ± 1.7 pg/mL, P = .03, respectively; Figure 11). Without stimulation, no IL-5 was detected in supernatants.
IL-5 release from BALf CD34+ and CD34– eosinophilic cells after 20 hours of culture with phorbol myristate acetate and calcium ionophore. Data are shown as means + SEM. *P < .05.
IL-5 release from BALf CD34+ and CD34– eosinophilic cells after 20 hours of culture with phorbol myristate acetate and calcium ionophore. Data are shown as means + SEM. *P < .05.
Discussion
Our study shows that allergen exposure induces an increase in CD135+ primitive myeloid progenitors within the bone marrow CD34+ cell population without significant changes in total number of bone marrow CD34+ cells or number of newly produced CD34+ cells. These primitive cells were not found in blood or BALf. The earliest eosinophil-lineage–committed CD34+ cells (CD34+/IL-5Rα+ cells in the first differentiation stage) are restricted to the bone marrow compartment. CD34+/IL-5Rα+ cells were not significantly affected by AW allergen exposure in bone marrow or blood and were not detected in BALf. In the bone marrow, allergen exposure increased the number of the CD34+ eosinophilic cells and CD34– mature eosinophils. In blood and AW, allergen exposure induces increases in CD34+ eosinophilic cells, CD34– mature eosinophils, and also newly produced CD34+ cells. In addition, allergen exposure induces increase in BALf CD34+ cells coexpressing stem cell antigen-1 (Sca-1). Furthermore, allergen exposure induces a shift in differentiation of BM, blood, and BALf eosinophil-lineage–committed CD34+ cells toward more mature eosinophils. Importantly, AW CD34+ cells from allergen-exposed mice produce eosinophil colonies and express IL-5 receptor α chain after stimulation with IL-5. Moreover, AW CD34+ eosinophilic cells themselves release high amounts of IL-5 after unspecific stimulation.
Increased number of CD34+ cells in bone marrow20,21 and AW13 in atopic individuals or individuals with ongoing asthma disease has previously been documented. To date, however, no simultaneous evaluation of changes in CD34+ cells in BM and AW has been presented. In this study, we evaluated not only the allergen-induced changes in CD34+ cells in BM, blood, and BALf but also allergen-induced changes in newly produced CD34+ cells. To assess these changes, we performed double immunocytochemical staining of CD34 antigen with BrdU nuclear staining. In the BM, allergen exposure did not induce significant changes in the relative number of CD34+ cells or in the number of newly produced CD34+ cells. This finding is in agreement with our previous report, in which no significant allergen-induced changes in total number of BrdU+ cells in BM were observed.7 However, allergen exposure induced a significant increase in CD34+ cells in blood and BALf, where newly produced CD34+ cells substantially contributed to the observed increase. After allergen exposure, newly produced CD34+ cells comprised around 75% of total BALf CD34+ cells and about 85% of total blood CD34+ cells. The observed increased number of BrdU+ cells in BALf is at least in part the result of increased recruitment from the bone marrow, because an increased number of these cells was observed in blood. The fact that we did not detect significant changes in BM progenitor numbers suggests that BM has a rapid turnover of CD34+ cells being released into the circulation, accumulated into the AW, and very rapidly produced de novo. Taking into account that CD34-deficient mice fail to establish AW eosinophilia after allergen exposure,11 it is most likely that an allergen-driven increased number of newly produced CD34+ cells in the AW represents an expanded pool of eosinophil precursors available for further differentiation. However, the observed magnitude of the increase in newly produced CD34+ cells in BALf implies that apart from recruitment from the BM there might be an additional source of newly produced CD34+ cells. This led us to the conjecture that the increased number of newly produced CD34+ cells in the AW can also be the result of local multiplication of these cells within the AW.
CD135 is a receptor tyrosine kinase expressed on primitive hematopoietic progenitors. Its interaction with ligand is required for normal development of stem and progenitor cells. Early commitment of CD34+ cells into myeloid lineage is accompanied by up-regulation of CD135 antigen expression.22,23 Here we show, for the first time, that allergen exposure induces expression of CD135 antigen on CD34+ cells in BM. Together with previously discussed results, this argues that allergen exposure stimulates very early myeloid progenitors within the BM CD34+ cell population, without significant changes in total number of BM CD34+ progenitor cells.
Recent data suggest that hematopoietic stem cells can reside in both CD34-positive and -negative compartments.24 Furthermore, CD34 expression is reversible in hematopoietic stem cells,25 and it reflects the activation/kinetic state of these cells.26 To investigate the relation between CD34+ cells from the bronchoalveolar fluid and a hematopoietic stem cell phenotype, we analyzed the expression of Sca-1 on BALf CD34+ cells. Sca-1 is the marker defining the hematopoietic stem cell in mice.27 A recent study using Sca-1–/– mice has shown that Sca-1 plays an important role in regulating the repopulating ability of hematopoietic stem cells and the development of committed progenitor cells.28 Our data show that allergen exposure induces significant increase in Sca-1 expression within the BALf CD34+ population. After allergen exposure, 30% of the CD34+ cells were positive for Sca-1, which argues that approximately one third of BALf CD34+ cells resemble a hematopoietic stem cell phenotype.
CD34+ cells expressing a membrane-bound IL-5 receptor α-chain (IL-5Rα) are considered to be early eosinophil precursors.12 Subjects with asthma have increased numbers of these double-positive cells in the bronchial mucosa,13 which argues that eosinophils could differentiate from CD34+ cells locally within the AW. Supporting this hypothesis, however, there is only indirect evidence, such as increased expression of IL-5Rα mRNA on CD34+ cells within asthmatic bronchial mucosa13 or simultaneous decrease in the number of CD34+/IL-5Rα+ cells and increase in the number of major basic protein positive (MBP+) cells in allergic nasal tissue explants in response to rIL-5 or allergen.14
In our study, we used 2 different methods to assess commitment of CD34+ progenitor cells into eosinophil lineage, namely, coexpression of CD34 antigen and IL-5Rα and, second, appearance of eosinophilic granulation on CD34+ cells. In addition, to establish the relationship between these 2 eosinophil lineage features and the stage of cell differentiation, we evaluated the phenotype of cells committed to the eosinophil lineage.17 Allergen exposure did not induce changes in the number of CD34+ cells expressing IL-5Rα in BM or blood, which suggests that this cell population is somewhat constant and independent of allergen exposure in mice; this is also consistent with our earlier observations.7 In the bone marrow, the CD34+/IL-5Rα+ cell population was composed of cells in the first, second, and third stages of differentiation. CD34+ cells in the 1st stage of differentiation expressing IL-5Rα are the earliest detectable eosinophil precursors and were found only in the BM. In the blood, the CD34+/IL-5Rα+ cell population was composed of cells in the second and third stages of differentiation. CD34+/IL-5Rα+ cells in the second differentiation stage were the earliest eosinophil progenitors detected in the blood, which argues that cells might be recruited to blood from the bone marrow only after they have reached the second differentiation stage. No IL-5Rα positive staining was detected in BALf, which confirms our previous study.7 It seems reasonable to assume that the lack of IL-5Rα expression on BALf cells is the result of a change from the membrane-bound form to the soluble form of receptor, a phenomenon that has been described in the human BALf eosinophils in response to allergen challenge.29 Furthermore, an increase in the number of CD34+/IL-5Rα+ cells in asthmatic AW has been described only in bronchial mucosa,13 and there are no data regarding the presence of these cells in BALf. In contrast, allergen exposure induces an increase in the number of CD34+ eosinophilic cells and CD34– eosinophils in bone marrow, blood, and BALf. Allergen-induced increase in CD34+ eosinophilic cells in the second and third stages of differentiation in BALf was associated with a parallel increase of respective cells in BM and blood, which suggests that these cells are recruited into the AW from the bone marrow via blood. On the other hand, the CD34– cell population in BM, blood, and BALf was composed of more mature eosinophilic cells in the third and fourth stages of differentiation, which argues that eosinophils begin to shed CD34 antigen starting from the third stage of differentiation and that expression of this antigen is lost on eosinophils in the fourth (terminal) stage of differentiation. However, in BALf, the population of eosinophilic cells in the fourth stage of differentiation contained not only CD34– cells, as in BM and blood, but also CD34+ cells. Because CD34+ eosinophilic cells per se were found only in the AW, it seems reasonable to assume that this cell type is the result of local eosinophil development, which can be either terminal differentiation of recruited eosinophil-lineage–committed precursor cells, or local production of eosinophils within the AW. No eosinophilic granulation was found in CD34+ cells in the 1st differentiation stage in any compartment, which shows that eosinophilic granulation appears as early as in the second cell-differentiation stage. In summary, there is a pre-existing pool of early eosinophil-lineage–committed precursors in the BM, a pool that is independent of allergen exposure. Also, allergen exposure induces a shift in differentiation of available precursors in BM and precursors recruited into blood and AW toward more mature eosinophils. Finally, the allergen-induced population of terminally differentiated eosinophils in BALf is composed of 2 distinct populations: 90% CD34– eosinophils and 10% CD34+ eosinophilic cells.
To evaluate the assumption that CD34+ cells cannot only differentiate but also retain the ability to multiply within the AW, we performed semisolid culture of BALf CD34+ cells. Culture of BM CD34+ cells from the same animals was used as a positive control. Culture of BALf CD34+ cells in the presence of rIL-5 resulted in growth of eosinophilic colonies. Because recent reports have shown that also CD34– cells can possess hematopoietic activity,24 we also cultured BALf CD34– cells. However, no CFU outgrowth from these cells was observed (data not shown). The number of Eo-CFUs grown from BALf CD34+ cells was lower compared with Eo-CFUs grown from BM CD34+ cells. This is not surprising, because the earliest (and apparently the most mitotically active) eosinophil progenitors were found only in the BM. Considering that the responsiveness of cells to IL-5 is controlled by IL-5Rα expression30 and that we did not detect IL-5Rα staining in BALf in in vivo experiments, we repeated immunocytochemical staining for IL-5Rα for cells recovered from cultures in the presence of rIL-5. We found that 19% of eosinophils grown from BALf CD34+ cells and 26% of eosinophils grown from BM CD34+ cells expressed IL-5Rα. Also, we found that approximately 50% of the eosinophils developed from BALf and BM CD34+ cells in vitro express CD34 antigen, which is consistent with our in vivo observations. Importantly, BALf CD34+ cells released markedly more IL-5 in response to unspecific stimulation compared with CD34– cells. Our in vitro data show that allergen-induced BALf CD34+ cells retain the ability to multiply, likely via up-regulation of membrane-bound IL-5Rα in response to rIL-5, as has also been described for human cord blood CD34+ cells.31 Furthermore, BALf CD34+ cells release quite high amounts of IL-5, suggesting that these cells have autocrine multiplication and/or differentiation pathways, which is in line with a previously suggested autocrine differentiation pathway of peripheral blood CD34+ cells in individuals with asthma.19
In conclusion, our study shows that AW allergen exposure induces an increase in early CD135+ bone marrow hematopoietic progenitors without a simultaneous increase of the total number of bone marrow CD34+ cells. In addition, allergen exposure increases the number of CD34+ cells in BALf, and these CD34+ cells not only can complete their differentiation into eosinophils but also have the capacity to produce eosinophil colonies, proving their ability to multiply. The fact that BALf CD34– cells failed to produce colonies implies that after allergen exposure all true AW hematopoietic progenitor cells belong to a CD34+ cell fraction rather than to a CD34– fraction. Together, our in vivo and in vitro data suggest that the CD34+ and CD34– populations of terminally differentiated eosinophils present in BALf after AW allergen exposure are the result of eosinophil development either in AW or in bone marrow, respectively. Thus, eosinophilic cells that only complete their differentiation in the AW shed CD34 antigen and, therefore, represent the CD34– fraction. On the other hand, CD34+ terminally differentiated eosinophilic cells in BALf represent a previously undetected cell population that might be the result of multiplication of CD34+ cells within the AW. Moreover, AW CD34+ eosinophilic cells have a much greater capacity to release IL-5 compared with CD34– eosinophils, showing that these eosinophil fractions are functionally different. Enhanced release of IL-5 also suggests that these CD34+ cells may maintain AW eosinophilic inflammation via autocrine IL-5–dependent multiplication and eosinophil maturation within AW.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-05-1618.
Supported in part by the Swedish Heart and Lung Foundation, the Vårdal Foundation of Sweden, and the Swedish Medical Research Council (K2001-71X-13492-02B).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Margareta Sjöstrand and Rille Pullerits for technical assistance during the progress of the study and Teet Pullerits for helpful discussion.