Abstract
The reduction in expression of the integral membrane protein CD47 in human red blood cells (RBCs) deficient in protein 4.2 suggests that protein 4.2 may mediate a linkage of CD47 to the membrane skeleton. We compared the fractions of membrane skeleton-attached CD47, Rh-associated glycoprotein (RhAG), Rh, and band 3 in normal and protein 4.2-deficient cells using fluorescence-imaged microdeformation. We found that CD47 attachment decreases from 55% in normal cells to 25% to 35% in 4.2-deficient cells. RhAG, which has been shown to have no significant variation in expression among the cells studied, shows a significant decrease in membrane skeleton attachment in 4.2-deficient cells from 60% to 40%. Both Rh and band 3, which have also been shown to have no change in expression, show a smaller decrease from 75% attached in normal RBCs to 55% attached in 4.2-deficient cells. In normal cells, Rh phenotype influences CD47 expression but not the level of membrane skeleton attachment of CD47. In contrast, the results indicate that protein 4.2 strongly influences CD47 levels as well as the extent of membrane skeleton attachment in the RBC, whereas protein 4.2 affects membrane skeletal attachment of RhAG, Rh, and band 3 to a lesser extent. (Blood. 2004;103:1131-1136)
Introduction
The role of the ubiquitously expressed transmembrane protein CD47 integrin-associated protein (IAP) on various cell types has been the focus of an increasing number of studies. On hematopoietic cells, surface expression of CD47 is involved in leukocyte activation and chemotaxis,1 T-cell adhesion,2 and macrophage multinucleation.3 On mature erythrocytes CD47 interacts with the signal regulatory protein α (SIRP-α) of splenic macrophages.4 Subsequent studies with red blood cells (RBCs) from CD47 knockout mice suggest that this association inhibits phagocytosis, thereby delaying erythrocyte clearance from the circulation and implicating CD47 as a “marker of self” in mice.5
In an attempt to further elucidate the function and interactions of CD47 in the human erythrocyte, we recently provided visual evidence for colocalization of CD47 with Rh and Rh-associated glycoprotein (RhAG) on the RBC surface.6 The results appear consistent with reduced expression of CD47 in Rhnull erythrocytes, which lack Rh and RhAG proteins.7 It has been shown recently that RBCs expressing no RhCE (D-- erythrocytes) or considerably reduced levels of RhCE (D.. and RN erythrocytes) also have reduced levels of CD47, suggesting that CD47 may be associated preferentially with the RhCE protein.8 However, our recent studies of Rhnull cells concluded that approximately 60% of CD47 on the RBC surface is connected to the membrane skeleton, also referred to in this work as the spectrin-actin cytoskeleton, independent of Rh or RhAG.6
In addition to cis interactions between CD47 and Rh, protein 4.2 has recently been suggested to have vertical interactions with CD47, linking it to the cytoskeleton in RBCs. Protein 4.2 binds to the cytoplasmic domain of band 3 and to spectrin in solution, suggesting that it stabilizes the connection of band 3 to spectrin mediated by ankyrin.9 Several different protein 4.2 mutations result in a loss of protein 4.2 expression, causing spherocytosis in the homozygous state,10 and 2 groups have shown that there is also a substantial reduction of CD47 on protein 4.2-deficient RBCs (4.2-Hammersmith11 ; 4.2-Lisboa and 4.2-Nancy8 ). Bruce et al11 noted that the absence of protein 4.2 in 4.2-deficient Hammersmith RBCs was associated with either a 75% reduction in CD47 using flow cytometry (FC) or an apparent absence of CD47 by Western blot. Mouro-Chanteloup et al8 also observed an approximately 80% reduction in CD47 in 4.2-deficient Lisboa and Nancy cells by FC. Unlike CD47, expression levels of band 3, RhAG, and Rh peptides in 4.2-deficient RBCs were reported to be mostly unchanged. The RhAG protein was slightly altered in that it showed decreased mobility in a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, suggesting an increased glycosylation.8,11 Membrane solubilization studies with the detergent Triton X-100 were inconclusive with respect to the skeleton-attached fraction of CD47 due to minimal residual protein. No qualitative change in the connection of Rh and RhAG to the cytoskeleton was seen in the 4.2-deficient cells.8
The present studies were undertaken to quantify the cytoskeletal attachment of CD47, RhAG, and Rh in normal and protein 4.2-deficient cells. Although previous studies have shown a severe reduction in surface expression of CD47 in protein 4.2-deficient RBCs,8,11 the sensitive biophysical technique used here, fluorescence-imaged microdeformation12 (FIMD), provides details of subtle changes in cytoskeletal attachment combined with reduced protein expression levels. Our most significant finding, perhaps, is that deficiency of protein 4.2 leads to a considerable increase in the mobile fractions of CD47 as well as RhAG and Rh proteins. Interestingly, CD47 levels, but not cytoskeletal attachment, appears to depend on Rh phenotype in normal cells, a feature that has received little attention to date.
Materials and methods
Erythrocytes
Control erythrocytes were collected at room temperature in phosphate-buffered saline (PBS; Sigma-Aldrich, St Louis, MO) and washed twice with PBS. Cytoskeletal connectivity experiments were performed on normal RhD-negative (ce/ce) and homozygous RhD-positive (DcE/DcE) cells as indicated. FC experiments were performed on normal cells of varying Rh phenotype; both CD47 and RhD expression levels are reported.
The 4.2-Nippon cells (Dce/DcE), which express less than 1% protein 4.2 compared to normal,13 homozygous (probable DCe/ce), heterozygous (probable DCe/ce) 4.2-Hammersmith cells,11 and control cells were all collected in lithium heparin tubes and shipped overnight on ice. Heterozygous 4.2-Hammersmith cells have previously been shown to have 95% of normal protein 4.2.11
Antibodies
The following murine monoclonal antibodies were used: R-phycoerythrin (PE)-labeled and unlabeled BRIC126 (IgG2b; IBGRL, Bristol, United Kingdom), B6H12 (IgG1; BD PharMingen, San Diego, CA), 6H914 (IgG1), and 2D3 (IgG1; Oncogene, San Diego, CA) anti-CD47; 2D1015 (IgG1) anti-RhAG; F8D8 (IgG1; Gamma Biologicals, Houston, TX) anti-RhD; BRIC-69 (IgG1; IBGRL) blood group Rh-related antibody; BRIC6 (IgG3; IBGRL) antiband 3; fluorescein-5-isothiocyanate (FITC)-labeled CBL-467F anti-CD59 (IgG2a; Cymbus Biotechnology, Chandlers Ford, United Kingdom); and FITC-labeled BRIC10 (IgG1; IBGRL) antiglycophorin C. Binding of unlabeled mouse antibody was detected with a FITC-labeled Fab fragment of goat antimouse IgG (Jackson Laboratories, West Grove, PA) for visual fluorescence and PE-conjugated goat antimouse IgG (Sigma-Aldrich) for FC. The secondary antibodies were tested to ensure no nonspecific binding to human erythrocytes. All antibody binding was done at room temperature.
Image acquisition and analysis
Microscopy images were acquired on a Nikon TE300 inverted microscope with a × 60 (oil, 1.4 NA) objective using a liquid nitrogen-cooled charge-coupled device (CCD) camera (Roper Scientific, Trenton, NJ). The liquid nitrogen-cooled CCD camera used for imaging is well known for its linear response as well as high sensitivity.16 Image acquisition was performed with Image Pro software (Media Cybernetics, Silver Spring, MD). Scion Image (Scion, Frederick, MD) was used to determine overall fluorescence intensity of labeled cells acquired using visual microscopy. Two rectangular sections of approximately 2 μm thick were measured across the fluorescent RBC images, and the edge brightness at 4 points was averaged and the average of 5 background intensities was subtracted.
FC
RBCs were incubated with saturating concentrations of CD47 antibodies BRIC 126, B6H12, and 6H9 or RhD antibody F8D8. Data were collected either on FACScan or FACSCalibur flow cytometers in the Flow Cytometry and Cell Sorting Shared Resource of the School of Medicine at the University of Pennsylvania.
FIMD
Images of labeled cells aspirated into micropipettes were obtained and fluorescence intensities were measured using similar methods described for image acquisition. Central features of FIMD methods and analyses are described in “Results.” Further details can be found in Discher et al12 and Discher and Mohandas.17 FIMD has been used previously to describe the cytoskeletal connection of CD47 and RhAG proteins in normal RBCs.6
The error associated with the FIMD analysis (fitting linear regressions to the data) outweighs sources of experimental error. The analysis error was estimated following Taylor,18 from the combined uncertainty of each data point in relation to a least-squares linear slope fit to the data and normalizing by the number of points to get an SEM.
Results
Immunodetection of CD47 on protein 4.2-deficient and normal cells
Because residual levels of CD47 in 4.2-deficient RBCs have differed quantitatively in past reports,8,11 the effects of potential variables such as antibody choice, detection method (fluorescence microscopy versus FC), and the Rh phenotype were tested. Fluorescence microscopy assessments are also important as a basis for FIMD experiments.
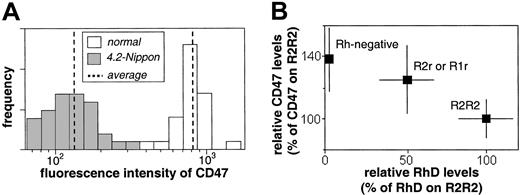
A histogram of fluorescence intensity measured by visual microscopy highlights the deficiency of CD47 on 4.2-Nippon RBCs measured at saturating antibody concentrations (Figure 1A). There is broad variation in fluorescence intensity among cells in the sample, which suggests differences in CD47 surface expression. However, the 4.2-deficient cells express mean levels of CD47 that are 17% of levels expressed on normal RBCs. These measurements are consistent with those obtained by FC, which are summarized in Table 1.
CD47 concentration on normal and 4.2-deficient RBCs. (A) Fluorescence image intensity analysis of 4.2-Nippon RBCs (n = 60 each) labeled with 6H9 shows a mean CD47 level that is 17% of normal with significant variation among both normal and 4.2-Nippon cells. (B) FC measures of CD47 expression for 2 homozygous RhD (R2R2), 2 heterozygous RhD (R2r or R1r), and 4 RhD-negative RBC samples. CD47 levels for R2R2 and Rh-negative RBCs are statistically different (P < .001). Cells were stained with the antibodies F8D8 (RhD) or BRIC 126 (CD47) and a fluorophore-conjugated antihuman or antimouse secondary antibody.
CD47 concentration on normal and 4.2-deficient RBCs. (A) Fluorescence image intensity analysis of 4.2-Nippon RBCs (n = 60 each) labeled with 6H9 shows a mean CD47 level that is 17% of normal with significant variation among both normal and 4.2-Nippon cells. (B) FC measures of CD47 expression for 2 homozygous RhD (R2R2), 2 heterozygous RhD (R2r or R1r), and 4 RhD-negative RBC samples. CD47 levels for R2R2 and Rh-negative RBCs are statistically different (P < .001). Cells were stained with the antibodies F8D8 (RhD) or BRIC 126 (CD47) and a fluorophore-conjugated antihuman or antimouse secondary antibody.
FC analyses of 4.2-Nippon RBCs labeled with 4 different antibodies (B6H12, 6H9, BRIC126, and 2D3) suggest that these cells express levels of CD47 that are 17% to 20% of normal. The 4.2-Hammersmith RBCs express slightly higher levels of CD47 (24%-25% of normal), consistent with previous reports.11 Although the analyses show that there is at least a 75% reduction in CD47 levels on 4.2-deficient RBCs, they confirm that there are sufficient levels of detectable protein to conduct biophysical studies (FIMD).
To determine if the slight difference in residual levels of CD47 on Hammersmith compared to Nippon cells might be related to their Rh phenotype, especially given the observation that CD47 levels are diminished on RBCs lacking expression of RhCE,8 we examined normal RBCs for correlation of expression of CD47 with Rh. It is well known that RhD expression differs in RBCs with different Rh phenotypes, and because the total Rh protein level is essentially constant,19 RBCs with the highest expression of RhD (homozygous R2R2) will have less RhCE. Because an appropriate monoclonal antibody for FC analysis that recognizes only RhCE was not available, an anti-RhD was used to look for a correlation between levels of RhD and CD47. RBCs homozygous for RhD (R2R2), heterozygous (R2r or R1r), or lacking RhD (RhD-negative) were tested for both RhD and CD47 by FC, and the analysis was done without prior knowledge of the Rh phenotypes. Indeed, average CD47 expression inversely correlated with RhD expression (Figure 1B). CD47 levels for homozygous (R2R2) compared to RhD-negative RBCs are statistically different (Student t test, P < .001) and differ by approximately 40%. CD47 expression levels on heterozygous (R2r or R1r) RBCs are intermediate to, but not statistically different from, the homozygous positive and RhD-negative RBCs. These data predict that CD47 levels do vary with Rh phenotype. However, the differences in residual CD47 between 4.2-Nippon (homozygous RhD) and the 4.2-Hammersmith RBCs (heterozygous RhD) exceed the differences seen in normal RBCs and cannot be completely accounted for by Rh phenotype. This suggests perhaps that the 4.2 mutation-specific differences between 4.2-Nippon and 4.2-Hammersmith RBCs might have an impact on CD47 levels.
FIMD and cytoskeletal connectivity
Combining immunofluorescence with micropipette aspiration, FIMD12 provides a quantitative, highly sensitive measure of cytoskeletal connectivity (or its converse, mobility) for surface proteins in intact RBCs. FIMD requires one image, and the cytoskeletal connectivity of extremely low levels of protein can be measured without significant photobleaching and loss of signal, which is a problem with fluorescent recovery after photobleaching (FRAP).
In FIMD, the fluorescence gradient along the projection of the RBC aspirated into a horizontally held micropipette is directly related to cytoskeletal connectivity.12 As seen from a simulation of an aspirated RBC (see also Discher et al20 ), the spectrin-actin network is compressed at the entrance of the pipette, e, and dilated at the cap, c (Figure 2A). Fluorescently labeled glycophorin C, which is nearly 100% cytoskeletally attached through protein 4.1,17 shows a maximum fluorescence intensity at the entrance of the pipette, denoted as e-intensity, and minimum intensity at the cap of the projection, c-intensity. These quantities reflect the cytoskeleton compression and dilation in the aspirated projection. Quantification of FIMD data are based on the ratio of e-intensity to c-intensity, plotted as a function of the projection length normalized by the pipette radius, L/Rp. An increase in e-intensity/c-intensity, with increased projection length or strain suggests greater network association. A plot of e-intensity/c-intensity versus L/Rp yields a nearly straight line with a slope, m, related to the degree of cytoskeletal connectivity, independent of protein concentration. This slope for glycophorin C in 4.2-deficient RBCs is less than 5% different than normal cells (Figure 2B), validating its use as a control.
FIMD analysis compares the fluorescence intensity at the entrance of a micropipette with the intensity at the cap. (A) Simulation of an aspirated RBC reference clearly shows the spectrin-actin network to be compressed at the entrance, e, and dilated at the cap, c. Membrane proteins associated with the spectrin-actin cytoskeleton such as glycophorin C show fluorescence profiles reflecting these network gradients. (B) Glycophorin C shows only a slight change in projection gradient or fitted slope for normal versus 4.2-Nippon (4.2-N) cells; asterisks indicate statistically different slopes. In contrast, CD47 on 4.2-deficient cells shows a significant and clear reduction in connectivity from the normal.
FIMD analysis compares the fluorescence intensity at the entrance of a micropipette with the intensity at the cap. (A) Simulation of an aspirated RBC reference clearly shows the spectrin-actin network to be compressed at the entrance, e, and dilated at the cap, c. Membrane proteins associated with the spectrin-actin cytoskeleton such as glycophorin C show fluorescence profiles reflecting these network gradients. (B) Glycophorin C shows only a slight change in projection gradient or fitted slope for normal versus 4.2-Nippon (4.2-N) cells; asterisks indicate statistically different slopes. In contrast, CD47 on 4.2-deficient cells shows a significant and clear reduction in connectivity from the normal.
The fraction of cytoskeletal connectivity, f*, is the ratio of cytoskeletally attached protein to total surface protein, and (1 - f*) represents the mobile fraction of protein. Connectivity is determined from the projection slopes of glycophorin C (nearly 100% network-linked) and GPI-linked CD59 (0% linked): f*i = (mi-mCD59)/(mGlyC-mCD59). The slight reduction in the projection slope of glycophorin C (Figure 2B) and CD59 (data not shown) between 4.2-deficient RBCs and normal cells suggests a slight reduction in overall cytoskeletal arrangement and rigidity, but alters the connectivity data for other proteins by less than 4%. The reproducibility of cytoskeletal attachment by FIMD is within 10% to 15%, but the greatest source of error is associated with determination of the least squares fit slope.
Cytoskeletal connectivity in normal cells
Despite the variations in expression level of CD47 on RhD-positive compared to RhD-negative normal cells, cytoskeletal attachment measured by FIMD does not seem to be affected by Rh phenotype within the uncertainty of the technique (Figure 3). FIMD of CD47 and RhAG in both RhD-negative and homozygous RhD-positive normal RBCs show similar connectivity, 55% and 60%, respectively, which is consistent with our previous results.6 Although there is a slightly higher cytoskeletal attachment for RhAG compared to CD47, the increase is not within an acceptable confidence level to make any conclusive argument. In contrast, Rh shows a cytoskeletal attachment level of over 70%, which is significantly higher than attachments of RhAG and CD47. For reference, band 3 in normal RBCs here is approximately 75% attached to the cytoskeleton, which is in good agreement with values previously cited by FIMD17 and by other methods.21
Cytoskeletal attachment of surface proteins in normal and 4.2-deficient RBCs. FIMD of CD47 on normal RhD-negative (-), normal homozygous RhD-positive (+), 4.2-Hammersmith (H), and 4.2-Nippon (N) cells shows significant changes in CD47 cytoskeletal attachment for 4.2-deficient RBCs. In 4.2-deficient RBCs, RhAG and Rh both have a reduced cytoskeletal connectivity compared to normal. The reduction of 30% in cytoskeletal attachment seen in band 3 is consistent with previous results for 4.2-Nippon RBCs.13
Cytoskeletal attachment of surface proteins in normal and 4.2-deficient RBCs. FIMD of CD47 on normal RhD-negative (-), normal homozygous RhD-positive (+), 4.2-Hammersmith (H), and 4.2-Nippon (N) cells shows significant changes in CD47 cytoskeletal attachment for 4.2-deficient RBCs. In 4.2-deficient RBCs, RhAG and Rh both have a reduced cytoskeletal connectivity compared to normal. The reduction of 30% in cytoskeletal attachment seen in band 3 is consistent with previous results for 4.2-Nippon RBCs.13
Changes of cytoskeletal connectivity in protein 4.2-deficient cells
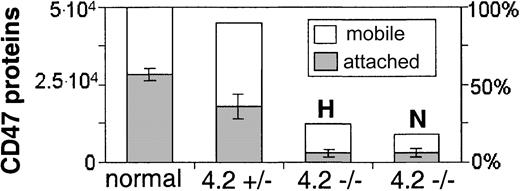
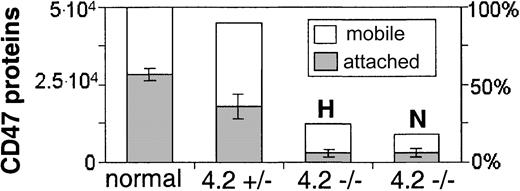
CD47, RhAG, Rh, and band 3 all show reduced cytoskeletal attachment in 4.2-deficient cells compared to normal cells (Figure 3). Band 3, used here as a reference for FIMD, has a 30% reduction in cytoskeletal connectivity in the 4.2-Nippon RBCs (from f* = 75% in normal to f* = 53%), which is consistent with measurements made by rotational mobility and detergent extraction.13 Cytoskeletal connectivity of CD47 in 4.2-Nippon is reduced by almost half, from f* = 55% in normal to f* = 32%. Changes in cytoskeletal attachment of CD47 are even more striking in 4.2-Hammersmith (f* = 24%), which may reflect a difference in the 4.2 mutation or CD47 surface concentration. Figure 4, which combines CD47 expression levels with cytoskeletal attachments, shows that both 4.2-Nippon and 4.2-Hammersmith have approximately the same number of cytoskeletally connected CD47 proteins.
Approximate number of mobile and cytoskeletally attached CD47 surface proteins per cell on normal and 4.2-deficient RBCs. Overall numbers of CD47 on normal RBCs7 partitioned into either mobile or cytoskeletally attached. CD47 levels for 4.2-Hammersmith heterozygote (4.2+/-), 4.2-Hammersmith homozygote (4.2-/- H), and 4.2-Nippon (4.2-/- N) RBCs are presented as relative to the normal referenced levels.
Approximate number of mobile and cytoskeletally attached CD47 surface proteins per cell on normal and 4.2-deficient RBCs. Overall numbers of CD47 on normal RBCs7 partitioned into either mobile or cytoskeletally attached. CD47 levels for 4.2-Hammersmith heterozygote (4.2+/-), 4.2-Hammersmith homozygote (4.2-/- H), and 4.2-Nippon (4.2-/- N) RBCs are presented as relative to the normal referenced levels.
In both 4.2-Nippon and 4.2-Hammersmith, the connectivity of RhAG is reduced from that seen in normal RBCs (from f* = 60% in normal to f* = 40% in 4.2-deficient cells), but the reduction is less severe than that of CD47. In contrast to RhAG and CD47, Rh proteins in 4.2-Hammersmith RBCs show less than a 30% decrease in cytoskeletal connectivity (from f* = 70% in normal to f* = 50%).
We also tested heterozygous 4.2-Hammersmith RBCs (data not shown) and found that they have an intermediate level of CD47 cytoskeletal connectivity (f* = 40%) compared to the homozygous 4.2-Hammersmith (f* = 24%) and normal RBCs (f* = 55%) despite having near normal CD47 expression (Table 1). This reduced connectivity combined with little change in expression shows an intermediate level of overall attached proteins (Figure 4). However, the error bars for FIMD measurements of the heterozygous RBCs were almost 4-fold larger than those of the homozygotes and suggest cell heterogeneity that is not amenable to single-cell techniques.
Discussion
Cytoskeletal attachments of the Rh-macrocomplex may vary during RBC maturation
Measurements of cytoskeletal attachments of the proteins CD47, RhAG, and Rh in normal, mature RBCs reported here supplement the wealth of information currently available on RBC cytoskeletal architecture. Proteins of the Rh complex have recently been reported to have cytoskeletal attachments,6,22 and these interactions also seem to vary with RBC maturation. For example, in K562 human erythroleukemia cells the cytoskeletally attached fraction of Rh and RhAG was found by detergent extraction to be 54% and 79%, respectively.23 Our results using FIMD on mature RBCs show the inverse trend suggesting a change in cytoskeletal attachments from precursors to mature cells.
Also, it has been shown in both K562 cells and early progenitor cultures that CD47 is expressed on the cell surface before band 3, RhAG, and Rh proteins,24,25 and CD47 has been shown by detergent extraction in progenitor cultures to be cytoskeletally linked before the presence of band 3.23 However, expression of band 3 is thought to be necessary for protein 4.2 association to the spectrin-actin cytoskeleton.26 Because progenitor cells do not have any spectrin-bound protein 4.2, but do have cytoskeletally attached CD47, it is likely that CD47 changes its cytoskeletal attachment sites during erythrocyte development and maturation.
Protein 4.2 deficiency and surface protein cytoskeletal attachment
Protein 4.2 has long been known to bind band 3,9 and our FIMD data corroborate the 30% decrease in cytoskeletal connectivity of band 3 seen previously in 4.2-Nippon cells.13 Ankyrin and protein 4.127 may serve to mediate residual band 3 connectivity in the absence of protein 4.2. Rh shows loss of cytoskeletal connectivity similar to band 3 in 4.2-deficient cells, and RhAG has a significantly greater loss of cytoskeletal connectivity. CD47 shows, by far, the most severe loss of cytoskeletal attachments, not just in attached fraction, but also in overall numbers (Figure 4).
Previously, Western blots of Triton X-100 extractions have suggested that Rh and RhAG are not altered in their cytoskeletal connectivity in 4.2-deficient RBCs (specifically 4.2-Lisboa and 4.2-Nancy) versus normal.8 The insensitivity of immunoblots to small changes in protein concentrations could be a confounding factor. We propose that our measured increase in mobile fraction for Rh and RhAG could be due in some part to the slight increase in mean expression of both of these proteins found consistently in previous studies8,11 ; the number of cytoskeleton-connected RhAG molecules stays relatively similar, whereas the overexpressed RhAG protein adds only to the mobile fraction.
Band 3, Rh, and RhAG are all thought to bind ankyrin9,22 and show a reduction in cytoskeletal attachment of near 30%. In contrast, 4.2-deficient RBCs showed a small (< 5%) decrease in the attachment of glycophorin C, which is known to bind the cytoskeleton independent of ankyrin.28 It is possible that the loss of 30% of the cytoskeletal attachments is related to indirect or direct stabilization of ankyrin complexes by protein 4.2. However, the severe loss of CD47 in 4.2-deficient cells suggests that CD47 cytoskeletal attachment is mediated most directly by protein 4.2.
Cis interactions of CD47 to band 3 or Rh
Previous work from our group showed a substantial cytoskeletal attachment of CD47 in Rhnull cells, indicating that Rh and RhAG do not mediate most CD47 attachment.6 Neither is CD47 attachment entirely dependent on band 3; the differential reduction of CD47 and band 3 in expression and cytoskeletal attachment suggests band 3-independent cytoskeletal linkage for at least a fraction of CD47. Also, band 3 is not preferentially recruited into induced clusters of CD47 and Rh complexes in normal RBCs.6
With the combined changes in residual surface expression and cytoskeletal connectivity, 4.2-Hammersmith and 4.2-Nippon both have 10% of the normal CD47 cytoskeletal attachments (Figure 4). It is thus possible that about 10% of attached CD47 in normal cells is not connected to the cytoskeleton via protein 4.2. The small number of CD47 molecules that remain cytoskeletally connected in 4.2-deficient cells may suggest either that CD47 has connections to the actin-spectrin cytoskeleton other than via protein 4.2, or the presence of some weak cis interactions with band 3, Rh, RhAG, or other proteins.
Protein 4.2 in overall spectrin-actin stability
Protein 4.2-deficient cells show a slightly decreased projection slope for glycophorin C and a slightly increased slope for CD59 when compared to normal cells. Combining the changes in glycophorin C and CD59 in the 4.2-deficient RBCs, there appears to be a 20% decrease in the fluorescence segregation (from e to c) associated with FIMD. Although this small variation does not noticeably alter connectivity results for intermediately connected proteins, there is some significance in the decrease of apparent network stability. Previous studies of membrane proteins, the spectrin-actin cytoskeleton, and other linking proteins have regarded protein 4.2 as insignificant to membrane stability.29 The change in response of glycophorin C, which has no linkage to protein 4.2, and CD59 by FIMD suggests that there may be some significance to the decrease of apparent network distension. This is supported by and may partially explain the reported morphology changes in protein 4.2-deficient RBCs.10
Minimum amount of CD47 required for circulation despite mechanism of reduced expression
Several RBC membrane protein deficiencies result in a considerable decrease in surface concentration of CD47. Rhnull, protein 4.2 deficiency, D--, D.., and RN all show reduction in CD47,8 but there is no reported deficiency that results in a reduction more severe than 90%. It is possible that human RBCs require a minimum CD47 expression level to allow continued circulation. This may be related to the “marker of self” hypothesis advanced for CD47 function in murine erythrocytes5 ; a nonzero amount of CD47 may be required on human RBCs to prevent premature clearance by macrophages.
Cytoskeletal linkage during erythropoiesis has been implicated as a determinant in the concentration of surface proteins in mature erythrocytes; the more mobile a protein, the more likely it is to be shed by vesiculation during maturation.30 A reduction in the cytoskeletal connectivity of CD47 in 4.2-deficient cells may indeed explain CD47's severe reduction in surface expression. Conversely, CD47 in Rhnull cells shows no loss of cytoskeletal connectivity,6 suggesting that CD47 may not be adequately trafficked to the surface during erythrocyte maturation in Rhnull cells.
In normal cells, the Rh phenotype may affect the level of CD47 expression, but we show here that it does not affect the cytoskeletal attachment of CD47, RhAG, or Rh. However, in 4.2-deficient RBCs, CD47 shows a large reduction not only in expression but also in cytoskeletal attachment with a decrease from 55% to 25% to 35%. RhAG, which shows no change in expression levels,8,11 also shows a significant decrease in cytoskeletal attachment in 4.2-deficient cells from 60% to 40%. Rh and band 3 both show a smaller decrease from 75% attached in normal RBCs to 55% attached in 4.2-deficient cells. These results clearly indicate that protein 4.2 influences CD47 levels as well as the extent of cytoskeleton attachment in the RBC membrane, whereas Rh phenotype modulates CD47 expression but not cytoskeletal connectivity. Underlying mechanisms may reflect the complexities associated with the timing of protein expression during erythropoiesis.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-04-1331.
Supported by grant R01 1-R01-HL-62352-01 (D.E.D.) from the National Institutes of Health and a Whitaker Graduate Fellowship (K.N.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Very helpful discussions with and initial supply of 4.2-Hammersmith RBCs and controls from Prof. M. Tanner and L. Bruce (Bristol University, Bristol, United Kingdom) are gratefully acknowledged. The authors thank T. Frame (Gamma Biologicals, Houston, TX) for the antibody F8D8, A. E. von dem Borne (Netherlands Red Cross, Amsterdam) for the antibody 2D10, M. Telen (Duke University, Durham, NC) for the antibody 6H9.