Abstract
Familial hemophagocytic lymphohistiocytosis (FHLH) is a rare, rapidly progressive disorder of early childhood characterized by uncontrolled activation of T cells and macrophages. Although perforin gene mutations have been described in a proportion of patients with FHLH, the genotype/phenotype correlation is still limited. Only a few patients with late onset clinical manifestations have been reported. The biochemical and immunologic alterations in the asymptomatic phase are not well known. We report on a family in which 2 fraternal twins both homozygous for a perforin mutation previously described as causative of the disease, markedly differed in phenotypic expression of FHLH. The twins also had a second novel heterozygous mutation. Natural killer (NK) activity was severely impaired in the patient and was normal in the asymptomatic fraternal twin. Our report highlights that FHLH may present after a long disease-free interval during which biochemical or immunologic alterations may be not evident, thus implying a role for interfering factors. (Blood. 2004;103:4610-4612)
Introduction
Familial hemophagocytic lymphohistiocytosis (FHLH) is a rare autosomal recessive disorder of the immune system whose clinical symptoms arise as a consequence of uncontrolled activation of T cells and macrophages and overproduction of inflammatory cytokines.1 Indeed, all the clinical and biochemical signs of the disease reflect a potent inflammatory reaction triggered by still-unidentified circumstances and, presumably, infections.2,3 In keeping with the clinical pattern of fever, hepatosplenomegaly, and pancytopenia, serum inflammatory cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin-1 (IL-1), and IL-6 are markedly elevated.4,5 Massive lymphocyte and macrophage activation, leading to hemophagocytosis, are the prominent pathologic findings.1,3 Most patients manifest symptoms of disease within the first months of life,6 although a few cases of late onset have been reported.7 Low natural killer (NK) and cellular cytolytic activities are generally considered a functional marker of the disease. Mutations of the perforin (PRF1) gene, implicated in the osmotic lysis of the target cells, have been described in some patients with FHLH.8-12
Although several reports greatly contributed to the understanding of the clinical features and the pathophysiology of the disease,1-5 several aspects still need to be clarified. In particular, it remains to be defined which are the functional and the biochemical abnormalities in the asymptomatic phase, and what are the roles, if any, of different viruses in triggering the overt phase of disease. Furthermore, a clear correlation between genotype and phenotype is still lacking.
In this report, we describe a family of 6 members with 2 fraternal twins carrying alterations of the perforin gene profoundly differing in the clinical phenotype. The first subject developed a rapidly progressive disease after a disease-free interval of many years, while the second is still asymptomatic. The biochemical, immunologic, and genetic features in these 2 subjects were compared.
Study design
We investigated 6 family members. The proband presented at age 13 years with persistent fever, hepatosplenomegaly, cytopenia, and lymph node enlargement. Low white and red blood cell counts were recorded. Thereafter, moderate grade fever, jaundice, and cholestasis developed. Biochemical investigations revealed hypertriglyceridemia, hypofibrinogenemia, hypoalbuminemia, and hyperferritenemia. Signs of hemophagocytosis on a liver biopsy specimen led to a temptative diagnosis of FHLH. The patient died soon after the diagnosis because of the rapidly progressive disease. Before the overt manifestation of the disease, the patient successfully cleared a few viral infections, such as herpes simplex virus I (HSV I), Rubella virus, and Morbilli virus. At the age of 11 years, she was affected with an Epstein Barr virus (EBV) infection, which was apparently self-limiting. All the remaining family members are healthy, their clinical histories are negative, and laboratory data were normal as detailed in Table 1.
Perforin mutation analysis
Genomic DNA was isolated from peripheral blood lymphocytes and the exons 2 and 3 of the PRF1 coding region amplified using standard polymerase chain reaction (PCR) conditions. PCR products were recovered from 1.5% agarose with a size marker, purified using the QIAquick Gel extraction kit (Qiagen, Hilden, Germany) and sequenced in an automatic ABI 377 DNA sequencer (Applied Biosystems, Foster City, CA).
Cytotoxic assay
Infected target cells (K562) were pulsed for one hour at 37°C with Cr51 (30 μCi [1.11 MBq]/106 cells; Amersham Pharmacia, Buckinghamshire,
England) and washed 3 times with culture medium before the addition of effector cells. Specific lysis was measured in a triplicate assay performed with 5 × 103 target cells mixed with effector cells at different ratios (E/T = 25:1 to 3.125:1) in a total volume of 200 μL. The percentage of specific lysis was calculated as follows: 100 × (specific release - spontaneous release)/(total release - spontaneous release).
Results and discussion
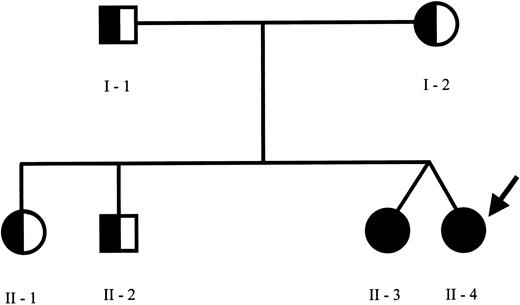
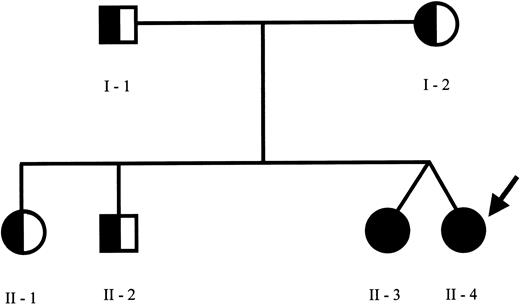
The pedigree of the family is shown in Figure 1. Sequence analysis of the perforin gene revealed the presence of 2 mutations in the proband. We found a C→T (C272T) homozygous substitution in exon 2 leading to an A91V amino acid change and a G→A (G695A) heterozygous substitution in exon 3 leading to an R231H change. The A91V mutation has been already reported in some patients with FHLH,7 while the R231H mutation represents a novel mutation. Sequence analysis of the family members revealed the presence of the A91V mutation at the heterozygous status in both parents, in the older sister and brother, and at the homozygous status in the fraternal twin sister. The R231H was found at the heterozygous status in the father and in the healthy twin. NK activity was normal in all family members, including the asymptomatic fraternal twin carrying the same genetic alterations of the proband (18%-30% of specific Cr51 release at the different E/T ratios) and markedly reduced in the patient, ranging between 2% and 5% of specific Cr51 release, in spite of a normal number of NK cells. This case report is interesting for several aspects. First, in the proband the overt clinical manifestations occurred after a disease-free interval longer than usually observed, as already reported in rare cases of adult presentation of HLH.7 The reason why some patients remain asymptomatic until adolescence or even adulthood remains unknown. The A91V PRF1 mutation occurs in the membrane attack complex extracellular domain of perforin, thus presumably modifying its binding to the target cell, required for pore formation. Notably, this mutation was frequently detected in association with FHLH in affected families from southern Italy.7 Thus far, nonsense, missense, and point mutations have been described in several domains but no correlation was found between PRF1 genotype and clinical manifestations.13 Therefore, it is unlikely that this particular mutation is associated with delayed clinical presentation. Remarkably, the same mutations were found in the 2 twins, only in one case being associated with a rapidly progressive and fatal clinical phenotype. The comparison of the clinical history of these subjects revealed that they share several similarities with regard to the encountered viral agents. The healthy sister had a normal clinical course during several infections, including an EBV infection, which is usually considered a triggering factor.14 This would suggest that virus-derived products are not sufficient per se to determine the accelerated phase of the disease. The functional studies in the 2 sisters revealed that NK activity was absent in the patient and normal in the healthy sibling with the same mutations. Previously, a reduction of the activity has been documented in heterozygous subjects, suggesting that a few mutations may exert a partial dominant-negative effect.15 In a large series of patients with HLH, NK activity was clearly impaired only in patients with central nervous system disease and normal in patients without brain involvement.16 Moreover, in a few cases NK activity was initially normal and subsequently decreased, thus implying that additional genetic or environmental factors may interfere with the immune function. The molecular mechanism underlying the different functional and clinical behavior of the disease in the 2 subjects here described remains unknown. It is possible that further genetic alterations may interfere in the phenotypic expression of the disease. Also, we cannot exclude with certainty that the fraternal twin could develop the disease in the future.
Family pedigree. Pedigree of the family members indicating the subjects carrying the C272T mutation of PRF1 gene at homozygous (closed circles) or at heterozygous (half circles) status. The proband is indicated by the arrow. The PRF1 sequence analysis revealed the presence of C272T (causing the A91V missense mutation) at the homozygous status in the proband and her fraternal twin and at the heterozygous status in the other family members. Furthermore, an additional G695A mutation (causing the R231H missense mutation) at the heterozygous status was found in the proband, in the healthy twin sister, and in the father.
Family pedigree. Pedigree of the family members indicating the subjects carrying the C272T mutation of PRF1 gene at homozygous (closed circles) or at heterozygous (half circles) status. The proband is indicated by the arrow. The PRF1 sequence analysis revealed the presence of C272T (causing the A91V missense mutation) at the homozygous status in the proband and her fraternal twin and at the heterozygous status in the other family members. Furthermore, an additional G695A mutation (causing the R231H missense mutation) at the heterozygous status was found in the proband, in the healthy twin sister, and in the father.
Our observation would also imply that in the overall approach to these patients sequence data should be evaluated with caution in the clinical decision-making process.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-10-3551.
Supported by grants “Ministero della Salute—Roma and Regione Campania, Legge 502” and Ministero Ricerca Scientifica—Progetto Rilevante Interesse Nazionale (PRIN) 2002 (no. 200 206 4893_003).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.