Abstract
Inappropriately low reticulocytosis may exacerbate malarial anemia, but the under-lying mechanism is not clear. In this study, naive and infected mice were treated with recombinant murine erythropoietin (EPO), and the upstream events of erythropoiesis affected by blood-stage Plasmodium chabaudi AS were investigated. Malaria infection, with or without EPO treatment, led to a suboptimal increase in TER119+ erythroblasts compared with EPO-treated naive mice. Furthermore, a lower percentage of TER119+ erythroblasts in infected mice were undergoing terminal differentiation to become mature hemoglobin-producing erythroblasts. The impaired maturation of erythroblasts during infection was associated with a shift in the transferrin receptor (CD71) expression from the TER119+ population to B220+ population. Moreover, the suboptimal increase in TER119+ erythroblasts during infection coincided with a blunted proliferative response by splenocytes to EPO stimulation in vitro, although a high frequency of these splenocytes expressed EPO receptor (EPOR). Taken together, these data suggest that during malaria, EPO-induced proliferation of early EPOR-positive erythroid progenitors is suppressed, which may lead to a suboptimal generation of TER119+ erythroblasts. The shift in CD71 expression may result in impaired terminal maturation of these erythroblasts. Thus, inadequate reticulocytosis during malaria is associated with suppressed proliferation, differentiation, and maturation of erythroid precursors.
Introduction
Malaria is an important etiologic factor for severe anemia among children and pregnant women in malaria-endemic areas, especially in sub-Saharan Africa.1-3 The mortality rate of malaria-related anemia is between 5.6% and 16% for children4-6 and 6% for pregnant women, especially in primigravidae.7,8 The cause of severe malarial anemia is considered to be multifactorial. It has been proposed that increased destruction of red blood cells (RBCs), including the rupture of infected RBCs by schizonts and increased clearance of infected and uninfected RBCs due to hemolysis and/or phagocytosis,9-11 is a major contributing factor.12 In addition, insufficient erythropoiesis, as evidenced by inappropriately low numbers of reticulocytes in the peripheral blood of some malaria patients with anemia,13,14 may fail to alleviate malarial anemia and aggravate the severity of the disease.
When anemia occurs, tissue hypoxia results in increased renal erythropoietin (EPO) production.15 Subsequently, EPO travels systemically to hematopoietic tissues, including bone marrow and spleen, to stimulate erythropoiesis via binding to the EPO receptor (EPOR), which is expressed on erythroid progenitors, especially the erythroid colony-forming unit (CFU-E) and proerythroblasts.16-18 As a result, an increased number of reticulocytes is released into the bloodstream to alleviate anemia. Therefore, inappropriately low reticulocytosis during malaria infection may be the result of inadequate EPO production and/or a suboptimal response of erythroid progenitors to EPO stimulation.
Studies in malaria-infected human adults indicate that EPO production is inadequate during malaria.19,20 In contrast, studies in infected children and in experimental animal models suggest that EPO production is robust and correlates inversely with the degree of anemia.14,21-26 Furthermore, a previous study from our laboratory demonstrated that blood-stage Plasmodium chabaudi AS infection in mice suppresses reticulocytosis in response to exogenous EPO administration.27 The degree of suppression was found to be proportional to the level of parasitemia. Hence, we postulated that a suboptimal response of erythroid progenitors to EPO stimulation, rather than inadequate EPO production, may be the underlying mechanism of insufficient erythropoiesis during blood-stage malaria. Here, we characterized the alterations in splenic erythroid compartments in naive mice and P chabaudi AS–infected mice following exogenous EPO treatment. We demonstrated that the development of late erythroid precursors is severely suppressed during blood-stage malaria, which may ultimately lead to the decreased production of reticulocytes observed in malaria-infected experimental animals and patients.
Materials and methods
Parasite and experimental infection
P chabaudi AS was maintained, as previously described, by weekly passage of parasitized RBCs (PRBCs).22 Male A/J mice, 8 to 10 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were infected intraperitoneally with 106 PRBCs. Blood smears were prepared by collecting tail-vein blood daily starting on day 4 after infection. RBC densities were scored by diluting 4 μL whole blood in 3 mL phosphate-buffered saline (PBS) and counted using a hematocytometer. For determination of the percentages of parasitemia, slides were stained with Diff-Quik Solution (Dade Behring, Düdingen, Switzerland). Parasitemia was determined by counting at least 400 cells. Because reticulocyte counts obtained from Diff-Quik–stained blood smears were similar to those obtained from New Methylene Blue staining, Diff-Quik staining was employed for scoring reticulocytes.28 All animal care and handling was carried out in accordance with institutional guidelines of the McGill University Health Centre Research Institute and McGill University.
In vivo EPO administration and splenocyte preparation
Recombinant murine EPO (rmEPO) (Boehringer Mannheim–Roche Diagnostics, Laval, QC, Canada) was prepared at a concentration of 50 U/mL in sterile Hanks balanced salt solution (HBSS) (Gibco Laboratories, Invitrogen, Burlington, ON, Canada) supplemented with 0.1% bovine serum albumin (BSA) (Gibco Laboratories, Invitrogen). When parasitemia reached 10% to 15%, typically on day 5 or 6 after infection, infected mice were injected intravenously with 200 μL equivalent to 10 units of rmEPO daily for 3 days. This treatment regimen was devised based on our previous observation that reticulocyte response to exogenous EPO stimulation is most severely suppressed in P chabaudi AS–infected mice when the parasitemia is high.27 Naive mice were also treated with the same amount of rmEPO for 3 days. Naive and infected mice without treatment or treated with 0.1% BSA solution for 3 days were used as controls. Data collected from mice treated with 0.1% BSA solution were not significantly different from nontreated controls. Therefore, only data from nontreated mice are reported here.
One day following the third injection, EPO-treated and control mice were humanely killed and spleens were aseptically removed. Spleen weights and body weights were recorded, and spleen index was calculated by dividing spleen weight by body weight and multiplying by 100%. Spleens were teased into small pieces prior to being gently pressed through sterile fine wire meshes. RBCs were lysed using buffered ammonium chloride and washed twice with PBS. Cell clumps were removed by passing through a 70 μm cell strainer (Falcon, Franklin Lakes, NJ). Single cell suspensions were prepared in various media and at the density described in the following sections of “Materials and methods” for each assay.
FACS analyses
For fluorescence-activated cell sorting (FACS), cells were resuspended in PBS supplemented with 1% FCS (HyClone, Logan, UT) and 0.05% sodium azide (Fisher Scientific, Nepean, ON, Canada) at a density of 20 × 106/mL. Cells were treated with Fc-Block (BD BioSciences, Mississauga, ON) to reduce nonspecific Fc-mediated adherence of antibodies to Fc receptor prior to staining. TER119+ erythroblasts were detected with phycoerythrin (PE)–conjugated or fluorescein isothiocyanate (FITC)–conjugated anti-TER119 antibody. Transferrin receptor (CD71) expression was detected using FITC-conjugated anti-CD71 antibody. EPOR-expressing erythroid progenitors were identified by staining the cells with rabbit antihuman EPOR antibody (Santa Cruz Biotechnology, Santa Cruz, CA) against the N-terminus of EPOR followed by staining with FITC-conjugated antirabbit immunoglobulin G (IgG) antibody (Sigma Chemical, St Louis, MO). B cells, T cells, granulocytes, and macrophages were identified by staining with peridinin chlorophyll protein (PerCP)–conjugated anti-B220, anti-CD3, anti–Gr-1, and anti–Mac-1 antibodies, respectively. Dead cells were excluded by propidium iodide (PI) staining (Sigma Chemical). Apoptosis of total splenocytes and enriched TER119+ erythroblasts was detected using the In Situ Cell Death Detection Kit–FITC (TUNEL [terminal deoxynucleotide transferase dUTP nick end labeling] assay) from Boehringer Mannheim–Roche Diagnostics as per the manufacturer's instructions. All fluorochrome-conjugated antibodies were purchased from BD BioSciences unless otherwise stated. For the cell-cycle distribution studies, cells were first stained with FITC-conjugated anti-TER119 antibody (eBioscience, San Diego, CA), washed, and fixed in 70% ethanol on ice for 2 hours, and then DNA content was determined by staining the cells with 40 μg/mL PI and 100 μg/mL RNase (Sigma Chemical) in PBS at 37° C for 30 minutes. Total splenocytes or TER119+ cells were gated, and cell-cycle distribution was determined with Modfit LT software (version 3.0, diploid option, Verity Software House, Topsham, ME). All FACS analyses were carried out using a FACScan equipped with CellQuest software (BD Biosciences).
Hemoglobin (Hb) staining
Hemoglobin (Hb)–producing erythroblasts were identified according to the method of Zhang et al.29 Briefly, cells were washed and 200 μL aliquots of cells at a density of 4 × 106/mL were plated in 48-well culture plates and stained with 20 μL freshly prepared staining solution consisting of 1 part benzidine (Sigma Chemical) at 1.5% in 90% glacial acetic acid (Sigma Chemical) plus 6 parts 5% H2O2. At least 1000 cells were counted for each sample.
TER119+ splenocyte enrichment
TER119+ erythroblasts were enriched by magnetic cell sorting system with antimouse TER119 MicroBeads purchased from Miltenyi Biotec (Auburn, CA). Nonspecific binding was blocked by incubating cells with normal mouse serum prior to the addition of microbeads. Enrichment was performed following the manufacturer's instructions. The purity of the enriched cells ranged from 75% to 95% based on FACS analysis.
Proliferation assay
The in vitro EPO-induced proliferation assay was modified from Zhang et al.29 Briefly, cells were resuspended at 2 × 106/mL in Opti-MEM I medium (Gibco Laboratories, Invitrogen) supplemented with 10% fetal calf serum (FCS) and 48 mg/L gentamicin (Schering Canada, Pointe-Claire, QC). Aliquots of 100 μL cell suspension were plated into 96-well plates with rmEPO ranging from 0 to 2 U/mL. After 22 hours of culture, 1 μCi (37 KBq) 3H-thymidine (specific activity, 6.7 Ci/mmol [248 GBq/mmol]; ICN Biomedicals, Irvine, CA) was added to each well and incubated for 2 hours. Cells were harvested with an automatic cell harvester, and 3H-thymidine incorporation was determined by scintillation counting.
Statistical analyses
Data are expressed as means ± standard error of the mean (SEM). The effects of infection on the response to exogenous EPO treatment were analyzed by analysis of variance (ANOVA) followed by Tukey studentization analysis. P values less than .05 were considered to be significant. Statistical analyses were performed using SAS-STAT software (SAS Institute, Cary, NC).
Results
Suppressed EPO-induced reticulocytosis during P chabaudi AS infection
Following infection, parasitemia became patent by day 4 after infection. Naive mice and infected mice were treated with 10 units of rmEPO per day on days 6 to 8 after infection as described in “Materials and methods.” Untreated naive mice and infected mice served as controls. Hematologic parameters were determined 1 day following the completion of treatment. EPO treatment did not significantly alter parasitemia in infected mice (Figure 1A). EPO-treated infected mice had similar levels of parasitemia compared with untreated infected controls. Naive mice, with or without EPO treatment, had comparable numbers of RBCs (Figure 1B). Infection led to the development of severe anemia, and EPO treatment did not change the RBC counts significantly. Interestingly, EPO treatment in naive mice resulted in significantly and markedly increased reticulocytosis (Figure 1C). In contrast, EPO treatment in infected mice did not result in a significant increase in reticulocytosis as compared with untreated infected controls. EPO-treated infected mice had approximately 91% fewer reticulocytes compared with EPO-treated naive mice. These data demonstrated that blood-stage malaria leads to a profoundly decreased reticulocyte response to exogenous EPO stimulation.
Hematologic parameters of naive or P chabaudi AS–infected mice, either untreated or treated with 10 units of recombinant murine EPO (rmEPO) per day for 3 days. Parasitemia and hematologic parameters were determined after the completion of EPO treatment. (A) Parasitemia; (B) total RBCs; (C) total reticulocytes. Data are means ± SEM for 5 mice per group. N indicates untreated naive controls; N+EPO, naive mice treated with EPO; I, untreated infected controls; I+EPO, infected mice treated with EPO. Lowercase letters above each bar represent the result of statistical comparison. When 2 bars are labeled with different letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results were obtained in 4 independent experiments. N/A indicates not applicable.
Hematologic parameters of naive or P chabaudi AS–infected mice, either untreated or treated with 10 units of recombinant murine EPO (rmEPO) per day for 3 days. Parasitemia and hematologic parameters were determined after the completion of EPO treatment. (A) Parasitemia; (B) total RBCs; (C) total reticulocytes. Data are means ± SEM for 5 mice per group. N indicates untreated naive controls; N+EPO, naive mice treated with EPO; I, untreated infected controls; I+EPO, infected mice treated with EPO. Lowercase letters above each bar represent the result of statistical comparison. When 2 bars are labeled with different letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results were obtained in 4 independent experiments. N/A indicates not applicable.
Suboptimal increase in late erythroid precursors during malaria
To investigate the mechanism underlying the decreased reticulocyte response to exogenous EPO stimulation during malaria, changes in the spleen indexes and cellularities among naive and infected mice after EPO treatment were examined. In naive mice, EPO treatment caused notable splenomegaly (Table 1). In contrast, spleens from infected mice were found to be significantly enlarged compared with naive mice, and EPO treatment did not significantly increase the spleen index as observed in naive mice. Changes in the spleen indexes were reflected in spleen cellularities. EPO treatment in naive mice increased the number of nucleated splenocytes considerably (Table 1). Compared with untreated naive mice, blood-stage malaria infection led to a significant increase in spleen cellularity, which was not significantly modulated by EPO treatment in infected mice.
Next, changes in the percentages and numbers of splenic TER119+ splenocytes among naive and infected mice after EPO treatment were investigated by FACS analysis. TER119 has been identified as an erythroid lineage–specific marker in mice expressed on cells undergoing differentiation from the stage of proerythroblasts to mature RBCs but not by early erythroid progenitors, such as erythroid burst-forming units (BFU-Es) and CFU-Es.30 Naive mice, without EPO treatment, had a low frequency of splenocytes expressing TER119 (Figure 2A). An approximately 11-fold increase in the percentage of TER119+ splenocytes was identified in naive mice after EPO treatment (Figure 2B). Compared with naive mice, infection resulted in a more than 3-fold increase in the percentage of splenocytes expressing TER119 (Figure 2C), and EPO treatment did not alter the percentage significantly (Figure 2D). Furthermore, EPO-treated infected mice had considerably fewer percentages of erythroblasts compared with the high frequency of erythroblasts identified in EPO-treated naive mice.
FACS analyses and hemoglobin (Hb) staining of splenocytes from naive or P chabaudi AS–infected mice, either untreated or treated with 10 units of rmEPO per day for 3 days. The erythroblast population was identified with PE-conjugated anti-TER119 antibody (A-D). Region M1 represents gating for TER119+ cells. Terminally differentiated mature erythroblasts were identified with benzidine staining for the presence of Hb as described in “Materials and methods” (E-H). Data presented are naive mice (A,E), naive mice treated with EPO (B,F), infected mice (C,G), and infected mice treated with EPO (D,H). Values represent percentages of TER119+ (A-D) or Hb-positive cells (E-H). Data are expressed as means ± SEM for 3 to 5 mice per group. When 2 sets of data are labeled with superscripts of different lowercase letters, it indicates that these 2 sets of data are statisitcally different (P < .05). Similar results were obtained in 3 independent experiments. Photos were taken at × 200 magnification.
FACS analyses and hemoglobin (Hb) staining of splenocytes from naive or P chabaudi AS–infected mice, either untreated or treated with 10 units of rmEPO per day for 3 days. The erythroblast population was identified with PE-conjugated anti-TER119 antibody (A-D). Region M1 represents gating for TER119+ cells. Terminally differentiated mature erythroblasts were identified with benzidine staining for the presence of Hb as described in “Materials and methods” (E-H). Data presented are naive mice (A,E), naive mice treated with EPO (B,F), infected mice (C,G), and infected mice treated with EPO (D,H). Values represent percentages of TER119+ (A-D) or Hb-positive cells (E-H). Data are expressed as means ± SEM for 3 to 5 mice per group. When 2 sets of data are labeled with superscripts of different lowercase letters, it indicates that these 2 sets of data are statisitcally different (P < .05). Similar results were obtained in 3 independent experiments. Photos were taken at × 200 magnification.
The effect of EPO treatment in increasing the erythroblast population in naive mice was even more notable when changes in spleen cellularities were taken into consideration. EPO-treated naive mice had a 70-fold increase in the number of TER119+ erythroblasts per spleen as compared with untreated naive controls (Table 1). Due to marked splenomegaly, infection led to an approximately 30-fold increase in the number of erythroblasts per spleen as compared with untreated naive controls, but EPO treatment during infection did not change the number of erythroblasts significantly. Importantly, EPO-treated infected mice had approximately 70% fewer total number of erythroblasts per spleen compared with with EPO-treated naive mice.
Next, terminally differentiated, mature erythroblasts were identified by benzidine staining for Hb (Figure 2E-H). Infection led to a more than 20-fold increase in the number of mature Hb-positive erythroblasts compared with untreated naive controls (Table 1). EPO-treated infected mice had similar numbers of Hb-positive erythroblasts per spleen compared with untreated infected controls. However, EPO treatment in naive mice resulted in a more than 160-fold increase in the number of splenic mature Hb-positive erythroblasts compared with untreated naive controls. Consequently, EPO-treated infected mice had 85% fewer mature Hb-positive erythroblasts per spleen compared with EPO-treated naive mice. By dividing the number of Hb-positive erythroblasts by the number of TER119+ erythroblasts, we determined that almost 75% of TER119+ erythroblasts in EPO-treated naive mice had undergone terminal differentiation to become mature Hb-producing erythroblasts (Table 1). On the other hand, only 29% to 30% of TER119+ erythroblasts in infected mice, with or without EPO treatment, were mature Hb-producing erythroblasts. These data suggest that erythroblasts from EPO-treated naive mice are more mature compared with erythroblasts from infected mice, with or without EPO treatment.
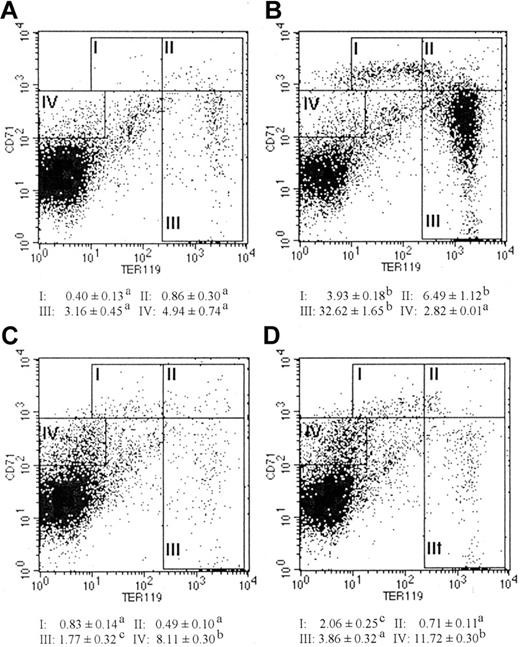
Shift in CD71 expression during malaria
It is well established that the expression of transferrin receptor (CD71) on erythroid lineage cells is required for iron uptake and Hb synthesis.31,32 Analysis of the expression intensities of CD71 and TER119 provides the opportunity to distinguish murine erythroblasts of different maturation stages.33 To further identify the specific stage in erythroblast maturation in which a defect occurs during malaria infection, splenocytes were immunostained with PE-conjugated TER119 and FITC-conjugated CD71 (Figure 3; Table 2). Region I (CD71highTER119low) represents the proerythroblast population or most immature erythroblasts. Region II (CD71highTER119high) represents the basophilic erythroblast population. Region III (CD71low-medTER119high) represents the polychromatic and orthochromatic erythroblast populations, which are Hb-producing cells. As shown in Figure 3, infected mice, with or without EPO treatment (Figure 3C-D), had significantly lower frequencies of erythroblasts in all 3 regions compared with EPO-treated naive mice (Figure 3B). When spleen cellularities were taken into consideration, infected mice, with or without EPO treatment, had approximately 1 × 106 to 5 × 106 fewer proerythroblasts (region I), approximately 9 × 106 to 10 × 106 fewer basophilic erythroblasts (region II), and approximately 46 × 106 to 53 × 106 fewer polychromatic/orthochromatic erythroblasts (region III) per spleen as compared with EPO-treated naive mice (Table 2). Consistent with the Hb staining results, these data suggest that the maturation of late-stage erythroid precursors is severely impaired during malaria infection.
FACS analyses of splenic erythroblasts at different maturation stages from naive or P chabaudi AS–infected mice, either untreated or treated with 10 units of rmEPO per day for 3 days. Erythroblasts at different maturation stages were identified by double staining with PE-conjugated anti-TER119 and FITC-conjugated anti-CD71 antibodies in naive mice (A), naive mice treated with EPO (B), infected mice (C), and infected mice treated with EPO (D). Region I: The CD71highTER119low population represents proerythroblasts, the least mature type of the erythroblasts. Region II: The CD71highTER119high population represents basophilic erythroblasts. Region III: The CD71low-medTER119high population represents polychromatic and orthochromatic erythroblasts, the mature Hb-producing cells. Region IV: CD71medTER119– represents the nonerythroblast population that expresses CD71. Values represent frequencies of splenocytes in each region. Data are means ± SEM of 3 to 5 mice per group. When 2 sets of data are labeled with superscripts of different lowercase letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results were obtained in 2 independent experiments.
FACS analyses of splenic erythroblasts at different maturation stages from naive or P chabaudi AS–infected mice, either untreated or treated with 10 units of rmEPO per day for 3 days. Erythroblasts at different maturation stages were identified by double staining with PE-conjugated anti-TER119 and FITC-conjugated anti-CD71 antibodies in naive mice (A), naive mice treated with EPO (B), infected mice (C), and infected mice treated with EPO (D). Region I: The CD71highTER119low population represents proerythroblasts, the least mature type of the erythroblasts. Region II: The CD71highTER119high population represents basophilic erythroblasts. Region III: The CD71low-medTER119high population represents polychromatic and orthochromatic erythroblasts, the mature Hb-producing cells. Region IV: CD71medTER119– represents the nonerythroblast population that expresses CD71. Values represent frequencies of splenocytes in each region. Data are means ± SEM of 3 to 5 mice per group. When 2 sets of data are labeled with superscripts of different lowercase letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results were obtained in 2 independent experiments.
Significant increases in the frequency and number of cells residing in region IV (CD71medTER119–) in infected mice, with or without EPO treatment, compared with naive mice, with or without EPO treatment, were also observed (Figure 3; Table 2). Further analysis revealed that most CD71+ cells in EPO-treated naive mice were TER119+ erythroblasts (71.74% ± 1.10%), whereas only 11.42% ± 1.87% and 16.68% ± 1.17% of CD71+ cells were TER119+ erythroblasts in infected mice, with or without EPO treatment, respectively (N+EPO versus I, P < .001; N+EPO versus I+EPO, P < .001). Analysis of the expression of leukocyte markers revealed that most CD71+ cells in untreated or treated infected mice were B220+ cells (68.08% ± 5.27% and 43.07% ± 2.17%, respectively) compared with the low frequency of B220+ cells in the CD71+ population in treated naive mice (17.28% ± 0.52%) (N+EPO versus I, P < .001; N+EPO versus I+EPO, P < .001). The shift in the frequencies of CD71+ cells from the TER119+ population to the TER119– population in infected mice compared with EPO-treated naive mice suggests that the primary cell population that uptakes iron in the hematopoietic tissue during infection may be shifted from the erythroblast to nonerythroblast population.
Apoptosis and cell-cycle distribution of erythroblasts in malaria
To determine whether the decreased production of late-stage erythroid precursors observed was a result of apoptosis in erythroblasts, we examined DNA breakage in enriched TER119+ erythroblasts using the TUNEL assay. Surprisingly, a significantly higher percentage of TER119+ erythroblasts from EPO-treated naive mice (Figure 4B) exhibited extensive DNA breakage compared with with infected mice, with or without EPO treatment (Figure 4C-D). Significantly lower frequencies of apoptotic erythroblasts observed in infected mice demonstrated that the suboptimal increase and impaired maturation of erythroblasts during malaria may not be the result of increased apoptosis.
Erythroblasts undergoing apoptosis. TER119+ erythroblasts were enriched by magnetic cell sorting system with antimouse TER119 MicroBeads purchased from Miltenyi Biotec. Apoptosis in erythroblasts from naive mice (A), naive mice treated with EPO (B), infected mice (C), and infected mice treated with EPO (D) was detected using the In Situ Cell Death Detection Kit–FITC (TUNEL assay) from Boehringer Mannheim–Roche Diagnostics. Numbers represent frequencies of TUNEL+ splenocytes. Data are means ± SEM of 3 mice per group except for panel A, where cells were pooled from 4 mice. When 2 sets of data are labeled with superscripts of different lowercase letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results were obtained in 2 independent experiments.
Erythroblasts undergoing apoptosis. TER119+ erythroblasts were enriched by magnetic cell sorting system with antimouse TER119 MicroBeads purchased from Miltenyi Biotec. Apoptosis in erythroblasts from naive mice (A), naive mice treated with EPO (B), infected mice (C), and infected mice treated with EPO (D) was detected using the In Situ Cell Death Detection Kit–FITC (TUNEL assay) from Boehringer Mannheim–Roche Diagnostics. Numbers represent frequencies of TUNEL+ splenocytes. Data are means ± SEM of 3 mice per group except for panel A, where cells were pooled from 4 mice. When 2 sets of data are labeled with superscripts of different lowercase letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results were obtained in 2 independent experiments.
In addition, the cell-cycle distribution of total splenocytes and TER119+ erythroblasts was analyzed. Without EPO treatment or infection, more than 90% of splenocytes from naive mice resided in G0/G1 phase and only about 2% were in S phase (Figure 5A). With EPO treatment, the percentage of splenocytes in S phase increased significantly from 2% to 17% in naive mice, which coincided with a significant decrease in the percentage of cells in G0/G1 phase from 90% to 71%. Compared with untreated naive mice, malaria infection also significantly decreased the percentage of splenocytes in G0/G1 phase and significantly increased the percentage of cells in S phase. EPO treatment in infected mice did not significantly change the percentages of splenocytes in G0/G1 phase or S phase. However, a slight yet significant decrease was observed in the percentage of splenocytes in G2 phase after EPO treatment in infected mice.
Cell-cycle analysis of total splenocytes and TER119+ erythroblasts from naive and infected mice with or without EPO treatment. For TER119+ erythroblasts, splenocytes were stained with FITC-conjugated anti-TER119 antibody prior to fixation and analysis. Cell-cycle distribution of total splenocytes (A) and TER119+ erythroblasts (B). Data are means for 4 to 5 mice per group. Standard errors were less than 10% for all means and are not shown. N indicates untreated naive controls; N+EPO, naive mice treated with EPO; I, untreated infected controls; I+EPO, infected mice treated with EPO. When 2 sets of data are labeled with superscripts of different letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results were obtained in 2 independent experiments.
Cell-cycle analysis of total splenocytes and TER119+ erythroblasts from naive and infected mice with or without EPO treatment. For TER119+ erythroblasts, splenocytes were stained with FITC-conjugated anti-TER119 antibody prior to fixation and analysis. Cell-cycle distribution of total splenocytes (A) and TER119+ erythroblasts (B). Data are means for 4 to 5 mice per group. Standard errors were less than 10% for all means and are not shown. N indicates untreated naive controls; N+EPO, naive mice treated with EPO; I, untreated infected controls; I+EPO, infected mice treated with EPO. When 2 sets of data are labeled with superscripts of different letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results were obtained in 2 independent experiments.
In contrast to the low percentage of cells in S phase among total splenocytes from untreated naive mice, 35% of TER119+ erythroblasts in untreated naive mice were in S phase (Figure 5B). Furthermore, EPO treatment significantly decreased the percentage of TER119+ erythroblasts in G0/G1 phase and, at the same time, significantly increased the percentage of TER119+ erythroblasts in S phase in naive mice. Malaria infection was shown to have similar effects on cell-cycle distribution among TER119+ splenocytes to EPO treatment. There was a significant shift of TER119+ erythroblasts from G0/G1 phase toward S phase in infected mice compared with untreated naive mice. However, there were no significant differences in the cell-cycle distribution of TER119+ erythroblasts between EPO-treated naive mice and EPO-treated infected mice. Taken together, these data suggest that malaria infection does not cause dysregulation of cell cycle in the TER119+ erythroblast compartment.
Alterations in early EPOR-positive erythroid progenitors during malaria infection
Hitherto, we demonstrated that there was a suboptimal increase in erythroblasts during anemia due to malaria, but this phenomenon could not be attributed to increased apoptosis or disrupted cell cycle of the TER119+ erythroblast population. Hence, we investigated changes occurring in the erythroid progenitors upstream of TER119+ erythroblasts. It is known that TER119 is expressed by erythroid lineage cells from proerythroblasts to mature RBCs but not by the less mature erythroid lineage cells, such as BFU-Es and CFU-Es.30 In contrast, expression of EPOR peaks on CFU-E and proerythroblast stages, although a lower level of EPOR expression can be detected on BFU-Es and erythroblasts.16-18
By FACS analysis, we observed that malaria infection resulted in a significantly increased percentage of early EPOR-positive erythroid progenitors as compared with untreated naive controls: 24.55% ± 0.99% for untreated naive controls; 28.95% ± 0.30% in naive mice treated with EPO; 36.33% ± 1.47% in untreated infected controls; and 37.46% ± 1.25% in infected mice treated with EPO. Although a significant increase in the frequency of EPOR-positive cells was demonstrated in EPO-treated naive mice compared with untreated naive controls, EPO treatment in infected mice did not significantly alter the percentage of EPOR-positive cells. However, EPO-treated infected mice had a significantly higher percentage of splenocytes expressing EPOR compared with EPO-treated naive mice. When spleen cellularities were considered, malaria infection, with or without EPO treatment, resulted in a significantly increased number of EPOR-positive cells per spleen by 12-fold compared with untreated naive controls (Figure 6A). EPO-treated naive mice had a significantly higher number of EPOR-positive cells per spleen as compared with untreated naive controls. However, the number of EPOR-positive cells in EPO-treated naive mice was not significantly different from those in EPO-treated infected mice.
Changes in early erythropoietin receptor–positive (EPOR+) erythroid progenitors determined by FACS analysis and in vitro EPO-stimulated proliferation assay. (A) Numbers of early EPOR-positive erythroid progenitors per spleen. (B) In vitro EPO-stimulated proliferation of unfractionated total splenocytes. Data are means ± SEM for 3 to 5 mice per group. N indicates untreated naive controls; N+EPO, naive mice treated with EPO; I, untreated infected controls; I+EPO, infected mice treated with EPO. When 2 sets of data are labeled with superscripts of different lowercase letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results are representative of 3 independent experiments.
Changes in early erythropoietin receptor–positive (EPOR+) erythroid progenitors determined by FACS analysis and in vitro EPO-stimulated proliferation assay. (A) Numbers of early EPOR-positive erythroid progenitors per spleen. (B) In vitro EPO-stimulated proliferation of unfractionated total splenocytes. Data are means ± SEM for 3 to 5 mice per group. N indicates untreated naive controls; N+EPO, naive mice treated with EPO; I, untreated infected controls; I+EPO, infected mice treated with EPO. When 2 sets of data are labeled with superscripts of different lowercase letters, it indicates that these 2 sets of data are statistically different (P < .05). Similar results are representative of 3 independent experiments.
Surprisingly, proliferation of total splenocytes in response to EPO stimulation in vitro revealed a very different pattern. Splenocytes from infected mice, with or without EPO treatment, exhibited little proliferation in response to EPO stimulation in vitro, similar to the lack of response by splenocytes from untreated naive controls (Figure 6B). In contrast, splenocytes from EPO-treated naive mice responded robustly to EPO stimulation in vitro. Thus, these data indicate that infected mice, with or without EPO treatment, have significantly higher frequencies of EPOR-positive cells yet markedly lower proliferation in response to EPO stimulation. The impaired proliferation of these early progenitors in infected mice may contribute to the suboptimal increase in late erythroid precursors (ie, erythroblasts) observed earlier in this study. This sequence of events may eventually lead to the insufficient generation of reticulocytes during blood-stage malaria infection.
Discussion
Inappropriately low reticulocytosis relative to the degree of anemia is often observed in malaria patients.13,14 Because reticulocytosis is the downstream event of erythropoiesis, reduced production of reticulocytes during malaria led to the proposal that erythropoiesis may be insufficient in infected individuals. Based on the results of a study in which the kinetics of red cell size, parasitemia, and EPO levels were determined in P falciparum–infected patients, Kurtzhals et al have proposed that malaria infection causes a rapidly reversible suppression of the bone marrow response to EPO.14 However, the extent of the suppression relative to the normal response to EPO has never been fully investigated. In a previous study, we demonstrated that the reticulocyte response following administration of exogenous EPO in P chabaudi AS–infected mice is suppressed proportionally to the level of parasitemia.27 However, it is not clear how changes occurring in the hematopoietic tissue due to malaria result in the reduced generation of reticulocytes.
Investigation of possible changes in bone marrow cellularity of the erythroid lineage cells in human malaria has been inconclusive. Depending on the duration of the disease, severity of anemia, involvement of cerebral complications, and age and geographic background of the patient, the bone marrow can be hypocellular, normocellular, or hypercellular with erythroid hypoplasia or erythroid hyperplasia.34-39 A study of erythroid lineage cells in Gambian children found no deficiencies in the numbers of early progenitor BFU-Es and CFU-Es in bone marrow and suggested that the perturbation of erythropoiesis in these children occurs mainly in the morphologically recognizable erythroblast populations.40 In addition, murine malaria models demonstrated depletion of BFU-Es and depleted or slight expansion of CFU-Es and erythroblasts in bone marrow, depending on the mouse strain and parasite species.21,22,41,42 However, these studies also revealed a dramatic increase in splenic CFU-E and erythroblast populations during infection. Interestingly, the increase in splenic erythroblasts is significantly lower in fatal infections compared with nonfatal infections. It has been proposed that the expansion of the late-stage splenic erythroid population is a main determinant of the fatal outcome of malaria infection.21,22 In spite of these studies, it is not clear whether the increases in these erythroid precursors during malaria are optimal. More importantly, very few studies have provided a mechanistic explanation for the paradox of increased erythroid precursors coinciding with decreased generation of reticulocytes during malaria infection. In this study, we infected mice with P chabaudi AS, which is similar to P falciparum, because both species of parasites invade RBCs at all stages and can cause severe anemia. It is not clear whether the defect in splenic erythropoiesis identified in this study can be extrapolated to human infection. Nevertheless, our study provides important insights into insufficient erythropoiesis caused by malaria.
We first provided evidence that, in association with the suppressed reticulocyte response to exogenous EPO stimulation during malaria infection (Figure 1), a suboptimal increase in splenic TER119+ erythroblasts occurred (Figure 2). TER119 is a glycoprotein expressed by erythroid lineage cells in mice from the stage of proerythroblasts to mature RBCs but not by the early progenitors, such as BFU-Es and CFU-Es.30 Therefore, the suboptimal increase in TER119+ erythroblasts is in accordance with the results of studies in Gambian children that the perturbation of erythropoiesis occurs in the morphologically recognizable erythroblast population.40 Furthermore, we showed that the maturation of TER119+ erythroblasts during infection was impaired as evidenced by the low frequency of erythroblasts that produced Hb (Table 1), an oxygen carrier protein composed of globin chains and iron, whose synthesis can generally be detected through the mature erythroblast stage.
The severely impaired development of TER119+ erythroblasts during malaria was further demonstrated by immunostaining of transferrin receptor (CD71) and TER119 (Figure 3; Table 2). Transferrin receptor is a membrane protein that plays a central role in iron metabolism.31,32 It is expressed at very high levels by proerythroblasts and basophilic erythroblasts while at lower levels by polychromatic and orthochromatic erythroblasts.33 By examining transferrin receptor expression in conjunction with TER119 staining, we demonstrated that P chabaudi AS infection drastically suppressed the development of erythroblasts across all maturation stages as compared with naive mice treated with EPO (Figure 3; Table 2). Consistent with the benzidine staining results (Figure 2; Table 1), the defect appeared to be most severe at the Hb-producing cells: polychromatic and orthochromatic erythroblast stages.
Additionally, our data reveal a novel observation that malaria infection significantly increased the frequency and absolute number of CD71+ cells, of which erythroblasts accounted for only a small percentage (Figure 3). It is known that in addition to erythroid lineage cells, transferrin receptor is also expressed by actively dividing cells.31 We identified the cells that expressed CD71 during P chabaudi AS infection to be mainly B220+ cells, in contrast to CD71 expression mainly on TER119+ cells in naive mice treated with EPO. Consistent with our finding, it has been demonstrated that P chabaudi infection in mice induces strong B-cell responses in the spleen.43 It is not known, however, whether CD71+TER119– cells compete with CD71+TER119+ cells for available iron during infection and compromise the production of Hb by erythroid lineage cells. Furthermore, the observation of a shift in transferrin receptor expression from erythroid to nonerythroid cells is also of clinical relevance given the use of serum transferrin receptor (sTrfR) level as an index of erythropoiesis in malaria and other hematologic disorders.44 Based on an assessment of sTrfR levels, Verhoef et al concluded that malarial anemia results in adequately increased erythropoiesis in asymptomatic Kenyan children.45 Based on our findings, however, we postulate that the sTrfR level may not accurately reflect the status of erythropoiesis during malaria. The increased sTrfR observed may have been due to shedding of CD71 from nonerythroid cells, such as B220+ cells, which increase during malaria infection. Hence, the use of sTrfR to estimate the adequacy of erythropoiesis in malaria should be employed with caution.
One potential explanation for the suboptimal increase in late erythroid precursors is the direct destruction of erythroblasts by Plasmodium parasites. However, to the best of our knowledge, there is no evidence suggesting that Plasmodium parasites invade erythroid precursors. Alternatively, Schmidt et al have suggested that abnormalities in CD71 expression and/or function may lead to premature cell death of erythroblasts.32 Furthermore, Dormer et al reported a marked loss of polychromatic erythroblasts in acute falciparum malaria.46 In addition, the dysplastic features of erythroblasts, namely dyserythropoiesis, such as deformed nuclei, chromatin bridges, and karyorrhexis,37,46,47 suggest that apoptosis might be an underlying mechanism for the ineffective erythropoiesis. We found no significant increase in apoptotic TER119+ erythroblasts as identified by TUNEL assay during malaria infection (Figure 4). In fact, EPO-treated naive mice had a significantly higher proportion of erythroblasts undergoing apoptosis as compared with EPO-treated infected mice. This suggests that apoptosis may not be a major mechanism underlying ineffective erythropoiesis during malaria. However, we cannot rule out the alternative possibility that the lower percentage of apoptotic erythroblasts in infected mice may have been a result of increased phagocytic activity by phagocytes in infected mice compared with naive mice.48 Active erythrophagocytosis is a long-standing observation during malaria infection in both experimental animals and humans36,39,47 and may result in the removal of apoptotic erythroblasts and in turn cause a decreased percentage of apoptotic cells and an overall reduced number of erythroblasts.
In addition to dyserythropoiesis and erythrophagocytosis, Wickramasinghe et al proposed that cell-cycle distribution of early polychromatic erythroblasts in malaria is abnormal, including an increased proportion of cells in the G2 phase and an arrest during the progress of some cells through the S phase.49 Here, we observed a shift in TER119+ erythroblasts from G0/G1 phase to S phase in infected mice as compared with untreated naive mice (Figure 5). Because erythroblasts from EPO-treated naive mice showed a similar pattern of cell-cycle distribution to those from infected mice, the shortened G1 phase and prolonged S phase in cell-cycle distribution during malaria may be due to an increase in EPO concentration during infection.26 The effect of EPO on the cell-cycle distribution has been demonstrated in the EPO-dependent cell line Ba/F3-EPO-R.50 The discrepancy between our findings and those of Wickramasinghe et al may be due to the difference in acute versus chronic phase of infection and/or the technique for defining cell-cycle distribution. In our study, we employed PI staining of DNA content in ethanol-fixed cells whereas Wickramasinghe and colleagues employed Feulgen microspectrophotometry and 3H-thymidine autoradiography.49
Although we did not identify either apoptosis or a disruption in the cell-cycle distribution of erythroblasts as potential mechanisms underlying the suboptimal increase in TER119+ erythroblasts during malaria, we demonstrated that proliferation of splenocytes from infected mice in response to EPO stimulation in vitro was blunted (Figure 6). By comparing EPOR expression on splenocytes from naive and infected mice, with or without EPO treatment, we observed that malaria infection resulted in a dramatic increase in the EPOR-positive population. EPOR is expressed mainly by erythroid progenitors preceding the stage of erythroblasts, including CFU-Es and proerythroblasts.16-18 The increase in the EPOR-positive population during malaria reported here is consistent with previous observations by our laboratory and other investigators that expansion of splenic CFU-Es occurs in infected mice.21,22,41,42 However, despite higher frequencies of splenocytes from infected mice with or without EPO treatment expressing EPOR, proliferation of splenocytes in response to EPO stimulation in vitro was lower in untreated and EPO-treated infected mice compared with EPO-treated naive mice. These data suggest that the proliferative activity of EPOR-positive cells is suppressed during infection. Alternatively, blunted EPO-induced proliferation in vitro displayed by splenocytes from infected mice may reflect that the cells were already under maximal endogenous EPO stimulation in vivo. Indeed, infected mice, with or without EPO treatment, had significantly higher levels of EPO in sera, measured as described previously,25 as compared with naive mice treated with EPO (data not shown). However, it has been shown that splenocytes from anemic mice proliferate robustly in response to EPO stimulation in vitro despite the high endogenous EPO levels.26,51 Hence, we propose that the blunted in vitro proliferation displayed by splenocytes from infected mice observed in our study is not due to high endogenous EPO levels in infected mice. Impaired proliferation of these early EPOR-positive erythroid progenitors may impede the subsequent differentiation of these cells and lead to the eventual reduction of TER119+ erythroblasts.
The data presented in this study demonstrated that EPO-treated infected mice had comparable numbers of EPOR-positive cells but 70% fewer TER119+ cells, 85% fewer Hb-positive cells, and 91% fewer reticulocytes compared with EPO-treated naive mice (Figure 7). The sharply reduced relative number of cells from EPOR-positive progenitors to TER119+ erythroblasts during malaria may be due to the suppressed proliferation of EPOR-positive progenitors in response to EPO stimulation. The further decreased generation of mature Hb-positive erythroblasts may be related to the redistribution of CD71 from the erythroblast to nonerythroblast population. Hence, malaria suppresses proliferation, differentiation, and maturation of erythroid precursors of various stages that results in an insufficient number of reticulocytes generated by infected animals, which may ultimately thwart the ability of the host to compensate for red cell loss during infection and exacerbate the severity of malarial anemia.
Summary of comparison between absolute numbers of various erythroid lineage cells from EPO-treated naive mice and EPO-treated infected mice. Absolute numbers of erythroid cells from EPO-treated naive mice were used as a reference point and set as 100% as indicated by the dotted line. Values are means ± SEM for 3 to 5 mice per group. Data are representative of 3 independent experiments.
Summary of comparison between absolute numbers of various erythroid lineage cells from EPO-treated naive mice and EPO-treated infected mice. Absolute numbers of erythroid cells from EPO-treated naive mice were used as a reference point and set as 100% as indicated by the dotted line. Values are means ± SEM for 3 to 5 mice per group. Data are representative of 3 independent experiments.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-08-2887.
Supported by the Canadian Institutes of Health Research (grant MOP-14663; M.M.S.). K.H.C. was a recipient of the Christine Gagnon Memorial Scholarship and MUHC studentship from McGill University and Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) Bridging Fund from the Centre for Host-Parasite Interactions, Quebec, Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Jaime Sanchez-Dardon for assistance in the cell-cycle analysis, Janet Laganiere for photographing benzidine staining of Hb-producing cells, and Rebecca Ing for critically reviewing the manuscript.