Abstract
The serine protease prolylcarboxypeptidase (PRCP), isolated from human umbilical vein endothelial cells (HUVECs), is a plasma prekallikrein (PK) activator. PRCP cDNA was cloned in pMT/BIP/V5-HIS-C, transfected into Schneider insect (S2) cells, and purified from serum-free media. Full-length recombinant PRCP (rPRCP) activates PK when bound to high-molecular-weight kininogen (HK). Recombinant PRCP is inhibited by leupeptin, angiotensin II, bradykinin, anti-PRCP, diisopropyl-fluorophosphonate (DFP), phenylmethylsulfonyl fluoride (PMSF), and Z-Pro-Proaldehyde-dimethyl acetate, but not by 1 mM EDTA (ethylenediaminetetraacetic acid), bradykinin 1-5, or angiotensin 1-7. Corn trypsin inhibitor binds to prekallikrein to prevent rPRCP activation, but it does not directly inhibit the active site of either enzyme. Unlike factor XIIa, the ability of rPRCP to activate PK is blocked by angiotensin II, not by neutralizing antibody to factor XIIa. PRCP antigen is detected on HUVEC membranes using flow cytometry and laser scanning confocal microscopy. PRCP antigen does not colocalize with LAMP1 on nonpermeabilized HUVECs, but it partially colocalizes in permeabilized cells. PRCP colocalizes with all the HK receptors, gC1qR, uPAR, and cytokeratin 1 antigen, on nonpermeabilized HUVECs. PRCP activity and antigen expression on cultured HUVECs are blocked by a morpholino antisense oligonucleotide. These investigations indicate that rPRCP is functionally identical to isolated HUVEC PRCP and is a major HUVEC membrane-expressed, PK-activating enzyme detected in the intravascular compartment. (Blood. 2004;103:4554-4561)
Introduction
Prolylcarboxypeptidase (PRCP; PCP, lysosomal carboxypeptidase, angiotensinase C, EC 3.4.16.2) catalyzes the hydrolysis of angiotensin II to produce angiotensin 1-7.1 Angiotensin II induces vasoconstriction and elevates plasminogen activator inhibitor-1 and tissue factor concentrations to influence vascular tone and the prothrombotic nature of the intravascular compartment.2,3 Angiotensin 1-7 formation by PRCP or angiotensin-converting enzyme 2 increases nitric oxide (NO) and prostacyclin formation and counterbalances the effects of angiotensin II.4,5 PRCP was originally recognized as an exopeptidase that cleaves small, biologically active peptides at carboxy terminus Pro-X, preferably Phe bonds, at low pH (less than 7.0).1,6-8 However, PRCP also cleaves proteins at neutral pH.6-8 Recently, a prekallikrein (PK)-activating serine protease at physiologic pH was isolated from cultured human umbilical vein endothelial cells (HUVECs) and their matrix and was identified to be PRCP.9,10 PRCP and heat shock protein 90 (Hsp90) have been proposed as PK activators on endothelial cells.11
The plasma kallikrein/kinin system (KKS) is a group of proteins that, when activated, influences vascular biology. High-molecular-weight kininogen (HK), the major cofactor and substrate of the enzymes of this system, has multiple activities. It is a cysteine protease inhibitor with antiangiogenesis and antiproliferative activity when cleaved.12,13 Its liberated biologically active peptides, bradykinin and bradykinin 1-5, have additional activity. Bradykinin is a vasodilator and a potent stimulator of tissue plasminogen activator release and NO and prostacyclin formation from endothelial cells.14-16 Bradykinin 1-5 (Arg-Pro-Pro-Gly-Phe) inhibits thrombin and thrombin activation of protease-activated receptor 1.17 PRCP contributes to these thromboprotective activities by being a physiologic activator of PK when bound to HK on endothelial cells.9 Formed plasma kallikrein from HUVEC PRCP activation has 3 substrates: it activates factor XII to factor XIIa, leading to increased PK activation.18 The formed kallikrein also results in kinetically favorable single-chain urokinase formation on HUVEC membranes, which promotes plasminogen activation.19 Last, the formed kallikrein digests its receptor HK to liberate bradykinin.17,18 These combined data suggest that plasma KKS contributes to the constitutive anticoagulant nature of the intravascular compartment. In addition, the ability of PRCP to activate PK, leading to bradykinin formation teleologically, is similar to its ability to degrade angiotensin II to angiotensin 1-7 because both products contribute to NO and prostacyclin formation.20 These data suggest that one function of the plasma KKS is to counterbalance the prothrombotic activity of angiotensin II, stimulating angiotensin receptor 1 of the renin angiotensin system.3,21,22
The current studies were undertaken to determine whether recombinant PRCP has the same substrate specificities as PRCP purified from endothelial cells and to determine whether PRCP is constitutively present on endothelial cell membranes and whether membrane-associated PRCP localizes with the binding proteins (putative receptors) of HK, cytokeratin 1, urokinase plasminogen activator receptor (uPAR), and gC1qR.23-25 Another aim of these investigations was to determine whether PRCP is a major PK activator associated with endothelial cells whose ability to activate PK can be down-regulated.
Materials and methods
Materials
Frozen human umbilical vein endothelial cells (HUVECs), endothelial cell growth medium (EGM), trypsin-EDTA (ethylenediaminetetraacetic acid), and trypsin-neutralizing buffer were purchased from Clonetics (San Diego, CA). Diethylaminoethyl (DEAE) cellulose was purchased from Whatman (Fairfield, NJ). Schneider insect (S2) cells were purchased from Invitrogen (Carlsbad, CA). Prestained and low-molecular-weight standards, nitrocellulose, and polyacrylamide were purchased from Bio-Rad (Richmond, CA). HK, PK, corn trypsin inhibitor (CTI), plasma kallikrein, factor XIIa, and antibody to human factor XII were purchased from Enzyme Research Laboratory (South Bend, IN). HD-Pro-Phe-Arg-paranitroanilide (S2302) was from DiaPharma (Franklin, OH). Chromogenic substrates H-Gly-Pro-pNA and H-Ala-Pro-pNA were purchased from Bachem (King of Prussia, PA). Peptides angiotensin II, angiotensin 1-7, bradykinin, and bradykinin 1-5 and chemicals EDTA, leupeptin, and Z-Pro-Pro-aldehyde-dimethyl acetate (Z-Pro-Prolinal) were purchased from Sigma (St Louis, MO). Diisopropylfluorophosphate (DFP) and phenylmethylsulfonyl fluoride (PMSF) were obtained from Calbiochem (San Diego, CA). Antibody to LAMP1 was obtained from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City). The Angiotensin II Enzyme Immunoassay Kit was purchased from Cayman Chemical (Ann Arbor, MI).
Methods
PRCP DNA expression constructs. Plasmid HKPCP-5 in pGEM7Z(-), which contains the full-length cDNA to human PRCP and was generously provided by Dr Randal Skidgel (University of Illinois), was used for polymerase chain reaction (PCR) cloning.26 After linearization of the plasmid, PCR cloning was performed to place SmaI and EcoRI restriction sites at the 5′ and 3′ ends of the cDNA. A sense primer (5′-TCCCCCGGGATGGCCCTCCGGCCGGCCTTAAGG-3′) and an antisense primer (5′-CCGGAATTCTCAGTGCTGCTTTCCCGCACTGTCATA-3′) were prepared to cover the full-length PCR product of the full-length, secreted protein (rPRCP) (Lys46-His496).26 The PCR product was then digested with the appropriate enzymes and cloned into the pMT/BIP/V5-HisC inducible/secreted expression vector (Invitrogen, Carlsbad, CA) after reciprocal digestion with SmaI and EcoRI and after gel purification and dephosphorylated with shrimp alkaline phosphatase, in accordance with the manufacturer's recommendations. Ligation was verified by enzyme digestion, and the nucleotide sequence confirmed that the recombinant expression vector was in frame with the nucleotide sequence of rPRCP using dideoxy sequencing.
Schneider 2 cell culture. The S2 cell line, which was derived from a primary culture of late-stage (20-24 hours old) Drosophila melanogaster embryos, was purchased from Invitrogen. Drosophila S2 cells were cultured according to the manufacturer's recommendation. Briefly, cells were cultured at 26°C in 6-well plates at 10 × 106 cells/mL in serum-free growth medium per well and were incubated overnight. On the day of transfection, medium was removed from each well and replaced with 300 μL solution containing 72 mM CaCl2 and 19 μg recombinant DNA in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered saline (50 mM HEPES, 1.5 mM Na2HPO4, 280 mM NaCl, pH 7.1). After incubation of the resultant solution at room temperature for 30 to 40 minutes, the cells were incubated for 24 hours at 26°C and induced by 500 μM copper sulfate. Seventy-two hours after induction, the cells were spun, the serum-free media were collected, and rPRCP was detected with the anti-PRCP antibodies.9
PRCP assays. Three assays were used to measure PRCP. The first assay was an indirect one that measured PK activation when bound to HK linked to microtiter plates. HK (2 μg) in 0.1 M Na2CO3, pH 9.6, was linked to 96-well microtiter plates and incubated overnight at 4°C. After incubation, the wells were washed with HEPES carbonate buffer (HCB) (137 mM NaCl, 3 mM KCl, 12 mM NaHCO3, 14.7 mM HEPES, 5.5 mM dextrose and 0.1% gelatin, pH 7.1, containing 10 μM CaCl2, and 1 mM MgCl2) and were then blocked with 1% gelatin. Samples containing rPRCP (usually 10-20 nM) were added in 100-μL aliquots to the cuvette wells in HCB in the absence or presence of 20 nM PK, mixed, and incubated for 1 hour at 37°C. The ability of purified rPRCP to activate PK bound to HK on plastic microtiter plates was determined in the absence or presence of 100 μM leupeptin, 1 mM DFP, 1 mM EDTA, 1 mM bradykinin, 300 μM angiotensin II, 1 mM bradykinin 1-5, or 300 μM angiotensin 1-7. DFP treatment of rPRCP consisted of treating the enzyme with the inhibitor followed by overnight dialysis of the enzyme and then its incubation with PK bound to HK on microtiter plates. Further studies determined the ability of increasing concentrations of goat anti-PRCP antibody, goat immunoglobulin G (IgG), Z-Pro-Prolinal, or CTI to inhibit PK activation when bound to HK. Goat anti-PRCP antibody was prepared by injection of peptides K66TFNQRYLVAD-KYWKK81 and R479HMKNWIRDFYDSAGKQH496 from mature PRCP (QCB Biochemicals, Hopkinson, MA).9 The antisera produced was affinity purified on a column coupled with the immunizing peptides. After incubation, the wells were washed to remove unbound rPRCP and PK. Any kallikrein activity was discerned through the addition of 100 μL S2302 (0.8 mM), and hydrolysis was observed at 405 nm for 1 hour at 37°C. The amount of kallikrein formed in the presence of the activator was determined by comparing it with the hydrolysis of S2302 by known amounts of plasma kallikrein (0.01-2.0 nM) under the same conditions.9
Additional studies determined the influence of 300 μM angiotensin II or 0.2 mg/mL neutralizing antibody to factor XIIa (Enzyme Research Laboratories) on 10 nM rPRCP or 40 pM factor XIIa proteolysis of PK bound to HK. At the completion of the incubation, 2 different kinds of investigations were performed. First, the reactions were stopped by the addition of sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the solubilized reactions were reduced with 2% β-mercaptoethanol, boiled, and applied to a 10% SDS-PAGE for electrophoresis. Samples were then transferred by electroblot onto nitrocellulose followed by immunoblot with a polyclonal antibody to human PK (Enzyme Research Laboratories), as previously reported.9 The formed kallikrein on reduced SDS-PAGE was detected by a second antibody conjugated with horseradish peroxidase followed by chemiluminescence and autoradiography. Second, simultaneous uninhibited samples were examined for their ability to hydrolyze 0.8 mM H-D-Pro-Phe-Arg-pNA, as described in the previous paragraph.
The second PRCP assay measured the ability of rPRCP to hydrolyze the chromogenic substrates H-Gly-Pro-pNA and H-Ala-Pro-pNA. In these assays, rPRCP in 0.2 M sodium acetate, 0.15 M KCl, pH 5.5, was incubated with 3 mM H-Gly-Pro-pNA or H-Ala-Pro-pNA in the absence or presence of 10 nM to 10 mM DFP, PMSF, Z-Pro-Pro-aldehyde-dimethyl acetate, leupeptin, or angiotensin II. The usual time for hydrolysis of the substrate was 1 to 2 hours of incubation at 37°C. The third assay measured the ability of rPRCP or DFP-treated rPRCP to hydrolyze angiotensin II such that it was not detected by a specific antibody to the carboxy terminus of angiotensin II. DFP-treated rPRCP was prepared by incubating 10 nM rPRCP with 100 nM DFP for 10 minutes followed by dialysis against HEPES carbonate buffer, pH 7.4, to eliminate any excess DFP. The Angiotensin II Enzyme Immunoassay (Cayman Chemical) was used according to the manufacturer's instructions to detect residual angiotensin II antigen.
Determining the mechanism by which CTI blocks rPRCP activation of prekallikrein. Simultaneous experiments determined the hydrolysis of 1 mM H-D-Pro-Phe-Arg-pNA or 3 mM H-Gly-Pro-pNA by plasma kallikrein or rPRCP, respectively. In experiments with plasma kallikrein, the reactions were performed in HEPES carbonate buffer, pH 7.4. In reactions with H-Gly-Pro-pNA, the assays were performed in 0.2 M sodium acetate, 0.15 M KCl, pH 5.5. In other experiments, biotinylated CTI was used to determine whether it directly bound to HK, PK, HK + PK, or HK + PK + rPRCP linked to microtiter-plate wells in sodium carbonate buffer, pH 9.6. CTI was biotinylated as previous reported for HK and PK.15,19
Endothelial cell culture. HUVECs were obtained and were cultured in EGM containing bovine brain extract, according to the recommendations of Clonetics. Cells between the first and third passages were subcultured onto fibronectin-treated T-175 flasks 2 days before the start of the experiment, as previously reported.9 Cell viability was determined using trypan blue exclusion.
Gel electrophoresis and immunoblot studies. Recombinant PRCP in the purification fractions was solubilized in 15 μL 2 × SDS sample buffer containing 2% β-mercaptoethanol and was boiled for 5 minutes. Proteins were separated on 10% SDS-PAGE stained with R-250 Coomassie blue. In certain experiments, proteins were separated on a 10% SDS-PAGE gel and then transferred to nitrocellulose membranes at 8 mA overnight. Membranes were then incubated in blocking buffer (5% [wt/vol] dry milk with 0.1% [wt/vol] bovine serum albumin [BSA], 0.05% Tween 20, 0.15 M NaCl, and 20 mM Tris-HCl, pH 7.4) for 1 hour.24 Nitrocellulose membranes were incubated with goat anti-PRCP peptide antisera (1:100) for 1 hour at room temperature.9 After washing, the nitrocellulose was incubated with an antigoat antibody horseradish peroxidase conjugate. The specific reactivity of antibody with the electroblotted sample was detected using the enhanced chemiluminescence (ECL) system from Amersham (Arlington Heights, IL).
Flow cytometry. Flow cytometry was performed using HUVECs (5 × 106/mL), as previously reported.27 One hundred microliters HUVEC suspension was incubated with 60 μg/mL goat anti-PRCP antibody or goat IgG in HCB containing 1 mg/mL human γ-globulin in the presence or absence of 100 μg/mL peptide K66TFNQRYLVADKYWKK81.9 Peptide K66TFNQRYLVADKYWKK81, prepared at QCB Biochemicals (Hopkinson, MA), was the immunogen for the goat antihuman PRCP antiserum.9 After 1-hour incubation at 37°C, the cells were washed 3 times by centrifugation at 400g and were resuspended in HCG containing 25 μg/mL sheep antigoat antibody labeled with fluorescein isothiocyanate (Sigma). After another 1-hour incubation, the fluorescein isothiocyanate (FITC)-labeled secondary antibody was monitored with an Epics-C flow cytometer (Coulter Electronics, Hialeah, FL), as previously reported.25
Laser scanning confocal microscopy. Monolayers of nonpermeabilized HUVECs grown on glass slides were washed and fixed with 2% paraformaldehyde for 15 minutes at 37°C. After washing the slides with 50 mM glycine in 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.4, (phosphate-buffered saline [PBS]) for 5 minutes at room temperature, the cells were incubated with 167 μg/mL goat anti-PRCP or normal goat IgG in PBS containing 1 mg/mL human γ-globulin and 1 mg/mL glucose for 1 hour at 37°C. The same cells were treated with 20 μg/mL monoclonal antibody to LAMP1. The goat antibody attached to the HUVECs was identified by incubating an Alexa Fluor 594-labeled donkey antigoat (H + L) conjugate (10 μg/mL) (Molecular Probes, Eugene, OR) or a sheep antimouse antibody conjugated with fluorescein isothiocyanate (10 μg/mL) (Calbiochem, San Diego, CA) for 1 hour at room temperature while protected from light to detect the antibody to LAMP1. In other experiments, HUVECs were permeabilized by incubating the cells with 0.1% Triton X-100 for 5 minutes at room temperature after fixation, followed by treatment with the antibodies to PRCP and LAMP1. Permeabilized cells were treated with 67 μg/mL anti-PRCP or normal goat IgG as the primary antibodies.
In other experiments, colocalization of PRCP was performed with the receptors of HK, gC1qR, uPAR, and cytokeratin 1. In these experiments, goat anti-PRCP (167 μg/mL) was incubated with cells in the presence of mouse antibody to gC1qR (2.4 μg/mL; clone 74.5.2; Covance Research Products, Richmond, CA), uPAR (2.4 μg/mL, monoclonal antibody 3B10FC [from Dr Robert F. Todd III, University of Michigan]), or antibody C180 (2.4 μg/mL; Sigma) to cytokeratins 1, 5, 6, and 8 for 1 hour at 37°C. After incubation, the cells were washed and incubated with an Alexa Fluor 594-labeled donkey antigoat IgG (H + L) conjugate (10 μg/mL) or sheep antimouse IgG conjugated with FITC (10 μg/mL) (Calbiochem) for 1 hour at room temperature while protected from light. Slides were covered with antifading mounting medium (Molecular Probes) and visualized using the laser scanning confocal microscopy, as previously described.25,27
Influence of morpholino oligonucleotides to PRCP on PRCP activity and antigen expression in cultured HUVECs. Morpholino oligonucleotides were synthesized by Gene Tools (Philomath, OR). Morpholino antisense oligonucleotides were prepared starting 6 nucleotides 5′ to the translation initiation site on PRCP (Table 1).28 Control morpholinos were also prepared consisting of an inverted antisense oligonucleotide and a partial sense oligonucleotide with a poly-A tail (Table 1). At the time of the experiment, HUVEC cells were seeded at 5000/cm2 in EGM supplemented with 2% fetal calf serum. In experiments inhibiting PK activation, the cells were allowed to become 80% to 90% confluent in a 96-well plate within 16 to 20 hours. Wells on the microtiter plate were divided into 4 treatment groups: control cells exposed to ethoxylated polyethylenimine (EPEI) special delivery solution alone (Gene Tools), cells exposed to the antisense morpholino (antisense), cells exposed to the inverted morpholino (inverted), and cells exposed to the morpholino that consisted of a partial sense sequence with a poly-A tail (sense). The amount of formed kallikrein on cells treated with the various morpholinos was assayed as described.9 At the conclusion of this incubation, the cells were washed and incubated with 20 nM HK for 1 hour at 37°C. At the end of the incubation, cells were washed again and incubated with 20 nM PK for 1 additional hour at 37°C. Afterward, 0.8 mM S2302 was added, and the hydrolysis of the substrate was monitored at 405 nm for 1 hour.
When the influence of the antisense oligonucleotide on PRCP antigen expression was examined, the cells were grown on glass slides for laser scanning confocal microscopy, and PRCP antigen was detected. After treatment with the various oligonucleotides, nonpermeabilized cells were fixed with 2% paraformaldehyde. Fixed cells were then incubated with anti-PRCP antibody (167 μg/mL) for 1 hour at 37°C. After washing, the amount of PRCP antigen was detected using secondary antibody labeled with FITC. The intensity of the FITC-labeled sheep antigoat antibody (25 μg/mL) detecting PRCP antigen on HUVEC membranes was determined. The relative amount of fluorescence of PRCP antigen per cell was determined by counting the number of cells on 32 to 34 high-powered fields with an average of 14 to 15 cells per high-powered field from 5 independent experiments. Data are expressed as mean ± SEM fluorescence per cell.
Morpholinos were delivered to the cells according to the manufacturer's recommendations, with minor modifications (Gene Tools). At the time of experimentation, media from cells were removed and washed 3 times with serum-free medium. Control cells were grown in medium supplemented only with EPEI special delivery solution. The final concentration of oligonucleotide and media with cells was 1 μM morpholino and 280 nM EPEI. Washed cells received the complete delivery solution followed by incubation for 4 hours. After incubation, the morpholino-containing medium were removed from each reaction and were replaced with fresh serum-containing medium. Cells were then incubated for 16 hours before assay for PRCP activity (microtiter-plate assay) or antigen (laser scanning confocal microscopy for antigen expression).
Protein assay. Protein concentration was determined by the method of Bradford using dye reagent from Bio-Rad (Hercules, CA) and BSA as a standard. Concentration of the purified PK activator also was determined by measuring ultraviolet absorption at 205 nm with the same BSA as the standard and the eluting buffer as the blank.
Results
rPRCP expression in insect cells
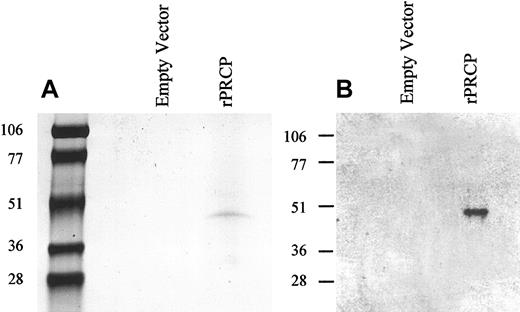
The cDNA for the full-length PRCP was designed using PCR in such a way as to contain 2 specific restriction sites (SmaI/EcoR1) for incorporation into the mammalian expression vector pMT/BiP/V5-His C. The vector has 18 amino acid residues upstream of the PRCP sequence as a signal sequence and 23 amino acid residues downstream that included a V5 epitope and a His6 -tag. The vector containing the gene for full-length, mature, secreted PRCP (rPRCP) was transfected in S2 cells. Recombinant PRCP under the influence of the metallothionein promoter was excreted into the serum-free culture medium and purified by DEAE ion exchange chromatography, followed by His-tag affinity chromatography.9 On SDS-PAGE, the rPRCP showed a single band with an apparent molecular mass of 49 kDa, consistent with the size of the protein based on the number of amino acids in rPRCP (Figure 1A). The identity of rPRCP was confirmed by Western blot analysis (Figure 1B). Using a polyclonal antibody prepared by immunizing a goat with unique peptide sequences from the amino and carboxy terminal ends of PRCP, a 49-kDa band was detected9 (Figure 1B). The migration of this protein was identical to that seen in the left panel of Figure 1B, where protein was stained by colloidal Coomassie blue. Alternatively, transfecting cells with empty vector did not produce any protein (Figure 1).
Recombinant PRCP. Schneider insect (S2) cells were transfected with either 19 μg pMT/BiP/V5-His C (empty vector) or 19 μg rPRCP-pMT/BiP/V5-His C (rPRCP). The full-length rPRCP was purified from serum-free medium as shown by colloidal Coomassie blue-stained SDS-PAGE (A) or immunoblot with goat antibody to PRCP (B).
Recombinant PRCP. Schneider insect (S2) cells were transfected with either 19 μg pMT/BiP/V5-His C (empty vector) or 19 μg rPRCP-pMT/BiP/V5-His C (rPRCP). The full-length rPRCP was purified from serum-free medium as shown by colloidal Coomassie blue-stained SDS-PAGE (A) or immunoblot with goat antibody to PRCP (B).
Small molecule inhibitors of rPRCP
Investigations were performed to demonstrate whether the rPRCP was active and, if so, to identify its inhibitors (Figure 2A). Recombinant PRCP, like HUVEC PRCP, had the ability to activate PK when bound to HK linked on microtiter plates9 (Figure 2A). Under the conditions of the assay, the assembly of HK + PK alone in plastic microtiter-plate cuvette wells did not generate hydrolytic activity on the substrate H-D-Pro-Phe-Arg-pNA. Similarly, material prepared from an empty vector had no enzymatic activity. Recombinant PRCP was inhibited by 100 μM leupeptin and 1 mM DFP. EDTA did not inhibit this enzyme; 1 mM bradykinin blocked 50% of rPRCP, and 300 μM angiotensin II completely inhibited the ability of rPRCPm to activate PK. However, equal concentrations of bradykinin 1-5 and angiotensin 1-7 had no inhibitory effect on rPRCP. These data indicated that rPRCP has the same inhibitory spectrum as PRCP isolated from HUVECs.9
Inhibitors of rPRCP. HK (2 μg/100 μL) was linked to cuvette wells in 0.1 M Na2CO3, pH 9.6, overnight at 4°C. After washing unbound HK, the cuvette wells were blocked with 1% gelatin for 1 hour at 37°C. After washing, rPRCP (10 nM) and PK (20 nM) were added. (A) Ability of rPRCP to activate PK bound to HK in the absence or presence of 100 μM leupeptin, 1 mM DFP, 1 mM EDTA, 1 mM bradykinin, 300 μM angiotensin II, 1 mM bradykinin 1-5, or 300 μM angiotensin 1-7 is shown. When the rPRCP was treated with DFP, it was dialyzed overnight before being incubated with PK bound to HK. rPRCP lane: combination of HK + PK + rPRCP added to microtiter cuvette wells. HK + PK lane: proteins added alone to microtiter cuvette wells without rPRCP. Empty vector lane: material from the culture supernatant of cells transfected with vector alone. (B) Ability of rPRCP (10 nM) to activate PK bound to HK was also examined in the absence or presence of increasing concentrations of goat IgG (IgG), goat anti-PRCP (Anti-PRCP), Z-Pro-Prolinal, or corn trypsin inhibitor (CTI). In both panels, after 1 hour of incubation at 37°C, the wells were washed and 0.8 mM S2302 was added. The amount of kallikrein activity on the microtiter plate well surface was measured by hydrolysis of S2302 and was monitored at 405 nm for 1 hour at 37°C. The error bars present the mean ± SEM of 3 or more experiments.
Inhibitors of rPRCP. HK (2 μg/100 μL) was linked to cuvette wells in 0.1 M Na2CO3, pH 9.6, overnight at 4°C. After washing unbound HK, the cuvette wells were blocked with 1% gelatin for 1 hour at 37°C. After washing, rPRCP (10 nM) and PK (20 nM) were added. (A) Ability of rPRCP to activate PK bound to HK in the absence or presence of 100 μM leupeptin, 1 mM DFP, 1 mM EDTA, 1 mM bradykinin, 300 μM angiotensin II, 1 mM bradykinin 1-5, or 300 μM angiotensin 1-7 is shown. When the rPRCP was treated with DFP, it was dialyzed overnight before being incubated with PK bound to HK. rPRCP lane: combination of HK + PK + rPRCP added to microtiter cuvette wells. HK + PK lane: proteins added alone to microtiter cuvette wells without rPRCP. Empty vector lane: material from the culture supernatant of cells transfected with vector alone. (B) Ability of rPRCP (10 nM) to activate PK bound to HK was also examined in the absence or presence of increasing concentrations of goat IgG (IgG), goat anti-PRCP (Anti-PRCP), Z-Pro-Prolinal, or corn trypsin inhibitor (CTI). In both panels, after 1 hour of incubation at 37°C, the wells were washed and 0.8 mM S2302 was added. The amount of kallikrein activity on the microtiter plate well surface was measured by hydrolysis of S2302 and was monitored at 405 nm for 1 hour at 37°C. The error bars present the mean ± SEM of 3 or more experiments.
Additional inhibitors of rPRCPm
Further studies showed that, as did wild-type PRCP, CTI inhibited rPRCP activation of PK when linked to HK with an IC50 of approximately 10 nM, a value similar to CTI inhibitory activity of wild-type PRCP9 (Figure 2B). Goat anti-PRCP IgG also inhibited rPRCP ability to activate PK bound to HK with an IC50 of 120 nM, whereas its IgG did not. Last, Z-Pro-Prolinal inhibited rPRCP ability to activate PK with an IC50 of 10 μM, a value similar to that seen with purified endothelial cell PRCP.9
Specificity of rPRCPm activation of prekallikrein
Additional investigations were performed to determine whether rPRCP activated PK as did wild-type PRCP isolated from HUVECs9 (Figure 3A). Recombinant PRCP proteolyzed PK to produce a 51-kDa heavy chain and 2 faint light chains at 39 and 36 kDa (Figure 3A). Angiotensin II at 300 μM, but not neutralizing antibody to factor XIIa (FXIIa), blocked rPRCP proteolysis of PK (Figure 3A). Alternatively, neutralizing antibody to FXIIa, but not angiotensin II, inhibited FXIIa cleavage and activation of PK (Figure 3A). These data indicated that rPRCP activated PK similarly to what has been reported for wild-type PRCP9 and that rPRCP and FXIIa activated PK near or at the same cleavage site.
Specificity of rPRCP activation of PK. Twenty nanomolar PK was incubated in HCB, pH 7.4, in microtiter plate cuvette wells precoated with 2 μg HK with either 10 nM rPRCP or 40 pM FXIIa in the absence or presence of 300 μM angiotensin II (AgII) or 0.2 mg/mL neutralizing antibody to FXIIa (Ab). (A) At the end of the incubation, the wells were washed, and the reactions were stopped by the addition of sample buffer for SDS-PAGE, reduced with 2% β-mercaptoethanol, boiled, and applied to 12% SDS-PAGE for electrophoresis followed by immunoblot with a polyclonal antibody to human PK. The formed kallikrein was detected by a secondary antibody conjugated with horseradish peroxidase and then by chemiluminescence and autoradiography. Numbers to the right of the gel represent molecular mass standards in kilodaltons. This experiment is representative of 1 of 3 experiments. (B) This experiment was performed simultaneously with the experiment depicted in panel A. At the end of the incubation, the wells were washed with HCB, pH 7.4, and then by 100 μL HCB, pH 7.4, containing 0.8 mM S2302 was added. The formed kallikrein was indicated by the hydrolysis of the substrate for 1 hour at 37°C. This experiment is the mean ± SEM of 3 experiments performed simultaneously with 3 PK cleavage experiments shown in panel A.
Specificity of rPRCP activation of PK. Twenty nanomolar PK was incubated in HCB, pH 7.4, in microtiter plate cuvette wells precoated with 2 μg HK with either 10 nM rPRCP or 40 pM FXIIa in the absence or presence of 300 μM angiotensin II (AgII) or 0.2 mg/mL neutralizing antibody to FXIIa (Ab). (A) At the end of the incubation, the wells were washed, and the reactions were stopped by the addition of sample buffer for SDS-PAGE, reduced with 2% β-mercaptoethanol, boiled, and applied to 12% SDS-PAGE for electrophoresis followed by immunoblot with a polyclonal antibody to human PK. The formed kallikrein was detected by a secondary antibody conjugated with horseradish peroxidase and then by chemiluminescence and autoradiography. Numbers to the right of the gel represent molecular mass standards in kilodaltons. This experiment is representative of 1 of 3 experiments. (B) This experiment was performed simultaneously with the experiment depicted in panel A. At the end of the incubation, the wells were washed with HCB, pH 7.4, and then by 100 μL HCB, pH 7.4, containing 0.8 mM S2302 was added. The formed kallikrein was indicated by the hydrolysis of the substrate for 1 hour at 37°C. This experiment is the mean ± SEM of 3 experiments performed simultaneously with 3 PK cleavage experiments shown in panel A.
These findings were confirmed on gel electrophoresis experiments and by simultaneous experiments whereby formed kallikrein activity was measured in additional cuvettes (Figure 3B). Recombinant PRCP alone produced plasma kallikrein activity from PK linked to HK on microtiter-plate wells that was abolished by angiotensin II, but not by neutralizing antibody to factor XIIa (Figure 3B). Alternatively, neutralizing antibody to factor XIIa blocked factor XIIa activation of PK, but angiotensin II did not (Figure 3B). These combined experiments indicated that rPRCP activation of PK was specific for that enzyme.
Characterization of rPRCPm as a serine protease
Next, investigations were performed to determine directly the enzymatic nature of rPRCP. Recombinant PRCP hydrolyzed H-Gly-Pro-pNA and H-Ala-Pro-pNA with a Km of 4 and 2.4 mM, respectively. Using H-Gly-Pro-pNA as a direct substrate of rPRCP, investigations determined its inhibitory profile. The serine protease inhibitors DFP and PMSF inhibited rPRCP with an IC50 of 50 nM and 600 μM, respectively (Figure 4A). Furthermore, Z-Pro-Prolinal, leupeptin, and angiotensin II inhibited rPRCP hydrolysis of H-Gly-Pro-pNA with an IC50 of 0.5, 0.8, and 3 mM, respectively (Figure 4A). Additional investigations showed that angiotensin II was proteolyzed by rPRCP, unlike DFP-treated rPRCP, such that the peptide was not recognized by an antibody to angiotensin II (Figure 4B).
Specificity of rPRCP activity. (A) Ten nanomolar rPRCP in 0.2 M sodium acetate, 0.15 M KCl, pH 5.5, was treated with 10 nM to 10 mM DFP, PMSF, Z-Pro-Prolinal, leupeptin, or angiotensin II (AgII), and 3 mM H-Gly-Pro-pNA was added. The entire mixture was then transferred to a microtiter plate cuvette well, and hydrolysis was measured for 2 hours at 37°C. (B) Hydrolysis of 300 μM angiotensin II by 10 nM rPRCP(AgII + rPRCP) or 10 nM DFP-treated rPRCP (AgII + DFP-rPRCP). DFP-rPRCP was prepared by treating 10 nM rPRCP with 100 nM DFP followed by dialysis against HCB, pH 7.4, to eliminate the free DFP. The amount of residual angiotensin II after the various rPRCP treatments was measured by radioimmunoassay, as indicated in “Materials and methods.” In this experiment, angiotensin 1-7 (AgII1-7) was added at 300 μM. In both panels, the figure is the mean ± SEM of 3 or more experiments.
Specificity of rPRCP activity. (A) Ten nanomolar rPRCP in 0.2 M sodium acetate, 0.15 M KCl, pH 5.5, was treated with 10 nM to 10 mM DFP, PMSF, Z-Pro-Prolinal, leupeptin, or angiotensin II (AgII), and 3 mM H-Gly-Pro-pNA was added. The entire mixture was then transferred to a microtiter plate cuvette well, and hydrolysis was measured for 2 hours at 37°C. (B) Hydrolysis of 300 μM angiotensin II by 10 nM rPRCP(AgII + rPRCP) or 10 nM DFP-treated rPRCP (AgII + DFP-rPRCP). DFP-rPRCP was prepared by treating 10 nM rPRCP with 100 nM DFP followed by dialysis against HCB, pH 7.4, to eliminate the free DFP. The amount of residual angiotensin II after the various rPRCP treatments was measured by radioimmunoassay, as indicated in “Materials and methods.” In this experiment, angiotensin 1-7 (AgII1-7) was added at 300 μM. In both panels, the figure is the mean ± SEM of 3 or more experiments.
When CTI was examined as a direct inhibitor of rPRCP, an unexpected result was found. Unlike the ability of CTI to inhibit PRCP activation of PK on cultured endothelial cells (Figure 2B), CTI did not directly inhibit rPRCP ability to hydrolyze H-Gly-Pro-pNA (Table 2). CTI was shown to inhibit rPRCP activation of PK bound to HK in microtiter plates without inhibiting rPRCP directly (Table 2). However, CTI also did not inhibit the active site of kallikrein (Table 2). In independent experiments, biotinylated CTI was found to bind to PK to prevent rPRCP activation (data not shown). Furthermore, CTI prevented PK activation by rPRCP, as seen on reduced SDS-PAGE (data not shown). These studies indicated that CTI was a unique inhibitor of rPRCP activation of PK. It bound to PK, preventing rPRCP from activating the PK catalytic site.
Properties of rPRCPm
The Km of 10 nM rPRCP activation of 20 nM PK bound to HK on microtiter plates was 17 nM. As did wild-type PRCP, rPRCP had a pH spectrum between 6.8 and 7.4.5 In addition, rPRCP activity was optimal in buffers containing 10 μM Ca2+ and 1 mM Mg2+, pH 7.1.
HUVEC PRCP flow cytometry
The presence of PRCP antigen on nonpermeabilized HUVECs in suspension was determined by flow cytometry (data not shown). When HUVEC suspensions were incubated with anti-PRCP antibody, a rightward shift resulted on the flow cytogram compared with cells incubated with a similar concentration of goat IgG (data not shown). When the cells were simultaneously incubated with anti-PRCP antibody and the peptide used to raise this antibody, its rightward shift on the flow cytogram was slowed and overlaid that of normal IgG (data not shown). These latter data indicated that the anti-PRCP antibody specifically identified PRCP antigen on the membranes of HUVECs in suspension.
PRCP expression and colocalization with LAMP1, gC1qR, uPAR, or CK1 on HUVECs
Investigations next were performed to determine the expression of PRCP on nonpermeabilized and permeabilized HUVECs in culture (Figure 5). Laser scanning confocal microscopy revealed that PRCP antigen was expressed on nonpermeabilized HUVEC membranes and did not colocalize with the lysosomal marker, LAMP1, on the membranes of cultured endothelial cells. When HUVECs were permeabilized with 0.1% Triton X-100, partial localization of PRCP and LAMP1 antigen occurred, indicating their lysosomal localization (Figure 5).
Laser scanning confocal microscopy of HUVEC PRCP. PRCP is shown in red and LAMP1 in green. HUVECs were grown on microscope slides for 2 hours and then fixed with 2% paraformaldehyde. Nonpermeabilized or permeabilized fixed cells were incubated with goat anti-PRCP and mouse anti-LAMP1 for 1 hour at 37°C. After washing, the PRCP and LAMP1 antigens were detected with a secondary antibody labeled with Alexa Fluor 594-labeled donkey antigoat antibody and FITC-conjugated sheep antimouse antibody, respectively, as described in “Materials and methods.” Original magnification × 60.
Laser scanning confocal microscopy of HUVEC PRCP. PRCP is shown in red and LAMP1 in green. HUVECs were grown on microscope slides for 2 hours and then fixed with 2% paraformaldehyde. Nonpermeabilized or permeabilized fixed cells were incubated with goat anti-PRCP and mouse anti-LAMP1 for 1 hour at 37°C. After washing, the PRCP and LAMP1 antigens were detected with a secondary antibody labeled with Alexa Fluor 594-labeled donkey antigoat antibody and FITC-conjugated sheep antimouse antibody, respectively, as described in “Materials and methods.” Original magnification × 60.
Additional investigations determined whether PRCP antigen colocalized with the various binding proteins, putative receptors, for HK (Figure 6). Investigations were performed on fixed, nonpermeabilized HUVECs grown on microscope slides. Using double-label experiments, PRCP antigen colocalized with gC1qR, uPAR, and cytokeratin 1, in decreasing order, on the membranes of fixed, nonpermeabilized HUVECs in culture (Figure 6). These data indicated that PRCP antigen colocalized with the proteins on the membranes of HUVECs that served as HK binding sites, the receptor for PK expression on HUVECs.
Colocalization of PRCP with HK receptors. HUVECs were grown on microscope slides for 2 hours and then fixed with 2% paraformaldehyde and not permeabilized. Fixed cells were then incubated with goat anti-PRCP and anti-gC1qR, anti-uPAR, or anti-CK1 for 1 hour at 37°C. After washing, the anti-PRCP antibody was detected with Alexa Fluor 594-labeled secondary antibodies. Anti-gC1qR, -uPAR, and -CK1 antibodies were detected with FITC-conjugated secondary antibodies, as described in “Materials and methods.” Original magnification × 60.
Colocalization of PRCP with HK receptors. HUVECs were grown on microscope slides for 2 hours and then fixed with 2% paraformaldehyde and not permeabilized. Fixed cells were then incubated with goat anti-PRCP and anti-gC1qR, anti-uPAR, or anti-CK1 for 1 hour at 37°C. After washing, the anti-PRCP antibody was detected with Alexa Fluor 594-labeled secondary antibodies. Anti-gC1qR, -uPAR, and -CK1 antibodies were detected with FITC-conjugated secondary antibodies, as described in “Materials and methods.” Original magnification × 60.
Modulating the membrane expression of PRCP activity and antigen
Next we performed investigations to determine whether the expression of external membrane PRCP was simply an artifact of culturing endothelial cells in plastic microtiter plates or on glass slides or was a true biologic expression of the protein. HUVECs grown to 80% to 90% confluence in microtiter-plate cuvette wells were treated for 4 hours with a morpholino antisense oligonucleotide to the translational initiation site of human PRCP (Figure 7A). After treatment, the cells were cultured for 16 hours. The PK-generating activity on cells treated with an antisense oligonucleotide was 65% lower than that seen on untreated cells (Figure 7A). The specificity of this interaction was shown by the finding that identical cells treated at the same time with a modified sense oligonucleotide or an inverted morpholino antisense oligonucleotide had no change in PK-generating activity from that of untreated cells (Figure 7A). These data indicated that the expression of PK generation on HUVECs was a biologic activity mostly arising from the expression of PRCP (Figure 7A).
Influence of antisense oligonucleotides to PRCP to block PK activation or antigen on HUVECs. (A) Eighty percent to 90% confluent monolayers of HUVECs in microtiter plates were incubated with various oligonucleotides for 4 hours, as described in “Materials and methods.” After incubation and washing, the cells were incubated with standard culture media for 16 to 20 hours before assay for formed plasma kallikrein (“Materials and methods”). Results presented are the mean ± SEM of 5 separate experiments. HK + PK lane: Nontreated cells incubated with these proteins. Sense, inverted, and antisense lanes: Wells incubated with modified sense, inverted, or antisense morpholino oligonucleotides, respectively. (B) The figure is a representative laser scanning confocal micrograph of untreated HUVECs (control) or antisense treated (Antisense) using a goat anti-PRCP antibody.9 Original magnification × 60. (C) The influence of antisense oligonucleotides to PRCP on PK antigen expression on HUVECs. HUVECs were grown on microscope slides and prepared for morpholino treatment, as described in “Materials and methods.” Data presented for each condition are the mean ± SEM of 32 to 34 fields of view with an average of 14 to 15 cells/field from 5 independent experiments. Results are expressed as arbitrary fluorescent units per cell. Control lane: untreated cells. Sense, inverted, and antisense lanes: HUVECs incubated with modified sense, inverted, or antisense morpholino oligonucleotides.
Influence of antisense oligonucleotides to PRCP to block PK activation or antigen on HUVECs. (A) Eighty percent to 90% confluent monolayers of HUVECs in microtiter plates were incubated with various oligonucleotides for 4 hours, as described in “Materials and methods.” After incubation and washing, the cells were incubated with standard culture media for 16 to 20 hours before assay for formed plasma kallikrein (“Materials and methods”). Results presented are the mean ± SEM of 5 separate experiments. HK + PK lane: Nontreated cells incubated with these proteins. Sense, inverted, and antisense lanes: Wells incubated with modified sense, inverted, or antisense morpholino oligonucleotides, respectively. (B) The figure is a representative laser scanning confocal micrograph of untreated HUVECs (control) or antisense treated (Antisense) using a goat anti-PRCP antibody.9 Original magnification × 60. (C) The influence of antisense oligonucleotides to PRCP on PK antigen expression on HUVECs. HUVECs were grown on microscope slides and prepared for morpholino treatment, as described in “Materials and methods.” Data presented for each condition are the mean ± SEM of 32 to 34 fields of view with an average of 14 to 15 cells/field from 5 independent experiments. Results are expressed as arbitrary fluorescent units per cell. Control lane: untreated cells. Sense, inverted, and antisense lanes: HUVECs incubated with modified sense, inverted, or antisense morpholino oligonucleotides.
We next investigated whether the antisense oligonucleotide treatment of HUVECs grown on glass slides also resulted in reduced antigen expressed as assessed by laser scanning confocal microscopy (Figure 7B-C). In these experiments, cells were left untreated or were treated with antisense, modified sense, or inverted oligonucleotides, as described. At 16 hours, the cells were fixed and incubated with a primary antibody to PRCP, followed by a secondary antibody conjugated with FITC. In Figure 7B, the relative immunofluorescence of untreated cells compared with antisense-treated cells is shown. Antisense-treated cells had reduced antigen expression. The amount of antigen was relatively measured by determining the mean fluorescence of each cell after examination 32 to 34 microscopic fields with an average of 14 to 15 cells per field for each condition in 5 separate experiments (Figure 7C). Untreated cells had mean ± SEM fluorescence per cell of 0.99 ± 0.06 U/cell compared with 0.56 ± 0.04 U/cell for the antisense-treated cells (Figure 7C). Antisense treatment resulted in a 43% reduction of the PRCP antigen intensity on laser scanning confocal microscopy. Similarly, cells treated with the modified sense or inverted oligonucleotide had fluorescence measurements of 105 ± 9.36 or 123 ± 12 U/cell, respectively (Figure 7C). Combined results of morpholino antisense experiments indicated that PRCP expression on HUVEC was down-regulated. Reduced PRCP expression on HUVEC membranes accounted for a major loss of the PK-activating activity associated with HUVECs.
Discussion
This study describes the successful cloning and secretion of a functional human rPRCP in S2 cells. The form of PRCP expressed in insect cells is the full-length secreted protein that encompasses Lys46-His496 (SWISS-PROT/P42785). This form of the protein does not have its 30- or 15-amino acid signal peptide or propeptide, respectively. Using an insect cell expression system, a functionally active form of rPRCP is expressed, unlike its expression in bacteria.23,29 It is unknown whether PRCP is expressed as an unprocessed precursor if it will be functionally active. The physiologic processing enzyme(s) of PRCP is also unknown. Although secreted from S2 cells under the regulation of a metallothionein promoter, PRCP is not normally a secreted protein. On SDS-PAGE, secreted rPRCP was approximately 49 to 51 kDa consistent with the size of the full-length protein.23 In addition, rPRCP expressed in insect cells retains its immunoreactivity with anti-PRCP antibodies, suggesting that any modification of its 12% carbohydrate does not substantively affect its immunologic reactivity.
Recombinant PRCP displays many of the biochemical properties of this protein when isolated from kidney and HUVECs.1,9 Recombinant PRCP is able to activate PK bound to HK linked to microtiter plates with a Km of 17 nM that is comparable to that seen with the enzyme isolated from HUVECs (Km, 7 nM).9 Recombinant PRCP appears to be a stoichiometric activator of PK because nearly equal molar concentrations of enzyme were added to substrate. However, additional studies are needed to confirm this assessment. The wide optimum range for pH observed with recombinant PRCP is in agreement with our previous findings that HUVEC PRCP is stable from pH 6.5 to 7.4.9 Using a different method for assaying PRCP activity and longer incubation times, PRCP also degrades angiotensin II at pH 5.8.1 Recombinant PRCP is inhibited by the same small molecule inhibitors and peptides (eg, Z-Pro-Prolinal) as HUVEC PRCP.9 The differences in the IC50 of rPRCP by Z-Pro-Prolinal to inhibit PK activation and H-Gly-Pro-pNA cleavage probably resulted from the pH of the reactions and the Km of the enzyme for each substrate. Both angiotensin II and bradykinin (RPPGFSPFR) are better substrate inhibitors for PRCP than des-Arg9 -bradykinin, which has a penultimate proline (data not shown). The reason for these substrate preferences is unknown. Recombinant PRCP also activates its physiologic substrate, PK, in an apparently identical manner to that of the wild-type enzyme seen on reduced SDS-PAGE.9 The actual PK activation site for PRCP must be characterized to determine whether it is identical to the Arg-Ile bond that factor XIIa cleaves. Finally, rPRCP is inhibited by the serine protease inhibitors DFP and PMSF. The inhibitory data with CTI on rPRCP activation of PK bound to HK is not the result of CTI interfering with the active site of rPRCP. Rather, CTI binds to PK and prevents rPRCP from activating PK without interfering with rPRCP or plasma kallikrein proteolytic activity. Interestingly, CTI also was found to bind Hsp90 when it was proposed as a PK-activating protein.11 However, the rPRCP preparation cannot contain Hsp90 because Hsp90 is not a serine protease and because Hsp90 activates PK with HK in solution.
Other investigations show that, though characterized as a lysosomal enzyme, PRCP is constitutively expressed on the membrane of cultured endothelial cells. Cell biology studies indicate that the lysosomal compartment of eukaryotic cells is an endomembrane system that is intimately involved in the export of internal constituents.30 LAMP1, another lysosomal protein, is present in membrane and intracellular compartments. The membrane and lysosomal distribution of PRCP and LAMP1, however, are different. There is no colocalization of PRCP and LAMP1 on nonpermeabilized HUVEC membranes and only partial colocalization on permeabilized cells. These data suggest that there are different mechanisms of expression of lysosomal constituents in and on cells.31,32 Last, the finding that PRCP colocalizes with each of the HK-binding proteins (cytokeratin 1, uPAR, and gC1qR) on the membranes of cultured HUVECs indicates that PRCP is physically placed in a position to be a PK activator.23-25
Finally, we sought to determine whether HUVEC membrane expression of PRCP activity and antigen is a biologically regulated activity or is an artifact of culturing endothelial cells in plastic microtiter plates or on glass slides. After using a morpholino antisense oligonucleotide at the translation initiation site of human PRCP, PRCP activity and membrane antigen expression decreased by 65% and 43%, respectively, compared with control cells. This indicated that, first, PRCP was a major PK activator on the membranes of endothelial cells and that, second, its membrane expression on cultured cells was a feature of the cells themselves and did not result from manipulations of culturing the cells on artificial surfaces.
Prepublished online as Blood First Edition Paper, March 2, 2004; DOI 10.1182/blood-2003-07-2510.
Supported by CVC McKay grant G002286 (Z.S.-M.), American Heart Association grant N004313 (Z.S.-M.), and National Institutes of Health grant HL52779 (A.H.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.