Abstract
The potential benefits of extended rituximab treatment have been investigated in a randomized trial comparing the standard schedule with prolonged treatment in 202 patients with newly diagnosed or refractory/relapsed follicular lymphoma (FL). All patients received standard treatment (rituximab 375 mg/m2 weekly × 4). In 185 evaluable patients, the overall response rate was 67% in chemotherapy-naive patients and 46% in pretreated cases (P < .01). Patients responding or with stable disease at week 12 (n = 151) were randomized to no further treatment or prolonged rituximab administration (375 mg/m2 every 2 months for 4 times). At a median follow-up of 35 months, the median event-free survival (EFS) was 12 months in the no further treatment versus 23 months in the prolonged treatment arm (P = .02), the difference being particularly notable in chemotherapy-naive patients (19 vs 36 months; P = .009) and in patients responding to induction treatment (16 vs 36 months; P = .004). The number of t(14;18)-positive cells in peripheral blood (P = .0035) and in bone marrow (P = .0052) at baseline was predictive for clinical response. Circulating normal B lymphocytes and immunoglobulin M (IgM) plasma levels decreased for a significantly longer time after prolonged treatment, but the incidence of adverse events was not increased. In patients with FL, the administration of 4 additional doses of rituximab at 8-week intervals significantly improves the EFS.
Introduction
Rituximab is the first commercially available monoclonal antibody for the treatment of lymphoma. It is a chimeric anti-CD20 immunoglobulin (Ig) comprising a human IgG1 and κ constant region with murine variable regions. Its mechanisms of action are thought to be complement-mediated lysis; antibody-dependent, cell-mediated cytotoxicity; and induction of apoptosis after binding to the CD20 antigen on the cell surface. As one of the most effective single-agent treatments for non-Hodgkin lymphoma (NHL) developed in the last decade, rituximab has rapidly become a common treatment for this malignancy, either as a single agent or, more recently, in combination with chemotherapy. Rituximab used as a single agent is very active in several types of indolent lymphomas with response rates (RRs) of 45% to 60% even in pretreated patients,1-3 while in more aggressive types of NHL RRs are approximately 30%.4,5 When added to chemotherapy, rituximab significantly improves the RR and in some studies has been shown to improve survival rates compared with chemotherapy alone.6,7
The standard rituximab treatment schedule (375 mg/m2 each week for 4 weeks) was based on the results of 2 phase 1 trials.8,9 Further trials using this schedule in patients with low-grade lymphoma confirmed its clinical activity, achieving a RR of approximately 50% and a response duration of 10 to 12 months.1,2 However, the relatively low proportion of complete responses (in the range of 5%) gave rise to the suggestion that this standard 375 mg/m2 weekly × 4 schedule might be enhanced to optimize the clinical efficacy of rituximab. In addition, a significant correlation between the median antibody concentration in blood and response to treatment has been observed.10 Finally, for the majority of anticancer drugs a treatment duration of several months is needed to attain the optimal result, and this could also apply to rituximab.
These considerations led us to propose that evaluation of various dosing schedules was warranted and that extended exposure to rituximab would be likely to augment its biologic effect and may thus achieve a higher partial and complete RR, longer time to progression, and longer duration of response. The present trial was therefore designed to test this hypothesis. We compared the standard schedule (375 mg/m2 weekly × 4) with a prolonged rituximab schedule in which the standard treatment was followed by a single 375-mg/m2 infusion every 2 months for 4 times. The purpose was to maintain a biologically active serum concentration for approximately 1 year. The 2-month interval was chosen based on preliminary pharmacokinetics data that demonstrated the persistence of potentially active drug levels in the serum of the majority of patients after this interval.10 To avoid randomizing rituximab-resistant patients into the prolonged treatment arm, the randomization was implemented after the end of the standard treatment (week 12 after initiation), when patients had proved not to be resistant to rituximab.
Patients and methods
Trial design
Trial sites. Patients were enrolled between January 1998 and February 2001 in 25 institutions in Switzerland, Italy, and South Africa. The trial was approved by the local ethics committee of each participating institution and conducted in accordance with the Declaration of Helsinki and currently applicable amendments. All patients gave written informed consent.
Treatment. All patients were initially treated with rituximab 375 mg/m2 per week for 4 weeks (“induction” phase). The drug was to be administered every 7 ± 1 days. Patients with stable disease (SD) or who were in partial (PR) or complete remission (CR) at week 12 were randomized in a 1:1 ratio to no further treatment (arm A, “no further treatment”) or to treatment with a single infusion of rituximab 375 mg/m2 at week 12 and again at months 5, 7, and 9 (arm B, “prolonged treatment”). The randomization was stratified according to status of disease at trial entry (first presentation vs refractory or relapsed disease), response to induction treatment (stable disease vs response), and center, using the minimization method. 11(pp84-86) Patients were randomized centrally by fax via the SAKK Trials Office in Bern. Upon progression or relapse, further treatment was at the treating physician's discretion.
Trial end points. Event-free survival (EFS) time was the primary end point and was calculated as the time from first induction infusion to progression, relapse, second tumor, or death from any cause. Remission duration was defined in the same way, but restricted to responding patients.
Sample size and interim analyses. For the randomized phase of the trial, a group sequential design with 2 interim analyses and 1 final analysis using EFS as the primary endpoint was adopted. The overall type I error probability was 5% and the statistical power was 80% for the 2-sided log-rank test, to detect an increase of EFS from 9 months in the no further treatment arm to 16.2 months in the prolonged treatment arm. A total of 99 events in about 135 patients were required for the final analysis. The first interim analysis was performed after 26 events in 59 patients and the second after 69 events in 132 randomized patients. Neither interim analysis called for early stopping of the trial.
Patients
Inclusion criteria were a biopsy-proven CD20+ follicular lymphoma grade I, II, or III according to the REAL classification, age of 18 years or older, and measurable disease defined as the presence of at least one previously unirradiated lesion with 2 measurable perpendicular diameters of which at least one should be of 2 cm. The interval since the last systemic anticancer treatment should not be shorter than 28 days (6 weeks if treated with nitrosureas). Patients should not have received corticosteroids within 28 days prior to trial entry unless chronically administered at a dosage of no more than 20 mg/day for indications other than lymphoma or lymphoma-related symptoms. Other inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less and, following assessment of the first 100 patients, in some of whom unexpected cardiac events had been observed,12 a cardiac ejection fraction (EF) of at least 50% as determined by echocardiography. Exclusion criteria included transformation to high-grade lymphoma, symptomatic central nervous system disease, prior antibody-based therapy, or a history of significant medical conditions, including previous malignancies within 5 years. Other exclusion criteria were a reduced renal function (creatinine > 2 times the upper limit of normal [ULN]) or liver function (bilirubin > 2 × ULN). Pregnant or lactating females, patients with active opportunistic infections, or patients with known HIV or hepatitis B or C infections were also excluded.
Trial assessments
Pretreatment evaluation. For all patients, a complete staging, including clinical examination, chest and abdomen computed tomography (CT) scan, bone marrow (BM) aspirate, and trephine biopsy, should be performed not more than 2 weeks before entering the trial. Any other clinically indicated tumor evaluations were at the discretion of the investigator. A central histology review was performed between registration and randomization to confirm the diagnosis.
Assessment of clinical response. A formal evaluation of all the involved sites was performed at 12 weeks and at 7, 12, 18, and 24 months, and then yearly or when clinically required. The first 100 patients had an additional staging procedure at week 8, but the first interim analysis showed similar RRs at weeks 8 and 12 and it was decided to limit the first restaging at week 12 for subsequent patients. Re-evaluation of the BM was required only at week 12 and month 12 if involved at trial entry.
Blood analyses. Routine blood counts and chemistries were assessed at baseline, before each rituximab administration, and at months 2, 3, 5, 7, 9, and 12. Serum immunoglobulins (IgG, IgA, IgM) were measured at baseline and again at months 3, 7, and 12, while blood samples for immunophenotyping were taken at baseline, week 12, and months 9, 12, 18, and 24. For the analysis of lymphocyte subsets, dimethyl sulfoxide (DMSO)-frozen cells were thawed and washed, stained with fluorescent antibodies, and analyzed on a FacsCalibur machine (Becton Dickinson, San Jose, CA) using the Multiset program. The antibodies used were anti-CD3, -CD4, -CD8, -CD19, -CD16 + 56, and -CD45. With a 4-color analysis on the lymphocyte gate, the following cells were counted: T cells (CD3+), T helper (CD3+/CD4+), T suppressor (CD3+/CD8+), natural killer (NK; CD3-/CD16+56+), and B cells (CD19+).
Molecular analysis. The presence of cells carrying the t(14;18) translocation was investigated at baseline in peripheral blood (PB) and BM using nested polymerase chain reaction (PCR)13 ; if the result was positive, the analysis was repeated at week 12 and month 12 for BM, while in the PB it was evaluated each time that a radiologic or clinical staging was performed. For the quantification of t(14;18) translocation-carrying cells with a major breakpoint region (MBR), real-time PCR analysis was performed using an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). For the detection of the t(14;18) translocation, 900 nM of the forward and reverse primer (identical to the internal primers used in the conventional PCR) and 300 nM of 5′-FAM-TTTCAACACAGACCCACCCAGAGCC-TAMRA-3′ as internal TaqMan probe were used; for the simultaneous amplification of the glyceraldehyde phosphate dehydrogenase (GAPDH) gene, 900 nM 5′-CTGGCACCCTATGGACACG-3′ as forward primer, 300 nM 5′-GGATGAGAAAGGTGGGAGCC-3′ as reverse primer, and 300 nM 5′-FAM-CCTGCACCACCAACTGCTTAGCACC-TAMRA-3′ as internal TaqMan probe were used. A total of 400 ng of genomic DNA, corresponding to 60 000 cells, was used in a final volume of 50 μL. For each sample the amplification of the MBR target gene and the GAPDH reference gene was performed in quintuplicates. The thermal cycling conditions comprised an initial uracil DNA glycosylase (UNG) Erase activation step at 60°C for 2 minutes, a denaturation step at 95°C for 10 minutes, and 50 cycles, each one consisting of 15 seconds at 95°C and 1 minute at 60°C. Genomic DNA from the t(14;18)-positive cell line Karpas 422,14 diluted into genomic DNA from t(14;18)-negative mononuclear cells (MNCs), was used to generate a standard curve for the t(14;18) translocation. After demonstrating that the amplification efficiencies of the target and reference gene were approximately equal (data not shown), the comparative Δ cycle threshold (ΔCt) method (ΔCt = Ct[t(14;18)] - Ct[GAPDH]) was used to calculate the t(14;18)-positive cell numbers in each sample.15 The calculated ΔCt standard curve showed an excellent correlation coefficient (r = 0.998) and slope (-3.29). The real-time PCR assay is highly reproducible with an interassay variation of 13% or less. At least 30 t(14;18)-positive cells per 106 MNCs could reproducibly be detected, which is in the range of other reports.16 If, in samples with 30 or less t(14;18)-positive cells per 106 MNCs, some of the 5 replicates failed to give a positive signal they were scored as 0.17
Toxicity. The evaluation of toxicity was conducted according to the World Health Organization toxicity grading.
Statistical methods
The comparisons between treatment arms or groups defined by other factors (as previously treated vs chemotherapy-naive or responders vs nonresponders) were carried out by Wilcoxon rank sum test for continuous variables and by chi-square or Fisher exact test for categoric variables. Odds ratios (ORs) were also calculated for associations between a binary response variable and a binary patient characteristic. Remission duration and EFS time were estimated by the Kaplan-Meier method and compared between groups by log-rank test. Hazard ratios (HRs) were also calculated as the ratio of hazard of experiencing an event in the nonreference group with respect to the reference group. The impact of some potential predictive variables on clinical response and EFS was analyzed by multiple logistic regression and multiple Cox regression, respectively, employing a stepwise model selection procedure. All tests were 2-sided. No adjustment for multiple comparisons in general was performed, except for the primary end point for which adjustment for multiple testing prescribed by the group sequential design was performed. For randomized patients, all between-arm comparisons were carried out by intention-to-treat principle.
Results
Patients' characteristics
A total of 202 patients were enrolled; their characteristics are summarized in Table 1. Seventeen patients were retrospectively judged ineligible for the trial: the pathology review could not confirm the follicular histology in 13 patients, while 4 patients did not have measurable disease as required per protocol. A further 3 patients had their last treatment less than 28 days prior to induction but this was considered a minor protocol violation and they were retained in the analysis.
Of the 185 eligible and evaluable patients, 35 were not randomized to the second phase of the trial: 26 due to disease progression during the induction phase, 5 due to major toxicity, and 4 due to other reasons. One patient was randomized prior to being found ineligible. The clinical and pathologic characteristics of the 151 randomized patients (78 in arm A and 73 in arm B) were similar in the 2 arms (Table 1) and, as expected, more favorable at randomization than at baseline. There was a tendency toward lower neutrophil counts at randomization. The proportions of patients having responded to any previous treatments and to rituximab induction treatment were similar for arms A and B. The only significant differences in baseline characteristics between the 2 arms were in lymphocyte subsets: NK- and B-cell counts were lower in arm A.
Description of treatment
Induction treatment. All except 5 patients received 4 infusions of rituximab 375 mg/m2. Four patients received only 1 infusion (1 patient died of a sudden cardiac event, 3 patients experienced infusion-related toxicity), and 1 patient did not receive the fourth infusion due to excessive infusion-related toxicity during the 3 previous infusions. The intervals between infusions in the induction phase were maintained in the planned range except in 2 patients. Reduced doses were administered to 2 patients.
Prolonged treatment. The majority of patients in arm B received the treatment according to the protocol. Treatment was discontinued before completing the planned schedule (4 infusions in 6 months) in 18 patients: 2 due to toxicity and 16 due to disease progression or relapse. In 1 patient the third prolonged treatment infusion was given at a reduced dose due to toxicity and in another patient the fourth prolonged treatment infusion was partially administered subcutaneously.
Objective response
Induction phase. At week 12 the RR for all 185 evaluable patients who received treatment was 52% (95% confidence interval [CI]: 45%-60%) with 8% CR. There was a significant difference between the RR of the 57 chemotherapy-naive patients (RR 67% with 9% CR) and the 128 previously treated patients (RR 46% with 8% CR) (OR = 2.34; P = .0097).
In addition to being chemotherapy naive, other favorable predictive factors identified by univariate analyses in evaluable patients included female sex (OR = 1.85; P = .040), disease diameter less than 5 cm (OR = 2.60; P = .0015), hemoglobin level at least 120 g/L (12 g/dL; OR = 2.12; P = .026), platelet count at least 100 × 109/L (OR = 2.68; P = .036), IgA level higher than 1.2 g/L (median) (OR = 2.24; P = .021), and IgM level higher than 0.6 g/L (median) (OR = 3.19; P = .0012). A multivariate analysis (due to missing values, only 149 patients were included in the model selection procedure) identified 2 significant independent predictors for clinical response (complete response [CR] and PR): previous chemotherapy (OR = 0.48; P = .05) and bulky disease (OR = 0.5; P = .039). Of the 97 evaluable patients who had BM involvement at baseline, 69 had BM re-evaluation at week 12 with 34 (49%) becoming morphologically negative. It is likely that the lack of BM evaluations for 28 patients has resulted in an underestimation of the CR rate, since patients assessed as achieving CR on the basis of clinical and radiologic evaluation, but who did not have a re-evaluation of a previously positive BM, were considered to have had a PR.
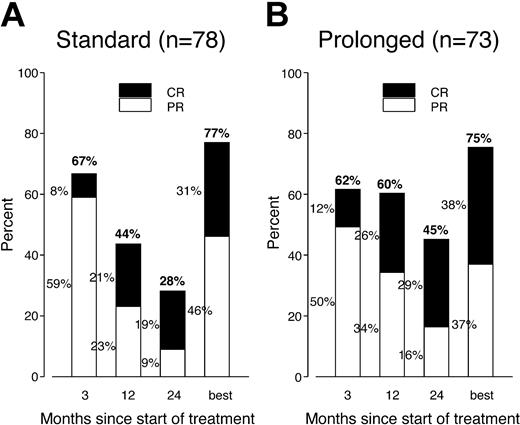
Postrandomization phase. Progressive disease was documented at around week 12 in 5 patients (4 in arm A, with no further treatment, and 1 in arm B, with prolonged treatment). The response assessments at week 12 and at months 12 and 24, as well as the overall best response rate (from randomization to last follow-up) for randomized patients, are summarized in Figure 1. In arm A the proportion of patients responding decreased steadily from 67% at week 12 to 44% at month 12 and 28% at month 24, while in arm B the proportion of responders remained initially stable, with 62% at week 12 and 60% at month 12, declining to 45% at month 24. In contrast, the proportion of patients in CR increased constantly in both arms, rising from 8% at week 12 to 19% at month 24 in arm A and from 12% to 29% in arm B. The number of patients improving their response in the first 2 years (from PR to CR, from SD to CR, or from SD to PR) was similar in both arms: 21 cases in arm A and 22 cases in arm B. Five patients in arm A and 8 patients in arm B had their first CR documented after month 12.
PR and CR rates (at intervals during follow-up and overall) in both study arms. The same proportion of patients responded in each arm and the number of complete responders gradually increased. The response rate remained elevated for a longer time in patients receiving prolonged treatment.
PR and CR rates (at intervals during follow-up and overall) in both study arms. The same proportion of patients responded in each arm and the number of complete responders gradually increased. The response rate remained elevated for a longer time in patients receiving prolonged treatment.
The between-arm difference in RR was not statistically significant at weeks 12 and month 7 but became significant after 1 year (P = .046). The between-arm difference in CR rate was not statistically significant at any time point. Among responders at week 12, the proportions of patients remaining in CR or PR at later time points were significantly lower in the no further treatment arm compared with the prolonged treatment arm: week 52, 56% versus 80% (P = .011); month 18, 40% versus 67% (P = .0097); and month 24, 35% versus 58% (P = .022). The overall best response was 77% (31% CR) in arm A and 75% (38% CR) in arm B (not statistically significant [NS]). The overall best response for chemotherapy-naive patients was 81% (31% CR) in arm A and 92% (52% CR) in arm B (NS).
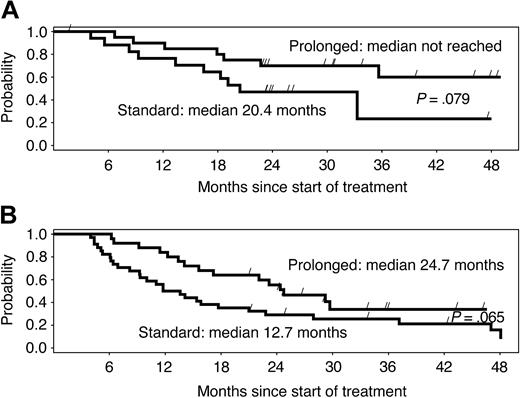
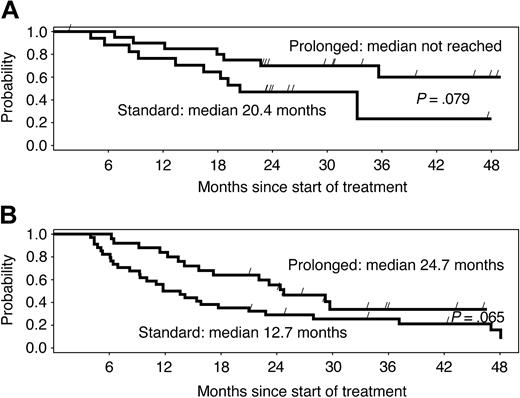
Among patients who reached a PR or CR, the median time to first response was 10 weeks in arm A and 9 weeks in arm B. The median remission duration among week-12 responders was 16 months in arm A and 36 months in arm B (P = .0039). The difference in remission duration was considerable and marginally significant for chemotherapy-naive versus pretreated patients (Figure 2A-B). Among patients who had BM involvement at baseline and BM re-evaluation during the postrandomization phase, 13 of 17 (77%) in arm A and 17 of 22 (77%) in arm B became morphologically negative for BM involvement.
Duration of response by study arm. Median duration of response is more than double with the prolonged schedule, independently of previous treatment, in (A) chemotherapy-naive and (B) pretreated patients. Differences are only marginally significant due to the small number of patients in subgroups.
Duration of response by study arm. Median duration of response is more than double with the prolonged schedule, independently of previous treatment, in (A) chemotherapy-naive and (B) pretreated patients. Differences are only marginally significant due to the small number of patients in subgroups.
Event-free survival
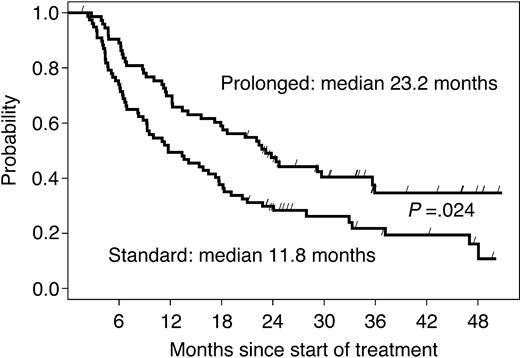
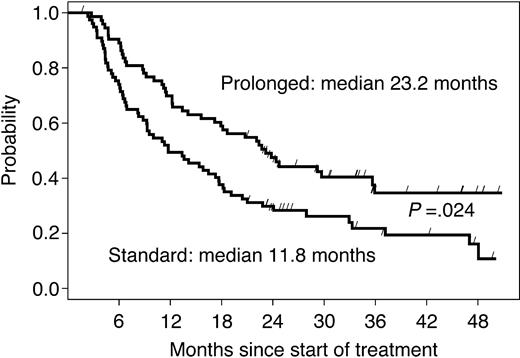
The median follow-up time in 126 living patients was 36 months. The median EFS time among 151 randomized patients was 11.8 months in arm A and 23.2 months in arm B (HR = 0.61, 95% CI: 0.40-0.93; P = .024, adjusted for multiple testing prescribed by the group sequential design; Figure 3). The difference was more pronounced in chemotherapy-naive patients, with a median EFS of 19 months in the no further treatment arm versus 36 months in the prolonged treatment arm (P = .009; Table 2). When EFS was stratified by response to the induction phase (Table 2), the prolonged treatment more than doubled the median EFS (from 16 to 36 months; P = .004) in responding patients, while the effect was not significant in patients with SD at first restaging (from 8 to 11 months; P = .35).
EFS of all randomized patients according to study arm. The curves are parallel, suggesting that the gain acquired during the 9 months of prolonged treatment is maintained over time.
EFS of all randomized patients according to study arm. The curves are parallel, suggesting that the gain acquired during the 9 months of prolonged treatment is maintained over time.
In univariate analyses not taking treatment effect into account, baseline characteristics significantly prognostic for EFS included disease diameter, Ann Arbor stage, previous radiotherapy or chemotherapy, number of previous chemotherapy regimens, and T-helper cell count. Characteristics at randomization with a prognostic impact on EFS were response at week 12, disease size (> 5 cm), BM infiltration, hemoglobin level, and lymphocyte count.
The multivariate analysis to identify factors independently prognostic for EFS in randomized patients (Table 3) confirmed that the prolonged treatment arm highly significantly increased EFS (HR = 0.40; P < .0001). Other independent prognostic factors included parameters related to biologic characteristics of the lymphoma (tumors with B-cell lymphoma-2 [bcl-2] immunoreactivity in > 80% of the tumor cells responded longer), amount of circulating monocytes, patient's performance status, and response to induction treatment.
Molecular response
Induction phase. In 70 patients with a major breakpoint cluster region t(14;18) in PB and/or BM analyzed by real-time PCR (analysis performed in PB only, n = 22; BM only, n = 3; BM + PB, n = 45), the median number of t(14;18)-positive cells was 374 (range, 0-579 004) per 106 MNCs in PB and 42 (range, 0-707 843) per 106 MNCs in BM. Among the 70 initially t(14;18)-positive cases after induction with rituximab, 56 and 43 patients could be re-evaluated by PCR for t(14;18) in PB and BM, respectively. Of those, 59% of PB samples and 39% of BM samples had become t(14;18)-negative; real-time PCR could be performed in only a subset of cases, and showed that the number of circulating t(14;18)-positive cells was effectively reduced: 85% of 52 PB samples and 64% of 28 BM samples had a reduction of at least 1 log in t(14;18)-positive cell numbers, and 42% of PB and 46% of BM samples had a greater than 2 log reduction. The number of t(14;18)-positive cells in PB and BM at baseline was significantly predictive of clinical response to rituximab (Table 4). Among patients with initially positive PCR results in PB samples, clearance of circulating t(14;18)-positive cells was significantly associated with clinical response: 84% PCR negative in 32 patients with CR or PR versus 11% PCR negative in 19 patients with SD or progressive disease (PD; P < .0001).
Postrandomization. Of the 70 patients with an initial PCR-detectable t(14:18) translocation, 59 were randomized. In the prolonged treatment arm, 5 of 12 PCR-positive patients became PCR negative, whereas none of 10 PCR-negative patients became PCR positive. In contrast, in the no further treatment arm, 1 of 3 PCR-positive patients became PCR negative (8 PCR-positive patients progressed so rapidly that no follow-up samples could be obtained) and 7 of 15 PCR-negative patients became PCR positive. PCR positivity was highly significantly correlated with disease progression. With both arms pooled together (due to the small number of observations), 11 of 16 patients remaining or becoming PCR positive had PD, whereas only 1 of 23 patients remaining or becoming PCR negative had PD (P < .0001). Therefore, patients who remained or became PCR positive after induction therapy with rituximab had a higher chance of disease progression in both arms.
Toxicity
All 202 patients were evaluable for toxicity. The incidence of nonhematologic and hematologic grade 3 and 4 toxicity is summarized in Table 5. In the induction phase, the majority of nonhematologic toxicities were mild infusion-related symptoms during the first infusion. Sixteen cases of serious adverse events were documented: 2 of them were life-threatening events, 1 patient died due to a cardiac event (he had no history of cardiac disease; autopsy revealed subacute myocarditis) and 1 developed acute myeloblastic leukemia. Two further patients had severe infusion-related symptoms resulting in permanent treatment discontinuation. Serious adverse events during prolonged treatment and follow-up period were reported in 4 cases in arm A and 9 cases in arm B. These included 2 malignancies, 1 infection, and 1 neuropathy in arm A, and 2 malignancies, 2 infections, 2 complications of surgery, 1 pneumopathy, and 1 agranulocytosis in arm B. The mean values of cardiac EF assessed in 83 patients at week 12 and in 51 patients at week 52 (20 in arm A and 31 in arm B) were the same as the baseline value (n = 107).
A total of 137 patients were evaluable for late toxicity beyond 1 year. The incidence was 7% in both treatment arms and included 5 cases of infection, 1 of teeth loss, 1 of asymptomatic neutropenia, 2 of leukocytopenia, and 1 of cachexia.
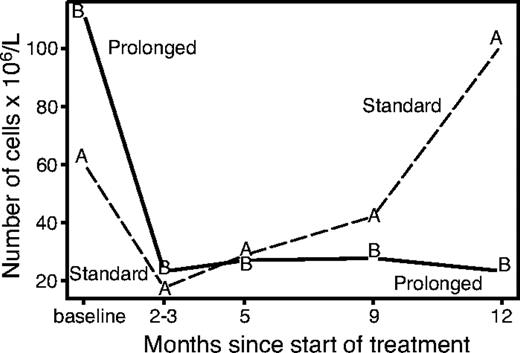
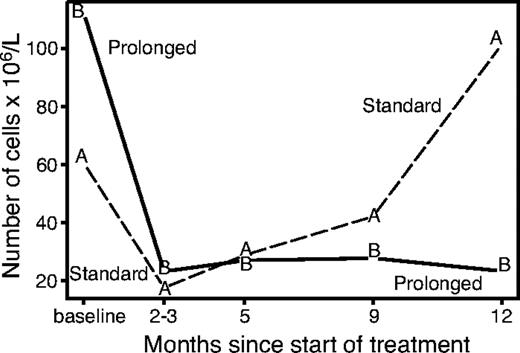
The lymphocytes subset analysis (excluding patients with baseline white blood cell count > 10 × 109/L who were suspected of having leukemic disease) showed a very early disappearance of circulating B cells, while T-helper, T-suppressor, and NK cells remained approximately stable during the induction phase. The evolution of circulating B lymphocytes for randomized patients over the first year of follow-up is shown in Figure 4. Median B-cell levels decreased rapidly in both study arms during the induction phase. At 1 year, the level rose above the baseline value in arm A while remaining depressed in arm B (P = .002). At further follow-up (despite the small number of available data) there appears to be a recovery of B cells in both arms (data not shown). The decrease in B cells was accompanied in arm B by a reduction of the IgM levels at 86% and 72% of baseline level at 3 and 12 months, respectively (P < .05).
Evolution of B lymphocytes according to study arm. The recovery of B cells starts 2 to 3 months after treatment and continues gradually with no further treatment, while the level of B cells remains suppressed with prolonged treatment.
Evolution of B lymphocytes according to study arm. The recovery of B cells starts 2 to 3 months after treatment and continues gradually with no further treatment, while the level of B cells remains suppressed with prolonged treatment.
Discussion
This trial shows that prolonged treatment with rituximab, compared with the standard rituximab schedule, improves the outcome of patients with follicular lymphoma in terms of both EFS and response duration, without causing additional toxicity. The standard rituximab schedule achieves a response rate of 50% lasting approximately 1 year, while the prolonged schedule nearly doubled the median EFS and remission duration attained with rituximab reaching the order of magnitude seen with chemotherapy or chemoimmunotherapy.7,18 It is noteworthy that patients in both arms continued to convert from partial to complete response even many months after the end of treatment, resulting in similar partial and complete response rates in both arms.
Other researchers have investigated the use of prolonged schedules or increased doses of rituximab including increasing the single dose or the number of infusions per week, increasing the number of consecutive weekly infusions up to 8, or repeating the weekly × 4 schedule every 6 months up to 2 years.19-22 Results from these trials suggest that increasing exposure to rituximab can improve efficacy. The trials with higher doses or more frequent administration were performed in chronic lymphocytic leukemia, demonstrating a RR that was higher than expected and suggesting that in this disease a dose-response or dose intensity-response correlation is likely. The weekly × 8 dosage was investigated in previously untreated low-grade lymphomas and showed an increased CR compared with the standard schedule, although this was not a randomized population.21 In that trial patients were highly selected with favorable characteristics and were not randomized against the standard schedule. Still, the weekly × 8 schedule did not seem to increase the overall RR or response duration compared to other series of previously untreated patients.
Hainsworth and colleagues22 investigated an extended dosing approach by administering the standard schedule every 6 months in previously untreated patients. The trial showed an increase of the remission rate over time that suggested, as in the present trial, that rituximab-sensitive follicular lymphomas remain sensitive and can be maintained in remission by continuous exposure to the drug. The clinical effect of this schedule is similar to that in our experimental arm (EFS in chemotherapy-naive patients of 34 and 36 months, respectively), although the amount of drug used in the 2 trials was significantly different (20 infusions vs 8 infusions). Whether a continuous rituximab challenge at low dose, as in the present trial, is more cost-effective than an intensive rechallenge every 6 months needs to be further investigated. The fact that 8 doses of rituximab given over 8 weeks are less effective on EFS than 8 doses given over 9 months suggests that the increased biological effect of our schedule is due more to the time of exposure to the drug than to peak serum levels. Another strategy that postpones the need for chemotherapy is to re-treat responders with the standard rituximab schedule at the time of their relapse. In a study by Davis and coworkers,23 it was observed that 40% of responders to the first exposure responded again at rechallenge, and the duration of the second response was longer than the first. The efficacy and cost-effectiveness of this strategy compared with those of a prolonged treatment schedule also need to be investigated in further studies. Preliminary data from a randomized trial addressing this question suggest that the time to chemotherapy is similar if patients receive rituximab for a prolonged time or at relapse only.24
Rituximab monotherapy is well tolerated. If compared with the toxicity of other chemotherapy schemes commonly used in follicular lymphoma, such as fludarabine or fludarabine combinations, CVP (cyclophosphamide, vincristine, and prednisone), or CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), rituximab treatment, even when prolonged, appears to cause fewer adverse events.25-28 The major benefit from the experimental arm of this trial was achieved in previously untreated patients. Although the subgroup of treatment-naive patients with follicular lymphoma who receive prolonged therapy is small (n = 30) and retrospectively defined, and the trial was not powered to study the effect of prolonged treatment in untreated patients, it is interesting to note that in this group the median EFS and response duration are doubled with the prolonged schedule. The results are comparable to the most aggressive regimens used for follicular lymphoma such as ProMACE-MOPP (prednisone, high-dose methotrexate, adriamycin, cyclophosphamide, and VP-16-mechlorethamine-vincristineprocarbazine-prednisone), ChVP (cyclophosphamide, low-dose doxorubicin, teniposide, and prednisone), CVP, or fludarabine.18,26-28 If it is confirmed that first-line therapy with 8 infusions of rituximab can achieve a 36-month remission accompanied by little toxicity, it is likely that many patients would prefer this approach compared with the adverse effects of aggressive polychemotherapy, especially considering that a good proportion of responding patients can respond again at relapse.23
In order to optimize the cost-benefit ratio for prolonged treatment with rituximab we tried to identify those patients who are more likely to benefit from such treatment. As expected, the parameters reflecting tumor burden or number of previous treatments are important for both response duration and EFS. Using quantitative PCR as a surrogate marker for tumor burden, we were able to show for the first time that low numbers of circulating lymphoma cells (ie, < 374 t(14;18)-positive cells/106 blood cells or < 42 t(14;18)-positive cells/106 BM cells at baseline) are highly predictive for clinical response to rituximab. The prognostic value of other parameters reflecting the immune status of the patient or biologic characteristics of the tumor is also an important new observation, although the interpretation of these findings is not straightforward. Lymphomas with bcl-2 immunoreactivity in greater than 80% of the tumor cells had a higher probability of responding. The prognostic impact of overexpression of the antiapoptotic bcl-2 protein is unclear, although, in the setting of rituximab treatment, it has been observed that in diffuse large B-cell lymphoma the overexpression of bcl-2 renders tumor cells more sensitive to chemotherapy.29,30
The results of this trial are encouraging and highly significant and support the potential for the prolonged schedule to become a treatment option for patients who have demonstrated sensitivity to rituximab. Continuing treatment for 9 months was an empirical decision, and further extension of regular administration of the drug (ie, until relapse) might substantially ameliorate remission duration. Considering the persistent depletion of B cells and the progressive reduction of serum IgM levels in the experimental arm, we would at present discourage such an approach outside a clinical trial setting. The follow-up trial to be conducted by our group aims to confirm the results of the present trial and explore the possibility of further prolongation of the treatment by comparing the 9-month schedule with a schedule of 2-monthly rituximab therapy administered until the time of disease progression.
Appendix
SAKK is a member of the Swiss Institute for Applied Cancer Research (SIAK).TBL6
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-10-3411.
Supported in part by research funding from Roche Pharma Schweiz AG to the Swiss Group for Clinical Cancer Research (SAKK).
A complete list of the members of SAKK appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Patricia Katz and Corinne Friedly for central data managing, Dina Delle Pezze for the FACS analysis of lymphocyte subsets, and Dr Doris Schmitter and Ursula Bolliger for assistance in molecular biology analysis.