We report a patient, a product of a consanguineous union, with liver hemosiderosis and severe congenital hypochromic microcytic anemia due to defective erythroid iron use. Iron is an essential element indispensable for the synthesis and the function of hemoglobin (Hb) and for normal red blood cell development. Iron deficiency can lead to decreased production of Hb and to a microcytic, hypochromic anemia. Erythroid iron uptake occurs via a receptor-mediated endocytosis of diferric transferrin (Tf) bound to the Tf receptor (TfR). Following acidification of internalized endosomes, iron is released from Tf and transported through the endosomal membrane by the transmembrane iron transporter divalent metal transporter 1 (DMT1), also known as natural resistance-associated macrophage protein-2 (Nramp2).1,2 Subsequent intracellular iron transport from DMT1 to mitochondria for heme synthesis is not completely understood.
Recently, it has been shown that the microcytic anemia (mk) mouse1 and the Belgrade rat2 carry the same missense mutation (G185R) in DMT1. Homozygous animals demonstrate diminished intestinal iron absorption and a severe deficit in erythroid iron use. The reduced rate of heme synthesis in reticulocytes from Belgrade rats can be corrected by the iron chelate salicylaldehyde isonicotinoyl hydrazone saturated with iron (Fe-SIH),3 which is known to bypass the TfR/DMT1 cycle of iron transport.4 These results indicate that DMT1 plays a crucial role in erythroid iron uptake, since the most prominent consequence of impaired DMT1 function is disturbed erythropoiesis. Even though the residual activity of this transporter in affected rodents allows the animals to remain viable, it is not sufficient to ensure appropriate rate of iron supply and subsequent heme synthesis in the erythroid cells.
The Czech proband is a 20-year-old female who is suffering from a severe congenital hypochromic microcytic anemia with high serum iron, normal total iron-binding capacity (TIBC), slightly elevated ferritin, and highly increased serum TfR (sTfR) values (Table 1). She is a child of a consanguineous union of second-degree relatives. Her Hb varied around 70 g/L, and she required the first blood transfusion after birth and 8 transfusions during infancy. Later she received transfusions only when her Hb levels dropped to less than 70 g/L; however, these averaged less than one per year (hence, the patient received < 4 g of iron, via transfusions, in her lifetime). She has had erythroid hyperplasia with defective hemoglobinization of intermediate and late normoblasts and absence of sideroblasts in the bone marrow. By the age of 19 years, she had gradually developed liver hemosiderosis with the liver iron content of 16 286 μg/g dry weight.
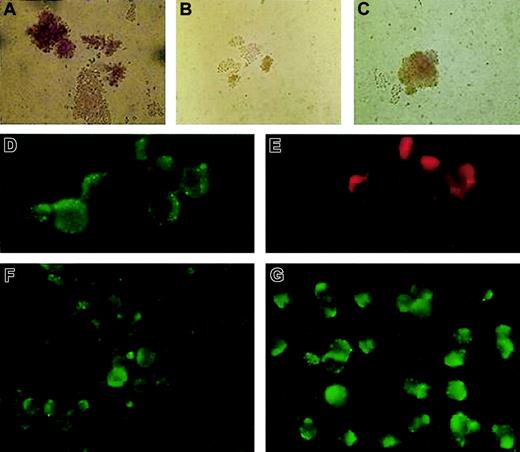
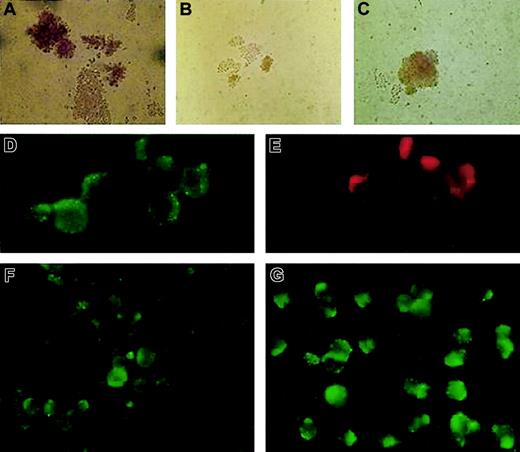
We analyzed isolated erythroid progenitors from peripheral blood and bone marrow of the patient using an in vitro colonyforming assay.5 The ethics committee of the Palacky University Hospital approved the study and the informed consent. In brief, we plated 3 × 105 light-density mononuclear cells to the methylcellulose media (H4531; StemCell Technologies, Vancouver, BC) with addition of 1 U or 2 U erythropoietin (Epo) per milliliter of media. The numbers and the size of the patient's erythroid colonies were smaller compared with the healthy controls and exhibited low cell content and abnormal morphology (Figure 1A-B). Based on the clinical observations and biochemical measurements, we postulated that addition of iron chelates to the cultures might correct the poor growth of the patient's erythroid colonies. Fe-SIH was added to the Epo-containing cultures at the final concentration of 10 μM. Indeed, addition of the iron chelate to the cultures rescued the numbers, cellularity, and defective hemoglobinization of the patient's erythroid burst-forming units (BFU-Es; Figure 1C). This result is congruent with impaired TfR/DMT1-dependent iron trafficking in erythroid precursors in this patient.
BFU-E growth in vitro and immunofluorescence staining of fixed and permeabilized patient's erythroblasts compared with healthy control. Healthy control (A) and the patient's (B) BFU-Es were grown in the presence of Epo, without Fe-SIH; the addition of 10 μM Fe-SIH rescued the growth of the patient's BFU-E (C). DMT1 (D) and TfR (E) were colocalized in EEA1–positive endosomes in the patient's erythroblasts. The expression of DMT1 in the patient's erythroblasts (F) was decreased compared with healthy control (G). Original magnifications: × 200 for panels A-C, × 1000 for panels D-G.
BFU-E growth in vitro and immunofluorescence staining of fixed and permeabilized patient's erythroblasts compared with healthy control. Healthy control (A) and the patient's (B) BFU-Es were grown in the presence of Epo, without Fe-SIH; the addition of 10 μM Fe-SIH rescued the growth of the patient's BFU-E (C). DMT1 (D) and TfR (E) were colocalized in EEA1–positive endosomes in the patient's erythroblasts. The expression of DMT1 in the patient's erythroblasts (F) was decreased compared with healthy control (G). Original magnifications: × 200 for panels A-C, × 1000 for panels D-G.
To further elucidate this defect at the protein level, we harvested erythroid colonies using cytospin, followed by immunofluorescence analysis. In addition, we isolated the patient's and 3 healthy volunteers' erythroid progenitors from a mononuclear fraction of bone marrow cells using labeling with glycophorin A antibody and magnetic cell sorting (Miltenyi Biotec, Bergisch Gladbach, Germany). Subsequent immunofluorescence analyses were performed with TfR antibody (CD71), DMT1/Nramp2 antibody (N-20; both from Santa Cruz Biotechnology, Santa Cruz, CA), and early endosomal antigen 1 (EEA1; Alexis, Montreal, QC, Canada) antibody. TfR expression in the patient's erythroblasts was normal (Figure 1E) and comparable with that seen in healthy controls (not shown). We thus hypothesized that the defect in this patient was most likely due to defective expression of DMT1. This was further supported by immunofluorescence analysis of glycophorin-positive erythroid cells. As shown in Figure 1F-G, the patient's erythroblasts showed decreased DMT1 expression, with the protein being localized within the cytoplasmic vesicles and on the surface of the blasts.
We conclude that the propositus was born with a homozygosity for an autosomal recessive defect of iron transport/use in erythroid cells. We hypothesize that this defect is caused by a malfunction of the endosomal iron transporter DMT1. In mk mice and Belgrade rats such a defect leads to a decrease in intestinal iron absorption. In contrast, the patient reported here has signs of iron overload that we explain by increased absorption of heme-derived iron in response to anemia. mk mice or Belgrade rats are unlikely to develop such a compensatory mechanism, since rodents have a low ability to use heme iron compared with inorganic iron6 ; moreover, neither laboratory mice nor laboratory rats have heme in their diets. The absorption of heme iron is poorly understood, but it likely involves heme binding to specific, yet-uncharacterized high-affinity binding sites in the mucosal brush border.7,8 It is plausible that a pathway involved in heme iron absorption is up-regulated in our patient.
Supported by the Czech Republic Ministry of Health grant NM/6739-3 and, in part, by The Canadian Institutes for Health Research.