Abstract
Similar to tumor necrosis factor (TNF), bacterial lipopolysaccharide (LPS) elicits parallel apoptotic and antiapoptotic pathways in endothelial cells. The overall result is that there is minimal endothelial cell death in response to LPS without inhibition of the cytoprotective pathway. While the TNF-induced death and survival pathways have been relatively well elucidated, much remains to be learned about LPS signaling events in this regard. It is known that the transcription factor nuclear factor-κB (NF-κB) provides a critical cell survival signal in response to TNF, but is not an essential component of the LPS-induced survival pathway. The TNF receptor-associated factor 6 (TRAF6) is a major effector of multiple LPS-induced signals, including a c-Jun N-terminal kinase (JNK)-mediated apoptotic response. In this report we demonstrate that following LPS stimulation, TRAF6 also transmits an important endothelial cell survival signal in a situation of complete NF-κB blockade. In response to LPS, TRAF6 activates the phosphatidylinositol 3′-kinase (PI3K)/Akt pathway, but not ERK1/2 mitogen-activated protein kinases (MAPKs) in endothelial cells. Activation of PI3K signals a critical antiapoptotic pathway in response to LPS in endothelial cells, whereas ERK1/2 does not. Thus TRAF6 acts as a bifurcation point of the LPS-initiated death and survival signals in endothelial cells. (Blood. 2004;103:4520-4526)
Introduction
Sepsis is the systemic response to severe infection in critically ill patients, and the endothelium plays a pivotal role in orchestrating the inflammatory response seen in this situation.1,2 Endothelial cell injury or apoptosis, and the accompanying loss of endothelial integrity, may result in dysregulation of the inflammatory response as well as the resulting capillary leak syndrome and hemodynamic instability seen in sepsis.1-5 Receptors of the innate immune system are expressed on various cell types including the endothelium.6 Because of the broad specificity of the innate immune system, receptors of this system have a wide spectrum of recognition for common structures on multiple pathogens.6 A structure that is common to Gram-negative bacteria is lipopolysaccharide (LPS). LPS is a critical glycolipid component of the outer wall of Gram-negative bacteria, and many of the cellular signals activated by gram-negative bacteria are attributed to LPS.7 A key set of innate immune receptors that recognize bacterial components belong to the Toll-like receptor (TLR) family.6 At least 10 members of the family have been described thus far, and TLR4 appears to be the main LPS receptor although coreceptors are also involved.8-12
Endothelial injury plays a critical role in the pathogenesis of sepsis due to Gram-negative bacteria.3 At the concentrations of LPS measured in patients' sera during sepsis, there is slight toxicity to cultured human endothelial cells, which is markedly increased when endothelial cells are concomitantly exposed to cycloheximide (CHX) or actinomycin D.13,14 A similar phenomenon has been described for tumor necrosis factor (TNF), which does not cause apoptosis on its own, but does when combined with CHX or actinomycin D.15 It has been shown that the concomitant induction of antiapoptotic pathways inhibits the parallel apoptotic pathway that is activated by TNF.13,15
Ligand activation of the TLRs results in recruitment of a complex of proteins to the membrane. These proteins include MyD88, the serine-threonine kinases of the interleukin 1 (IL-1) receptor-associated kinase (IRAK) family, and TNF receptor-associated factor 6 (TRAF6).16-19 TRAF6 through a series of kinases transmits the LPS signal to activate the transcription factor, nuclear factor-κB (NF-κB), as well as other downstream kinases, such as c-Jun NH2-terminal kinase (JNK).17,20-22 More recently it has been shown that in 293 cells CD40-activated TRAF6 stimulates extracellular signal-regulated kinase (ERK) activity in a Ras-independent manner.23 As well, phosphatidylinositol 3′-kinase (PI3K) activation initiated by TRANCE is also routed through TRAF6.24 Thus activation of TRAF6 by LPS may potentially activate several apoptosis-related pathways.
We have previously shown that TLR signaling activates inflammatory and apoptotic signaling pathways in endothelial cells via TRAF6.22 In particular, we have shown that LPS can signal an apoptotic pathway through TRAF6-mediated activation of JNK.22 Although NF-κB is known to activate a critical survival pathway, and LPS-induced activation of NF-κB up-regulates the expression of various antiapoptotic genes, inhibition of NF-κB activation is not sufficient to sensitize endothelial cells to LPS-initiated apoptosis.25 Thus, although TRAF6 can potentially serve as a bifurcation point for the apoptotic and antiapoptotic signals induced by LPS, NF-κB activation does not serve as a critical viability signal. Hence, other antiapoptotic signaling pathways transmitted by LPS remain to be elucidated. In this report, we demonstrate that TRAF6 can signal an NF-κB-independent survival pathway in response to LPS stimulation. We further show that TRAF6 can activate Akt but not ERK1/2 in response to LPS stimulation of endothelial cells. PI3K is necessary for TRAF6-related Akt phosphorylation, but MEK/ERK1/2 activation is independent of TRAF6 in endothelial cells exposed to LPS. Further, only PI3K/Akt activation is required for the LPS-induced, TRAF6-dependent, NF-κB-independent survival activity.
Materials and methods
Cell lines and reagents
Human microvascular endothelial cells (HMECs) were provided by the Center for Disease Control and Prevention, Atlanta, GA, and were cultured in MCDB-131 medium supplemented with 10% fetal calf serum and 10 μg/mL epithelial growth factor (EGF).
TRAF6-/- murine embryonic fibroblasts were provided by T. Mak and W. C. Yeh (University of Toronto) and cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum.21 Flag-tagged TRAF2 and TRAF6 cDNAs were provided by Tularik (South San Francisco, CA), and Flag-IκBmt (IκBαS32/36A) was a gift of D. Ballard, Vanderbilt University.17,26 The various constructs were cloned into the retroviral vectors LNCX or MSCVpac. Transient transfections of the Ampho Phoenix packaging cell line were carried out by calcium phosphate precipitation or lipid-mediated transfection. Viral supernatants were used to transduce HMECs, and cell lines were selected in G418 or puromycin as previously described.15 Expression of the various proteins was confirmed by immunoblotting with the anti-Flag (M5) antibody as described.15 Polyclonal cell lines were used to avoid artifacts due to retroviral integration site.
Antibodies against total and phosphorylated Akt (Ser 473) and phosphorylated ERK1/2 (Thr202/Tyr204) MAP kinase were obtained from Cell Signaling Technology (Mississauga, ON, Canada). The kinase inhibitors LY294002 and U0126 were obtained from Calbiochem (La Jolla, CA).
Viability assay
To quantitate the proportion of viable cells, HMECs were seeded on 96-well plates at a density of 30 000 cells/well. On the following day, cells were incubated in TNF or LPS with or without CHX (50 μg/mL) for 16 hours. Viable cell numbers were estimated by neutral red uptake as described.27
Electrophoretic mobility shift assay
HMEC nuclear extracts were prepared following incubation with LPS at 100 ng/mL for 30 minutes. Cells were washed twice in ice-cold phosphate-buffered saline (PBS), scraped into buffer A (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]-KOH pH 7.8, 1.5 mM MgCl2, 10 mM KCl), pelleted briefly, and lysed in buffer A plus 0.5% Nonidet P-40 (NP-40) for 10 minutes on ice. Nuclei were isolated by centrifugation for 10 minutes at 12 000g, then washed, repelleted, and incubated for 20 minutes in buffer C (50 mM HEPES-KOH pH 7.8, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA [ethylenediaminetetraacetic acid], 10% glycerol). Nuclear membranes were then removed by centrifuging for 10 minutes at 12 000g and the supernatant/nuclear extract removed and frozen at -70°C until needed.
Nuclear extract (15 μg) was diluted in 4 volumes of binding buffer (10 mM Tris-HCl, 50 mM NaCl, 1 mM EDTA pH 8.0, 4% glycerol), 2 μg poly-dIdC (Sigma, St Louis, MO), and 1 μL double-stranded 32 P-labeled oligonucleotide corresponding to the consensus NF-κB binding site and incubated at room temperature for 20 minutes. This binding reaction was then loaded onto a 5% nondenaturing 0.5x Tris borate EDTA-polyacrylamide gel that had been pre-run for one hour at 80 V. Electrophoresis was performed at 80 V for 1.5 to 2 hours at room temperature before drying the gel and carrying out autoradiography.
Caspase activity
Asp-Glu-Val-Asp (DEVD)-p-nitroaniline (pNA) cleavage assay for caspase 3/7 activity was performed with a colorimetric assay kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN) as previously described.27 Briefly, 200 μg of whole cell lysates from HMECs exposed to CHX (50 μg/mL) or CHX and LPS (100 ng/mL) for 8 hours was combined with 200 μM DEVD-pNA in a 96-well plate and incubated at 37°C. The release of the chromophore by active caspases was quantitated at 405 nm and normalized to untreated cell lysates.
Northern analysis
Northern blotting was peformed as previously described.13 Briefly, HMECs were stimulated with LPS or TNF for various times as indicated. Total cellular RNA (10 μg) was separated on agarose-formaldehyde gels, blotted onto nitrocellulose filters, and hybridized overnight with random-primed 32 P-labeled probes as indicated. The final washing conditions were 0.1X standard saline citrate (SSC), 0.1% sodium dodecyl sulfate (SDS) for 15 minutes at room temperature. Blots were stripped in boiling water prior to reprobing. A 3′ probe was used to identify the approximately one-kb human A1 transcript and a 300-bp HindIII fragment of A20 was used to identify the approximately 4.5-kb A20 mRNA. A glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was used to confirm equivalent loading of RNA samples.
Immunoblotting
Proteins from total cellular extracts were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and assessed by immunoblotting as previously described.27
Annexin V binding
Cells in confluent 12-well plates were preincubated for one hour in assay medium supplemented with vehicle, the MEK inhibitor U0126 (25 μM) or the PI3K inhibitor LY294 002 (50 μM). CHX or CHX and LPS were added to 50 μg/mL and 100 ng/mL respectively and incubated for an additional 13 hours. Monolayers were harvested by trypsinization and apoptotic cells were stained with annexin V conjugated to Alexa 594 (Molecular Probes, Eugene, OR) following the manufacturer's protocol. Cells were analyzed on a Coulter Elite flow cytometer. Annexin V-positive cells were quantitated with the FCS Express 2 program (De Novo Software, Thornhill, ON, Canada).
Statistical analysis
Differences between groups were determined by analysis of variance (ANOVA) followed by a Tukey test to determine significant differences between multiple groups.
Results
NF-κB activity is dispensable for LPS-induced cytoprotective activity
TNF activates parallel apoptotic and antiapoptotic pathways, with the antiapoptotic pathway requiring the expression of new genes.15,28 We and others have demonstrated that similar to TNF, LPS also activates parallel death and survival pathways.13,14,28,29 In order to potentiate either the TNF- or LPS-activated apoptotic pathways in endothelial (and other) cells, expression of cytoprotective genes must be blocked with either CHX or actinomycin D.13,14,28 The survival signals that protect endothelial cells from LPS-induced apoptosis appear to include both an inducible and constitutively active component.13,30 However, the LPS-induced survival pathways have not been well studied. A major TNF-induced antiapoptotic pathway is routed through activation of NF-κB. However, we have shown that inhibition of NF-κB sensitizes endothelial cells to TNF- but not LPS-induced apoptosis.25,31-33
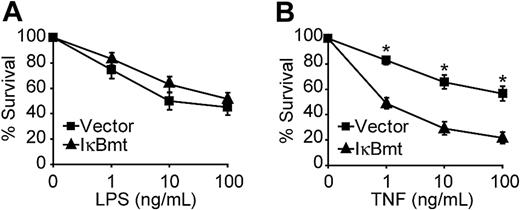
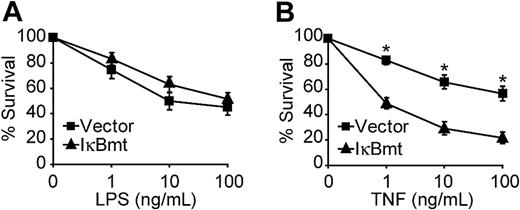
Because LPS signals parallel apoptotic and antiapoptotic pathways, it is possible that in some cases inhibition of NF-κB alone is not sufficient to reveal the cytoprotective activity of this pathway. Other studies have shown that, in some cases, the addition of CHX or actinomycin D is required to demonstrate that TNF-mediated NF-κB activation is important for cellular protection.33,34 We thus tested whether enforced expression of a superrepressor IκB mutant molecule (IκBmt), to block NF-κB nuclear translocation, would result in greater LPS-induced death in the presence of CHX. We have previously shown that the IκBmt protein, which is mutated to prevent stimulus-dependent phosphorylation and subsequent ubiquitin-dependent proteolysis, completely blocks NF-κB nuclear translocation and sensitizes endothelial cells to TNF-induced apoptosis.13,27 Figure 1A demonstrates that NF-κB blockade does not sensitize endothelial cells to LPS-triggered apoptosis in the presence of CHX. However, inhibition of NF-κB does cause greater cell death in response to TNF and CHX (Figure 1B).
NF-κB activation is not essential for LPS-induced survival signals. Empty vector- or IκBmt-transduced endothelial cells (as labeled) were treated with LPS (A) or TNF (B), in the presence of CHX (50 μg/mL) for 16 hours. Cell survival was measured by neutral red incorporation and normalized to cells treated with CHX alone. Data shown are the mean plus or minus the standard error of the mean (SEM) of 6 independent experiments, each done in triplicate. *P < .001.
NF-κB activation is not essential for LPS-induced survival signals. Empty vector- or IκBmt-transduced endothelial cells (as labeled) were treated with LPS (A) or TNF (B), in the presence of CHX (50 μg/mL) for 16 hours. Cell survival was measured by neutral red incorporation and normalized to cells treated with CHX alone. Data shown are the mean plus or minus the standard error of the mean (SEM) of 6 independent experiments, each done in triplicate. *P < .001.
TRAF6 signals an NF-κB-independent antiapoptotic pathway in endothelial cells exposed to LPS
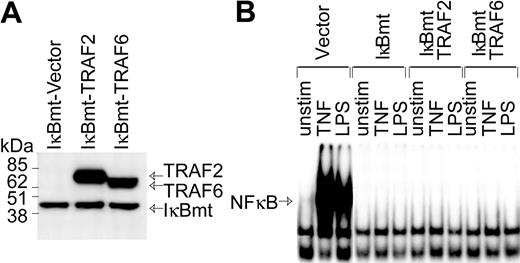
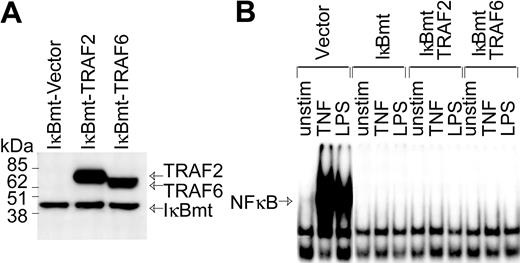
In part, LPS signals an endothelial death pathway through TRAF6-mediated activation of JNK.22 However, TRAF6 also mediates LPS-induced NF-κB activation.20,22 Since TRAF6 appears to integrate LPS-generated signals that could potentially act in opposing fashions to modulate cell viability, we attempted to determine whether TRAF6 acts as a bifurcation point of the LPS-induced cell survival and apoptotic pathways.17,20,21 Although NF-κB appears to be dispensable as a survival pathway for LPS-signaled apoptosis, LPS can induce antiapoptotic molecules via NF-κB.13 Thus, to determine whether TRAF6 can signal an NF-κB-independent survival pathway, HMEC-IκBmt cells were cotransduced with either TRAF6 or TRAF2 as a control (Figure 2A). While both TRAF6 and TRAF2 play a role in TNF-mediated signals, thus far only TRAF6 is implicated in cell signaling by the TLRs/LPS.22 Hence, TRAF2 serves as an additional negative control in these experiments. NF-κB activation in response to LPS or TNF was completely blocked in IκBmt cotransduced endothelial lines, as measured by NF-κB electrophoretic mobility shift assay (Figure 2B).
Enforced expression of IκBmt protein inhibits NF-κB activation in the presence of TRAF6. (A) Expression of Flag-tagged IκBmt, TRAF2, and TRAF6 in retrovirally transduced endothelial cell lines was verified by immunoblotting of total cell extracts using the M5 anti-Flag monoclonal antibody. (B) Electrophoretic mobility shift assay was performed on nuclear extracts from endothelial cells transduced with the empty vector IκBmt or a combination of IκBmt and TRAF2 or TRAF6. Cells were left unstimulated (unstim), or stimulated with TNF (10 ng/mL) or LPS (100 ng/mL) for 30 minutes prior to harvesting nuclear extracts.
Enforced expression of IκBmt protein inhibits NF-κB activation in the presence of TRAF6. (A) Expression of Flag-tagged IκBmt, TRAF2, and TRAF6 in retrovirally transduced endothelial cell lines was verified by immunoblotting of total cell extracts using the M5 anti-Flag monoclonal antibody. (B) Electrophoretic mobility shift assay was performed on nuclear extracts from endothelial cells transduced with the empty vector IκBmt or a combination of IκBmt and TRAF2 or TRAF6. Cells were left unstimulated (unstim), or stimulated with TNF (10 ng/mL) or LPS (100 ng/mL) for 30 minutes prior to harvesting nuclear extracts.
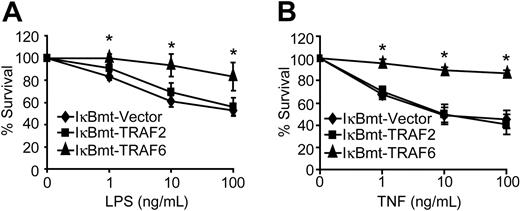
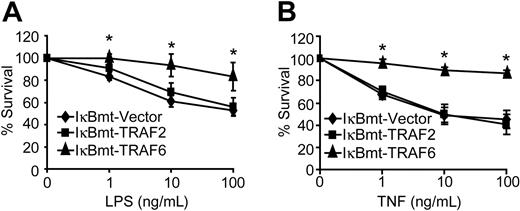
Despite complete blockade of NF-κB activation, TRAF6 was able to protect endothelial cells against LPS/CHX-induced cell death (Figure 3A). In contrast, TRAF2 showed no protective activity against LPS/CHX-induced cell death (Figure 3A). Because TRAF6 appears to be involved in the TNF signaling cascade, and we have shown that TNF alone (in the absence of CHX) is sufficient to cause endothelial apoptosis when NF-κB is blocked,27 we attempted to determine whether TRAF6 is also able to protect against TNF-induced death. Interestingly, TRAF6 provided an NF-κB-independent survival activity in response to TNF-induced death in the absence of CHX (Figure 3B). This is consistent with our finding that a dominant-negative TRAF6 sensitizes endothelial cells to TNF-induced death.22 Again TRAF2 was not able to protect endothelial cells from TNF-initiated apoptosis when NF-κB activity was repressed (Figure 3B). Although TRAF2 has previously been suggested to signal NF-κB-independent survival activity in response to TNF, in neither of the 2 previous studies was there direct evidence showing that TRAF2 was able to protect cells when NF-κB was inhibited.35,36
TRAF6 inhibits endothelial apoptosis independently of NF-κB activation. Double-transduced endothelial cells (as labeled) were treated with LPS and CHX (50 μg/mL; A) or TNF (without CHX; B) for 16 hours. Cell survival was measured by neutral red incorporation and normalized to cells treated with CHX alone (A) or to vehicle-treated cells (B). Data shown are the mean plus or minus SEM of 6 independent experiments, each done in triplicate. *P < .05.
TRAF6 inhibits endothelial apoptosis independently of NF-κB activation. Double-transduced endothelial cells (as labeled) were treated with LPS and CHX (50 μg/mL; A) or TNF (without CHX; B) for 16 hours. Cell survival was measured by neutral red incorporation and normalized to cells treated with CHX alone (A) or to vehicle-treated cells (B). Data shown are the mean plus or minus SEM of 6 independent experiments, each done in triplicate. *P < .05.
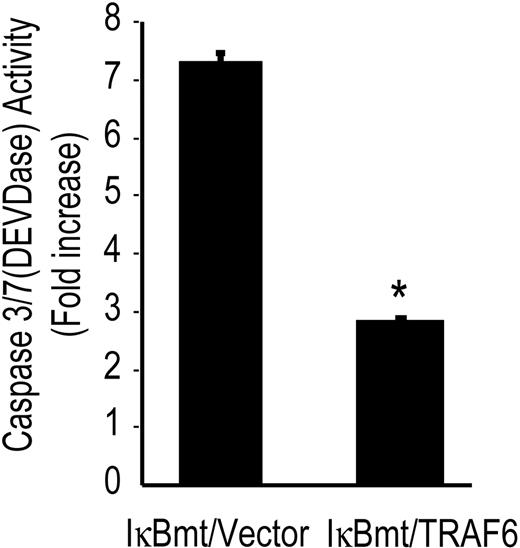
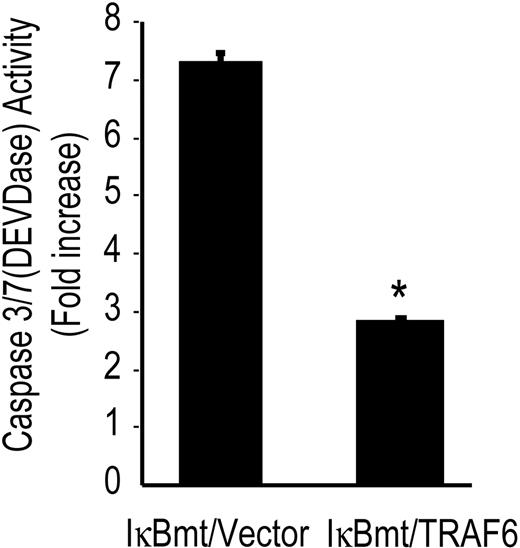
To confirm that TRAF6 initiates an antiapoptotic pathway in response to LPS, we determined whether TRAF6 was able to block caspase activation independently of NF-κB. Using the tetrapeptide caspase substrate, DEVD-pNA, in chromogenic assays of caspase activation, we observed that TRAF6 inhibited DEVDase activity (caspase 3/7) in the absence of functional NF-κB (Figure 4).
TRAF6 inhibits LPS-induced caspase activation independently of NF-κB activation. Double-transduced endothelial cells (as labeled) were treated with LPS (100 ng/mL) and CHX (50 μg/mL) for 8 hours and assayed for DEVD-pNA cleavage activity. Results are reported as fold increase over CHX-only-treated cells. Results shown are the mean plus or minus SEM of 3 independent experiments, each done in duplicate. *P < .01.
TRAF6 inhibits LPS-induced caspase activation independently of NF-κB activation. Double-transduced endothelial cells (as labeled) were treated with LPS (100 ng/mL) and CHX (50 μg/mL) for 8 hours and assayed for DEVD-pNA cleavage activity. Results are reported as fold increase over CHX-only-treated cells. Results shown are the mean plus or minus SEM of 3 independent experiments, each done in duplicate. *P < .01.
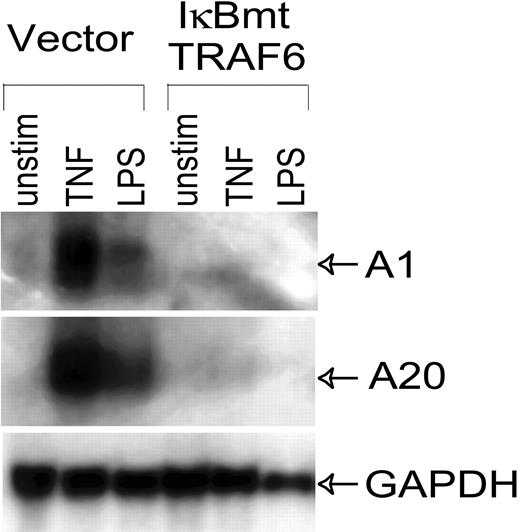
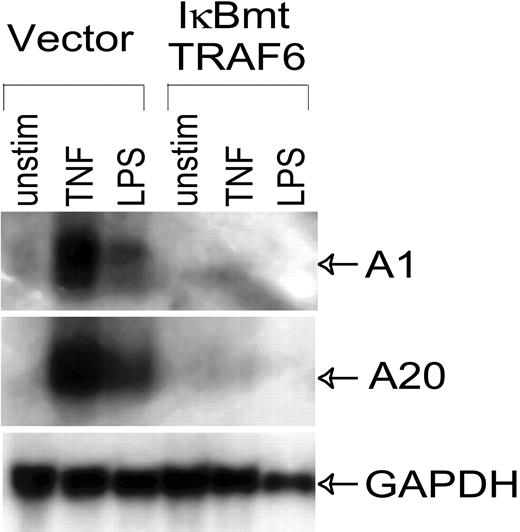
Even though LPS does not cause endothelial cell death when NF-κB activation is blocked, LPS stimulation of NF-κB activity results in the up-regulation of NF-κB-dependent cytoprotective factors.13 Enforced expression of these genes, such as the Bcl-2 homologue A1, and the zinc finger protein A20, is able to protect endothelial cells from LPS/CHX-induced apoptosis.13 Thus we performed Northern analysis to confirm that the NF-κB-dependent cytoprotective genes, A1 and A20, were not up-regulated through alternate pathways in these endothelial cell lines. As shown in Figure 5, TRAF6 was not able to induce A1 or A20 in the absence of NF-κB, thus ruling these genes out as possible mechanisms of LPS-induced, NF-κB-independent cytoprotective activity.
LPS-induced expression of antiapoptotic genes is inhibited by IκBmt/TRAF6 double-transduced cells. Northern analysis was performed on total RNA (20 μg) extracted from control or IκBmt/TRAF6 double-transduced endothelial cells, which were left unstimulated (unstim), or exposed to TNF (10 ng/mL) or LPS (100 ng/mL) for 3 hours. Membranes were hybridized to probes for the NF-κB-inducible antiapoptotic genes, A1 and A20, or to a GAPDH probe used as a loading and transfer control.
LPS-induced expression of antiapoptotic genes is inhibited by IκBmt/TRAF6 double-transduced cells. Northern analysis was performed on total RNA (20 μg) extracted from control or IκBmt/TRAF6 double-transduced endothelial cells, which were left unstimulated (unstim), or exposed to TNF (10 ng/mL) or LPS (100 ng/mL) for 3 hours. Membranes were hybridized to probes for the NF-κB-inducible antiapoptotic genes, A1 and A20, or to a GAPDH probe used as a loading and transfer control.
TRAF6 is required for LPS-induced activation of Akt, but not ERK1/2, in endothelial cells
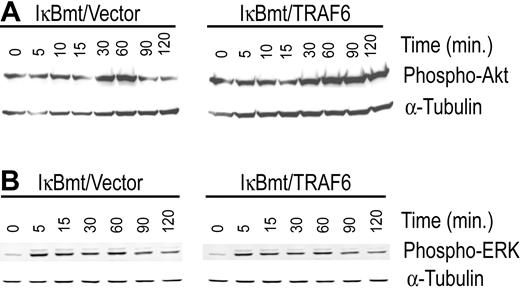
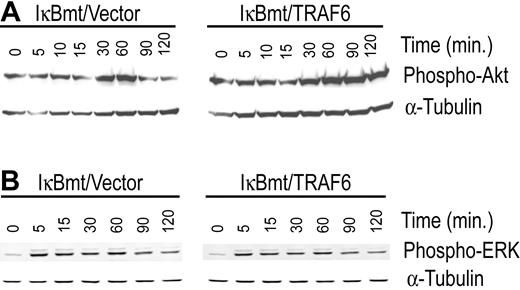
In an attempt to identify the NF-κB-independent, TRAF6-induced survival signals we tested the cotransduced cells for differential activation of other major survival pathways that have also been shown to be mediated through TRAF6. In this regard, PI3K/Akt and the MAPK/ERK pathways have both been shown to signal antiapoptotic pathways in various cell types.23,24 In 293T cells, the PI3K/Akt pathway has been shown to be activated by TRANCE through a signaling complex involving TRAF6 and c-Src.24 Ligation of CD40 in 293 cells results in TRAF6-dependent activation of ERK activity independent of Ras.23 We thus tested whether TRAF6/IκBmt-transduced endothelial cells demonstrated heightened Akt and/or ERK activation compared with controls, in response to LPS. As shown in Figure 6A, enforced expression of TRAF6 promotes activation of Akt by LPS as demonstrated by the increased and prolonged phosphorylation of Akt. In contrast, enforced TRAF6 expression in endothelial cells does not result in enhanced activation of ERK1/2 as determined by phosphorylation of these proteins in response to LPS (Figure 6B).
Enforced expression of TRAF6 enhances LPS-induced activation of Akt in the absence of functional NF-κB. Double-transduced endothelial cells (as labeled) were treated with LPS (100 ng/mL) and CHX (50 μg/mL) for various times as indicated. Activation of Akt (A) and ERK (B) was determined by immunoblotting for phospho-Akt or phospho-ERK, respectively. Membranes were stripped and reprobed for α-tubulin as a loading control. Results are representative of 3 independent experiments.
Enforced expression of TRAF6 enhances LPS-induced activation of Akt in the absence of functional NF-κB. Double-transduced endothelial cells (as labeled) were treated with LPS (100 ng/mL) and CHX (50 μg/mL) for various times as indicated. Activation of Akt (A) and ERK (B) was determined by immunoblotting for phospho-Akt or phospho-ERK, respectively. Membranes were stripped and reprobed for α-tubulin as a loading control. Results are representative of 3 independent experiments.
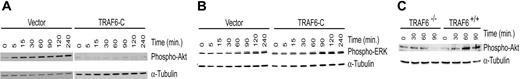
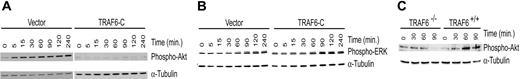
Previously we have shown that a truncated TRAF6 construct (TRAF6-C) acts in a dominant-negative fashion to inhibit LPS-initiated, TRAF6-mediated signals.22 To confirm the requirement for TRAF6 in LPS-induced Akt phosphorylation, we expressed the dominant-negative TRAF6-C construct in endothelial cells and determined whether Akt activation was inhibited. Immunoblotting demonstrates that TRAF6-C abrogates Akt phosphorylation in response to LPS (Figure 7A), but does not inhibit ERK1/2 activation (Figure 7B). Interestingly, in some experiments TRAF6-C appeared to enhance ERK1/2 activation (data not shown). Thus, TRAF6 is required for Akt, but not ERK, activation. To verify that TRAF6 is required for Akt activation, we tested TRAF6-deficient murine embryonic fibroblasts (MEFs).21 As seen in Figure 7C, TRAF6-/- MEFs were not able to activate Akt in response to LPS compared with wild-type MEFs. Similar results were seen when cells were stimulated with LPS and CHX (data not shown).
Inhibition of TRAF6 abolishes LPS-induced Akt activation. Endothelial cells transduced with a dominant-negative TRAF6 (TRAF6-C) were exposed to LPS (100 ng/mL) and CHX (50 μg/mL) for various times as indicated. Activation of Akt (A) and ERK (B) was determined by immunoblotting for phospho-Akt or phospho-ERK, respectively. Membranes were stripped and reprobed for α-tubulin as a loading control. (C) Wild-type or TRAF6-/- MEFs were stimulated with LPS only (100 ng/mL), and activation of Akt was determined by immunoblotting for phospho-Akt. Membranes were stripped and probed with α-tubulin as a loading control. Results are representative of 3 independent experiments.
Inhibition of TRAF6 abolishes LPS-induced Akt activation. Endothelial cells transduced with a dominant-negative TRAF6 (TRAF6-C) were exposed to LPS (100 ng/mL) and CHX (50 μg/mL) for various times as indicated. Activation of Akt (A) and ERK (B) was determined by immunoblotting for phospho-Akt or phospho-ERK, respectively. Membranes were stripped and reprobed for α-tubulin as a loading control. (C) Wild-type or TRAF6-/- MEFs were stimulated with LPS only (100 ng/mL), and activation of Akt was determined by immunoblotting for phospho-Akt. Membranes were stripped and probed with α-tubulin as a loading control. Results are representative of 3 independent experiments.
PI3K/Akt signals a TRAF6-dependent antiapoptotic pathway in endothelial cells stimulated with LPS
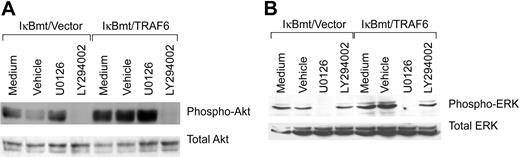
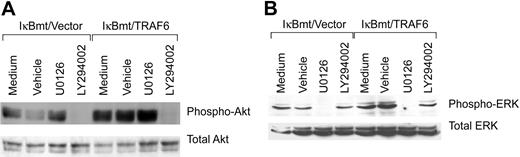
Activation of the cytoprotective kinase, Akt, provides a potent antiapoptotic signal in endothelial and other cells by phosphorylation of various downstream substrates.37 Akt is phosphorylated and activated downstream of PI3K, and PI3K has been shown to be activated downstream of TRAF6 in complex with c-Src.24 Thus, inhibition of PI3K with the inhibitor LY294002 should block TRAF6-initiated Akt phosphorylation. As demonstrated in Figure 8A, LY294002 does prevent LPS-induced Akt phosphorylation even in the TRAF6-expressing endothelial cells. This inhibition is specific in that ERK phosphorylation is not blocked by LY294002. ERK activation has also been shown to protect against various apoptotic stresses.38 Dominant-negative MEK has been shown to inhibit TRAF6-independent, as well as TRAF6-initiated, ERK1/2 activation. We thus tested whether the MEK inhibitor U0126 was able to block LPS-initiated ERK activation. As shown in Figure 8B, activation of ERK in response to LPS-TRAF6 signaling can be blocked by the MEK inhibitor U0126. Again, the inhibition is specific in that Akt activation is not blocked by U0126. Thus TRAF6 appears to act upstream of or parallel to PI3K to activate Akt in response to LPS. In contrast, LPS-induced ERK activation in endothelial cells is downstream of MEK, but does not appear to require TRAF6.
Inhibition of PI3K or MEK inhibits LPS-induced Akt or ERK activation, respectively. Double-transduced endothelial cells (as labeled) were pretreated for 60 minutes with specific inhibitors, vehicle only, or left untreated. Cells were subsequently treated with LPS (100 ng/mL) and CHX (50 μg/mL) for 60 minutes (Akt) or 30 minutes (ERK). Activation of Akt and ERK was determined by immunoblotting for phospho-Akt or phospho-ERK, respectively. Membranes were stripped and reprobed for total levels of ERK or Akt.
Inhibition of PI3K or MEK inhibits LPS-induced Akt or ERK activation, respectively. Double-transduced endothelial cells (as labeled) were pretreated for 60 minutes with specific inhibitors, vehicle only, or left untreated. Cells were subsequently treated with LPS (100 ng/mL) and CHX (50 μg/mL) for 60 minutes (Akt) or 30 minutes (ERK). Activation of Akt and ERK was determined by immunoblotting for phospho-Akt or phospho-ERK, respectively. Membranes were stripped and reprobed for total levels of ERK or Akt.
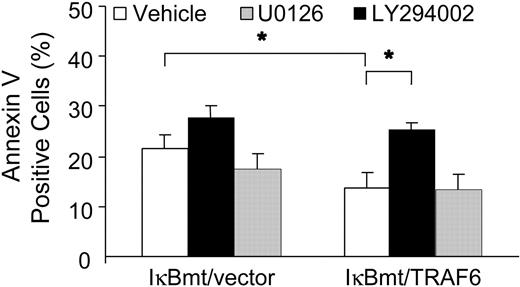
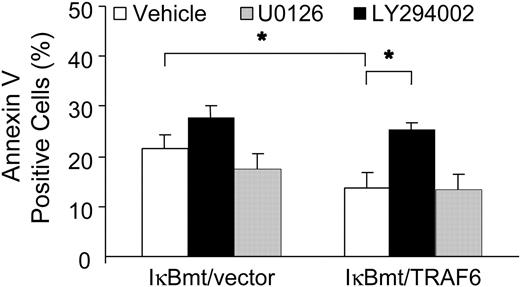
To determine whether TRAF6 was activating an NF-κB-independent antiapoptotic pathway through PI3K/Akt activation, we used annexin V labeling to determine whether inhibition of PI3K inhibited the TRAF6-related cytoprotection in response to LPS. In parallel, we also tested whether the MEK/ERK1/2 pathway plays a cytoprotective role against LPS-induced apoptosis. As seen in Figure 9, inhibition of PI3K activation abrogated the endothelial cytoprotective activity provided by TRAF6, whereas inhibition of MEK activity did not increase the apoptotic fraction in response to LPS, as measured by annexin V binding. These findings were corroborated in a viability assay using neutral red to detect viable cells (data not shown).
Inhibition of PI3K abrogates the TRAF6 protective effect. Double-transduced endothelial cells (as labeled) were treated with LPS (100 ng/mL) and CHX (50 μg/mL) for 8 hours following a 60-minute pretreatment with vehicle or specific inhibitor as indicated. Apoptotic cells were quantitated by labeling with Alexa 594-conjugated annexin V. Results shown are the increase in percentage of annexin V-positive cells over CHX treatment alone, and represent the mean plus or minus SEM of 3 independent experiments. *P < .05.
Inhibition of PI3K abrogates the TRAF6 protective effect. Double-transduced endothelial cells (as labeled) were treated with LPS (100 ng/mL) and CHX (50 μg/mL) for 8 hours following a 60-minute pretreatment with vehicle or specific inhibitor as indicated. Apoptotic cells were quantitated by labeling with Alexa 594-conjugated annexin V. Results shown are the increase in percentage of annexin V-positive cells over CHX treatment alone, and represent the mean plus or minus SEM of 3 independent experiments. *P < .05.
TRAF6 inhibits LPS-induced apoptosis in primary endothelial cells
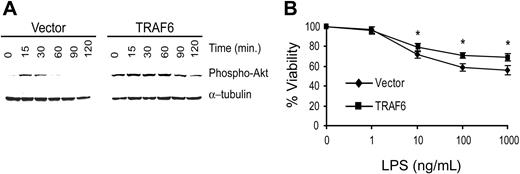
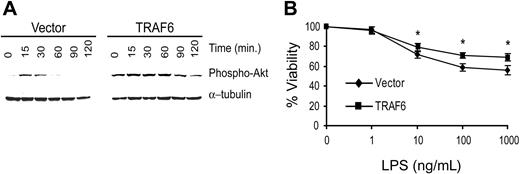
To confirm that TRAF6 signals cytoprotective activity in primary endothelial cells, we transduced primary bovine aortic endothelial cells (BAECs) with TRAF6 or the empty vector. Because LPS induces greater apoptosis in BAECs, we performed the following studies in the absence of CHX.22 As seen in Figure 10A, BAECTRAF6 demonstrates greater and more prolonged Akt activation in response to LPS (100 ng/mL) than vector-transduced cells. Further, BAEC-TRAF6 was protected from LPS-induced death compared with vector-transduced BAECs (Figure 10B). These findings confirm the above studies suggesting that LPS induces a TRAF6-dependent, PI3K/Akt-initiated antiapoptotic pathway in primary endothelial cells.
TRAF6 inhibits LPS-induced apoptosis in primary endothelial cells. BAECs were transduced with TRAF6 or empty vector. (A) Cells were stimulated with LPS for various times as indicated and activation of Akt was determined by immunoblotting for phospho-Akt. Membranes were stripped and reprobed for α-tubulin as a loading control. (B) Transduced BAECs were treated with LPS at various concentrations for 16 hours. Cell survival was measured by neutral red incorporation and normalized to cells treated with vehicle alone. Data shown are the mean plus or minus standard deviation (SD) of 4 independent experiments, each done in triplicate. *P < .05.
TRAF6 inhibits LPS-induced apoptosis in primary endothelial cells. BAECs were transduced with TRAF6 or empty vector. (A) Cells were stimulated with LPS for various times as indicated and activation of Akt was determined by immunoblotting for phospho-Akt. Membranes were stripped and reprobed for α-tubulin as a loading control. (B) Transduced BAECs were treated with LPS at various concentrations for 16 hours. Cell survival was measured by neutral red incorporation and normalized to cells treated with vehicle alone. Data shown are the mean plus or minus standard deviation (SD) of 4 independent experiments, each done in triplicate. *P < .05.
Discussion
It is thought that the manifestations of sepsis result from the dysregulation of innate immunity.1,2,6 The endothelium plays a pivotal role in orchestrating the early inflammatory response seen in sepsis by recruiting leukocytes, and facilitating their transmigration into tissues.29,39 The microcirculation undergoes major alterations during sepsis often resulting in systemic vascular collapse, disseminated intravascular coagulation, and vascular leak syndrome.1,2,39 One common element to these complications is endothelial cell injury and/or dysfunction. LPS is the prototypic activator of innate immune responses, and many of the inflammatory and clinical effects of Gram-negative sepsis are elicited by LPS.1,2,6,39 Widespread endothelial cell apoptosis has been demonstrated in LPS-challenged mice.3,5 This endothelial apoptosis is only partially prevented by inhibiting the activity of TNF.3 LPS can directly disrupt endothelial monolayer integrity and cytoprotective signaling events through caspase cleavage of adherens junction proteins.40 The endothelial response to LPS also includes actin reorganization and cell detachment from the underlying matrix, thereby causing a breach in the endothelial barrier.5,40
While it is clear that TLRs transmit signals that activate and propagate inflammatory responses, there is also significant evidence that these receptors can concomitantly signal both pro- and antiapoptotic pathways.13,14,22,41,42 The apoptotic response may be a protective strategy in some cases. It may also be a “byproduct” of inflammatory signaling in the absence of sufficient antiapoptotic signals, or due to the presence of overwhelming proapoptotic triggers. In endothelial cells LPS has been shown to trigger both pro- and antiapoptotic pathways.13,14,22 Thus one could speculate that in an appropriately “controlled” blood-borne bacterial infection, endothelial viability may be preserved, whereas in sepsis with multiorgan dysfunction, there may be increased endothelial apoptosis resulting in many of the clinical sequelae of sepsis.
In cultured endothelial cells, LPS induces a minimal, but detectable, amount of apoptosis.22 To enhance LPS-initiated endothelial death in vitro CHX was added to sensitize cells to apoptosis in this study. Although this model cannot directly be applied to in vivo findings during sepsis, there is evidence to suggest that the endothelial survival pathway may fail due to inhibition by other inflammatory mediators such as interferon-γ, or that the death pathway overwhelms the survival pathway by synergism with other cytokines during sepsis.43,44 It has also been suggested that LPS-induced nitric oxide may synergize with IL-1 to render endothelial cells more susceptible to apoptosis.45 The situation in vivo is further complicated by the fact that LPS has functional effects on leukocytes that can then directly or indirectly affect the viability of endothelium.46 For instance, it has been shown that cytokine-activated neutrophils and LPS-activated mononuclear cells can cause endothelial damage.46,47 However, even a small breach in endothelial barrier function may cause significant deleterious effects in vivo. Additionally, apoptotic endothelial cells demonstrate increased thrombogenecity and increased leukocyte adhesion.48,49 As we and others have previously shown, primary endothelial cells will undergo apoptosis in the absence of CHX, and as demonstrated in Figure 10, enforced expression of TRAF6 enhances Akt activation and inhibits LPS-induced endothelial death.22,50,51
The model described herein provides significant insight into endothelial signaling initiated by LPS. Our findings demonstrate that LPS can activate both MEK/ERK signaling and PI3K/Akt activation. However, only the PI3K/Akt pathway appears to require TRAF6, and plays a significant survival role in response to LPS in endothelial cells. PI3K has been suggested by various groups to activate NF-κB, either through activation of the IκB kinases or through transcriptional activation in the nucleus.52-54 However, our studies clearly demonstrate that the protective effect of PI3K activation is independent of NF-κB activation, since the cytoprotective activity is evident in the face of complete blockade of NF-κB nuclear translocation. Of interest, CD40 ligation also provides an endothelial cytoprotective function and induces PI3K/Akt activation associated with direct interaction of CD40 with TRAF6.55 The PI3K/Akt pathway has been shown to signal cell survival through transcription-dependent and -independent pathways.37 The transcription-independent pathways may act prior to the release of cytochrome c from mitochondria, by regulating proapoptotic Bcl-2 family members, or subsequent to the release of cytochrome c.37,56-61 Interestingly, inhibition of total TRAF6 activity also blocks endothelial apoptosis, because the inhibition of JNK activation abrogates the LPS apoptotic signal.22 The findings described in this manuscript suggest that enforced expression of TRAF6 provides greater antiapoptotic activity by enhancing Akt activity, but that JNK activation is likely already at a maximal level. To determine how the TRAF6 pathway activated by LPS functions in endothelial cell viability and apoptosis in vivo will require further studies.
We have previously shown that inhibition of TRAF6 by a truncated dominant-negative mutant inhibits NF-κB and JNK activation in endothelial cells.22 The functional result of inhibiting TRAF6 is that endothelial cells are protected against LPS-induced apoptosis even though NF-κB activity is also inhibited. In part the protective effect of inhibiting TRAF6 is due to the inhibition of a JNK-induced apoptotic signal.22 That TRAF6 also activates a PI3K-dependent antiapoptotic pathway suggests that TRAF6 acts as a bifurcation point of the parallel LPS-induced survival and death pathways (Figure 11). Recently it has been shown in THP-1 cells that the PI3K/Akt pathway inhibits JNK activity, suggesting one possible way that LPS-induced PI3K blocks the concomitant JNK-activated apoptotic pathway.62 In response to TNF stimulation, the serine-threonine kinase RIP functions in a similar fashion to activate survival and death pathways.63,64 In the case of RIP, however, enforced expression results in apoptosis, despite the critical role of NF-κB in signaling antiapoptotic pathways that protect against TNF-induced death.63,64 Our findings indicate that even though TNF and LPS evoke similar functional responses in endothelial cells, in some cases the signaling pathways utilized are distinct when cell responses are triggered through TNF receptors versus Toll-like receptors.
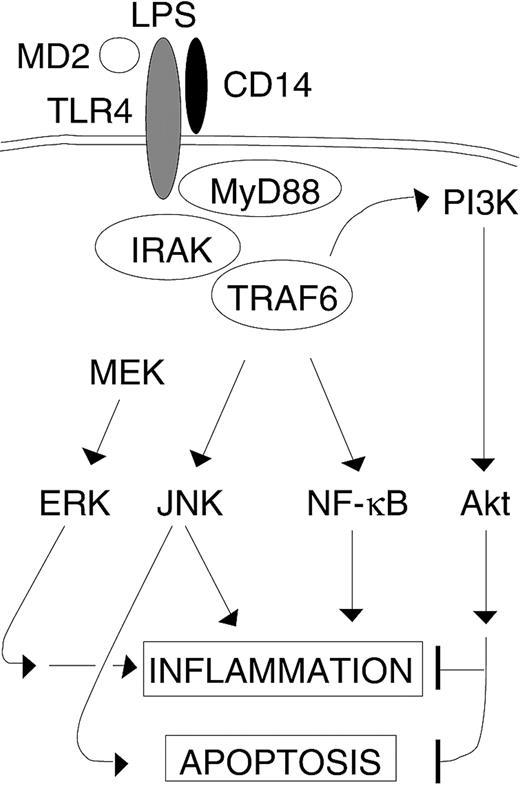
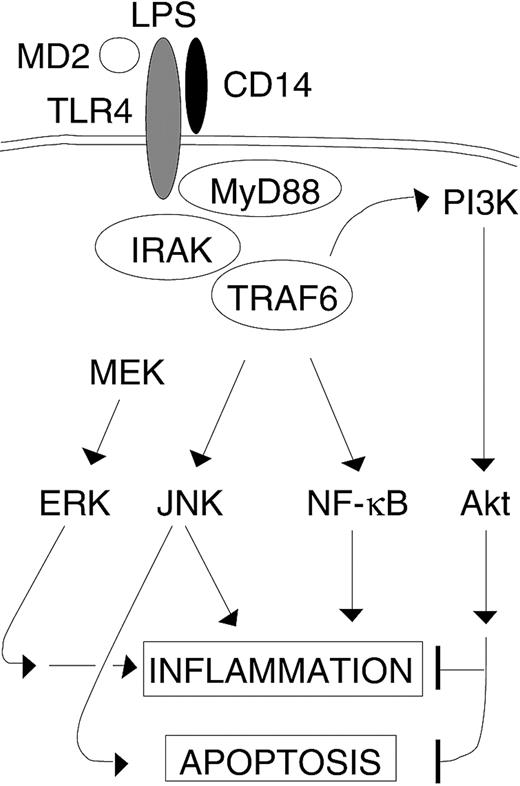
The role of TRAF6 in LPS-stimulated endothelial cells. LPS-induced activation of TRAF6 results in activation of multiple downstream signaling pathways involved in inflammation. Additionally, TRAF6 routes parallel apoptotic (JNK) and antiapoptotic (PI3K) signals that are triggered by LPS. However, LPS-induced ERK activation in endothelial cells is TRAF6 independent.
The role of TRAF6 in LPS-stimulated endothelial cells. LPS-induced activation of TRAF6 results in activation of multiple downstream signaling pathways involved in inflammation. Additionally, TRAF6 routes parallel apoptotic (JNK) and antiapoptotic (PI3K) signals that are triggered by LPS. However, LPS-induced ERK activation in endothelial cells is TRAF6 independent.
Prepublished online as Blood First Edition Paper, March 2, 2004; DOI 10.1182/blood-2003-06-2118.
Supported by grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of British Columbia and the Yukon. A.K. is a Clinician-Scientist of the Canadian Institutes of Health Research and a Scholar of the Michael Smith Foundation for Health Research.
F.W. and C.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.