Abstract
Ras gene mutations occur in 30% to 40% of patients with multiple myeloma (MM), and farnesylation is the first and most important step in the posttranslational modification of Ras proteins. R115777 is a newly synthesized potent farnesyl transferase inhibitor (FTI) and has recently demonstrated significant antitumor activities in vitro and in vivo. Therefore, we examined the effect of R115777 on the growth of fresh and cloned myeloma cells in vitro. R115777 inhibited the growth of fresh and cloned myeloma cells dose dependently, and effects were not dependent on the status of N-Ras mutation in fresh myeloma cells. Flow cytometric analysis using annexin V and 7-aminoactinomycin D (7AAD) showed that R115777 induced apoptosis of 2 of 3 myeloma cell lines at a concentration of 1.0 × 10-8 M. R115777 appears to be a potent inducer of apoptosis, and its effects depend on the status of Ras mutation in cloned myeloma cells but not on the status of N-Ras mutation in fresh myeloma cells. This is the first report that demonstrates the relationship between the N-Ras mutation in fresh myeloma cells and the effect of R115777. R115777 might have some benefit in the treatment of myeloma patients.
Introduction
Multiple myeloma (MM) is a malignancy of B cells characterized by the monoclonal proliferation of malignant plasma cells and osteolytic bone destruction. Several chemotherapeutic regimens have been used to improve the survival of patients with MM. Although high-dose melphalan supported by autologous stem cell transplantation (auto-SCT) has improved the survival of patients with MM, many patients were found to relapse during a long follow-up period.1,2 Allogeneic stem cell transplantation (allo-SCT) is possibly the only genuinely curative therapy for MM, and a graft-versus-myeloma effect by immunocompetent donor lymphocytes has been demonstrated but the treatment-related mortality is high.3,4 Recently, nonmyeloablative SCT has been reported to provide a stable engraftment of allogeneic stem cells and tolerable toxicity in patients with MM, but a longer follow-up period is necessary to evaluate late mortality.5,6 Therefore, there is an obvious need for new therapeutic strategies.
Ras proteins are prototypical G proteins that have been shown to play a key role in signal transduction, cell proliferation, and malignant transformation. Ligand-stimulated activation of the cell-surface receptor, receptor-associated tyrosine kinase, or agonist mediated through G protein-coupled receptors results in the activation of Ras protein. The Ras gene family consists of 3 functional genes, H-Ras, N-Ras, and K-Ras. Activating point mutations in all 3 Ras genes have been detected in a wide variety of human malignancies and are one of the most common alternations in hematologic malignancies.7-9 In MM, Ras gene activation is observed in 30% to 40% of cases and one of the most frequent point mutations.10-13 Although Ras protein undergoes several steps of posttranslational modification, farnesylation is the first and most important step for its membrane localization and cell-transforming activity. Thus, farnesyltransferase (FTase) is a very attractive target for the development of anticancer agents. R115777 (Zarnestra, Johnson & Johnson, Raritan, NJ), a potent and selective farnesyl transferase inhibitor (FTI), has recently demonstrated significant antitumor activities in vitro and in vivo.14-16 Phase 1 and pharmacokinetic studies of R115777 in advanced cancer or hematologic malignancies have been reported.17-20 In MM, Alsina et al19 reported that 50% of patients had a reduction in monoclonal proteins of less than 25% compatible with disease stabilization, but on the other hand Cortes et al20 reported that only 1 of 10 patients had a reduction of monoclonal protein. Moreover, the relationship between Ras gene mutations and inhibitions of myeloma cell proliferation by R115777 is still unknown. We therefore examined the effect of R115777 on the growth of fresh and cloned myeloma cells, and the relationship between N-Ras mutation and the effect of R115777 in vitro.
Patients, materials, and methods
Cell lines
Used in this study were 3 human myeloma cell lines: RPMI 8226, U266, and NCI-H929. All cell lines were obtained from the American Type Culture Collection (Rockville, MD) and were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Flow Laboratories, McLean, VA), 100 units/mL penicillin, and 100 μg/mL streptomycin.
Detection of N-, H-, K-Rasgene point mutations in myeloma cell lines
A polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was adopted for the detection of N-, H-, and K-Ras point mutations in myeloma cell lines as previously described.21 Briefly, cell lysate DNA was used for PCR amplification of sequences encompassing codons 12, 13, and 61 of the N-, H-, and K-Ras genes using previously described primers (Table 1). The PCR product was digested with the appropriate restriction enzymes. Digesters of PCR products were resolved on 3% agarose gels containing ethium bromide. DNA bands that were resistant to digestion were excised, and the DNA was recovered and sequenced using an ABI-PRISM 377 analyser (Biosystems, Foster City, CA).
Chemicals
R115777 was kindly supplied by Johnson and Johnson Pharmaceutical Research and Development Foundation. A solution of 10 × 10-3 M R115777 was prepared in dimethyl sulfoxide.
Isolation of fresh myeloma cells from patients
Included in this study were 9 patients diagnosed with MM and 1 with plasma cell leukemia (PCL) (Table 2). Mononuclear cells were isolated from bone marrow aspirates by Ficoll-Hypaque density centrifugation after informed consent was obtained. Myeloma cells were purified from the patients using CD138 microbeads by magnetic cell sorting (MACS) and separation of biomolecules as described.22 CD138+ myeloma cells were cultured in RPMI 1640 medium, supplemented with 20% FCS, 100 units/mL penicillin, 100 μg/mL streptomycin, and 20 ng/mL interleukin-6 (IL-6) (Chugai Pharmaceutical, Tokyo, Japan). Study approval was obtained from the Kyoto Prefectural University of Medicine institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Mutation analysis of exons 1 and 2 of N-Rasgene on fresh myeloma cells
Mutation analysis of exons 1 and 2 of N-Ras gene on fresh myeloma cells from 10 patients was performed. Exons 1 and 2 of N-Ras coding region were amplified from the genomic DNA with AmpliTaq Gold (Biosystems) using PCR primer (exon 1: 5′-TCT AGA ACT AGT GGA TCC TTG CTG GTG GTG TGA AAT GAC TG-3′ and 5′-CGA GGT CGA CGG TAT CGG GGC CTC ACC TCT ATG GTG G-3′; exon 2: F, 5′-CCA GGA TTC TTA CAG AAAAC-3′ and R, 5′-TCC TAG TAC CTG TAG AGG TT-3′) and the following PCR conditions: 94°C for 30 seconds (denature), 60°C for 60 seconds (annealing), and 72°C for 60 seconds (extension) for 30 cycles. The PCR products were purified and sequenced directly using the PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Biosystems) with PCR primer, and an automated ABI PRISMTM 3100 Genetic Analyzer (Biosystems). Finally, the sequence was compared with the wild-type N-Ras sequence.
Cell proliferation assay
The cell proliferation was examined by the rapid colorimetric Alamar blue assay as previously described.23 Briefly, the reduction of Alamar blue is proportional to the number of viable cells. The cells were cultured in quadruplicate at a density of 2.5 × 105 cells/mL in 96-well flat-bottomed tissue-culture plates with concentrations of 0 (control) and 1.0 × 10-6 Mto 1.0 × 10-12 M of R115777 for 72 hours at 37°C in a humidified atmosphere of 5% CO2 in air. Alamar blue (Alamar Bioscience, Sacramento, CA) was added and incubation continued for 4 to 8 hours. The optical density (OD) of these samples was directly measured with an enzyme-linked immunosorbent assay reader (International Reagents, Kobe, Japan) using test and reference wavelengths of 570 and 600 nm, respectively. The OD of control samples was regarded as 1.0.
Assay of apoptosis
Cells were prepared and cultured for the proliferation analysis in the presence or absence of R115777 at a concentration of 1.0 × 10-8 M. The concentration used was determined using the data from the phase 1 clinical laboratory trial.17,18 At 0, 12, 24, 48, and 72 hours of incubation, cells were washed and stained with fluorescein isothiocyanate (FITC)-labeled annexin V (AN) and 7-amino-actinomycin D (7AAD) as described previously.24 There were 5000 cells analyzed by flow cytometry (FACS Calibur; Becton Dickinson, Franklin Lakes, NJ), and the AN-/7AAD-, AN+/7AAD-, and AN+/7AAD+ populations were enumerated. The 3 populations of AN-/7AAD-, AN+/7AAD-, and AN+/7AAD+ have been found to correspond to live cells, early apoptotic cells, and both late apoptotic and necrotic cells, respectively.
Statistical analysis
The Fisher protected least-significant difference test was used to determine the statistical significance of differences between the means of several experiments. A probability value less than .05 was considered to be statistically significant.
Results
Effects of R115777 on the growth of myeloma cell lines
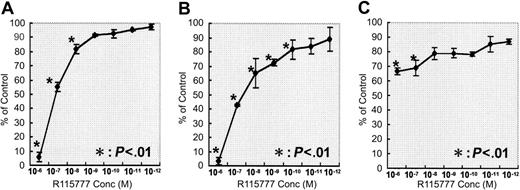
The effects of R115777 on the growth of 3 myeloma cell lines were analyzed using the Alamar blue assay. R115777 inhibited the growth of U266 and NCI-H929 cell lines dose dependently at concentrations ranging from 1.0 × 10-6 M to 1.0 × 10-8 M or 1.0 × 10-10 M (Figure 1A-B) but weakly inhibited the growth of RPMI 8226 cells (Figure 1C). The IC50, the concentration at which R115777 inhibits 50% of the growth of myeloma cells, ranged from 1.0 × 10-7 M to 1.0 × 10-8 M in U266 and NCI-H929 cell lines. The inhibitions of the growth of myeloma cell lines by R115777 depended on the status of N-, H-, and K-Ras gene mutations in cloned myeloma cells, as no Ras gene mutation was observed in the RPMI 8226 cell line (Table 1).
Effects of R115777 on the growth of 3 myeloma cell lines. The cells were cultured in quadruplicate at a density of 2.5 × 105 cells/mL in 96-well flat-bottomed tissue-culture plates with R115777 at concentrations of 0 (control) and 1.0 × 10-6 Mto1.0 × 10-12 M for 72 hours at 37°C in a humidified atmosphere of 5% CO2 in air. R115777 inhibited the growth of the U266 (A) and NCI-H929 (B) cell lines dose dependently but weakly inhibited the growth of RPMI8226 cells (C). Values represent means ± SDs of quadruplicate cultures. *P < .01.
Effects of R115777 on the growth of 3 myeloma cell lines. The cells were cultured in quadruplicate at a density of 2.5 × 105 cells/mL in 96-well flat-bottomed tissue-culture plates with R115777 at concentrations of 0 (control) and 1.0 × 10-6 Mto1.0 × 10-12 M for 72 hours at 37°C in a humidified atmosphere of 5% CO2 in air. R115777 inhibited the growth of the U266 (A) and NCI-H929 (B) cell lines dose dependently but weakly inhibited the growth of RPMI8226 cells (C). Values represent means ± SDs of quadruplicate cultures. *P < .01.
Effects of R115777 on the induction of apoptosis in myeloma cell lines
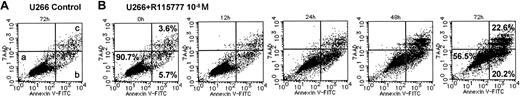
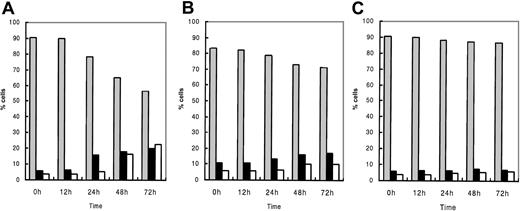
The effects of R115777 on the induction of apoptosis in the 3 myeloma cell lines were examined by flow cytometry. Using annexin V and 7AAD, 2-color flow cytometric analysis discriminated 3 populations: viable (lower left), early apoptotic (lower right), and both late apoptotic and necrotic cells (upper right) (Figure 2A). Percentages of these populations in each cell line treated with R115777 at a concentration of 1.0 × 10-8 M are shown in Figure 3. R115777 at 1.0 × 10-8 M induced early apoptosis in the U266 and NCI-H929 cell lines but not the RPMI 8226 cell line. In the U266 cell line, R115777 at 1.0 × 10-8 M induced apoptosis time dependently, and the percentage of late apoptotic and necrotic cells increased gradually. Finally, more than 40% of cells showed signs of apoptosis or necrosis (Figures 2B,3A). In the NCI-H929 cell line, R115777 at 1.0 × 10-8 M induced apoptosis time dependently, with more than 20% of cells undergoing apoptosis or necrosis (Figure 3B). No apoptosis was induced in the RPMI 8226 cell line, which has no N-, H-, or K-Ras gene mutations, by R115777 at a concentration of 1.0 × 10-8 M (Figure 3C).
Effects of R115777 on the induction of apoptosis in U266 cells. Cells were cultured with R115777 at 1.0 × 10-8 M and then examined using flow cytometry. (A) There were 3 populations revealed by 2-color flow cytometric analysis using FITC-annexin V and 7-amino-actinomycin D (7AAD): viable (lower left; a), early apoptotic (lower right; b) and both late apoptotic and necrotic cells (upper right; c). U266 cells cultured without R115777 for 72 hours are mostly viable (90.7%) (a). (B) U266 cells treated with R115777 at 1.0 × 10-8 M after incubation for 72 hours showed viable (56.5%), early apoptotic (20.2%) and late apoptotic and necrotic (22.6%) cells.
Effects of R115777 on the induction of apoptosis in U266 cells. Cells were cultured with R115777 at 1.0 × 10-8 M and then examined using flow cytometry. (A) There were 3 populations revealed by 2-color flow cytometric analysis using FITC-annexin V and 7-amino-actinomycin D (7AAD): viable (lower left; a), early apoptotic (lower right; b) and both late apoptotic and necrotic cells (upper right; c). U266 cells cultured without R115777 for 72 hours are mostly viable (90.7%) (a). (B) U266 cells treated with R115777 at 1.0 × 10-8 M after incubation for 72 hours showed viable (56.5%), early apoptotic (20.2%) and late apoptotic and necrotic (22.6%) cells.
Effect of R115777 on the induction of apoptosis in 3 myeloma cell lines. Cells were cultured with R115777 at a concentration of 1.0 × 10-8 M. After incubation for 0, 12, 24, 48, and 72 hours, cells in 500 μL cultured suspension were washed and stained with fluorescein isothiocyanate (FITC)-labeled annexin V (AN) and 7-amino-actinomycin D (7AAD) at each time point and analyzed with a FACS Calibur flow cytometer. R115777 at 1.0 × 10-8 M induced apoptosis in the U266 (A) and NCI-H929 (B) cell lines time dependently but did not induce apoptosis in the RPMI 8226 cell line (C). ▦ indicates viable; ▪, early apoptosis; and □, late apoptosis and necrosis.
Effect of R115777 on the induction of apoptosis in 3 myeloma cell lines. Cells were cultured with R115777 at a concentration of 1.0 × 10-8 M. After incubation for 0, 12, 24, 48, and 72 hours, cells in 500 μL cultured suspension were washed and stained with fluorescein isothiocyanate (FITC)-labeled annexin V (AN) and 7-amino-actinomycin D (7AAD) at each time point and analyzed with a FACS Calibur flow cytometer. R115777 at 1.0 × 10-8 M induced apoptosis in the U266 (A) and NCI-H929 (B) cell lines time dependently but did not induce apoptosis in the RPMI 8226 cell line (C). ▦ indicates viable; ▪, early apoptosis; and □, late apoptosis and necrosis.
Effects of R115777 on the growth of fresh myeloma cells from patients
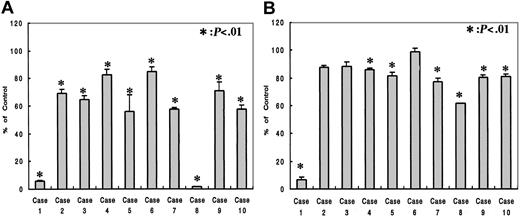
The effects of R115777 on the growth of fresh myeloma cells from 10 patients were analyzed using the Alamar blue assay. Patient characteristics, the percentage of purified CD138+ cells, and the status of N-Ras mutation are shown in Table 2. R115777 inhibited the growth of fresh myeloma cells at a concentration of 1.0 × 10-7 M in all patients and 1.0 × 10-8 M in 7 of 10 patients examined, and the degree of inhibition depended on the concentration of R115777 (Figure 4). N-Ras mutation was detected in case 5 (Table 2), in which the growth of myeloma cells was inhibited to 58% and 81% at a concentration of 1.0 × 10-7 M and 1.0 × 10-8 M of R115777, respectively. These observations suggested that the inhibition of R115777 did not depend on the status of N-Ras mutation in fresh myeloma cells.
Effects of R115777 on the growth of fresh myeloma cells from patients. The cells were cultured in quadruplicate at a density of 2.5 × 105 cells/mL in 96-well flat-bottomed tissue-culture plates with concentrations of 0 (control) and 1.0 × 10-7 M (A) or 1.0 × 10-8 M (B) of R115777 for 72 hours at 37°C in a humidified atmosphere of 5% CO2 in air. R115777 inhibited the growth of fresh myeloma cells in all patients examined at 1.0 × 10-7 M and 7 of 10 patients at 1.0 × 10-8 M, but the degree of inhibition differed among the patients. Values represent means ± SDs of quadruplicate cultures. *P < .01.
Effects of R115777 on the growth of fresh myeloma cells from patients. The cells were cultured in quadruplicate at a density of 2.5 × 105 cells/mL in 96-well flat-bottomed tissue-culture plates with concentrations of 0 (control) and 1.0 × 10-7 M (A) or 1.0 × 10-8 M (B) of R115777 for 72 hours at 37°C in a humidified atmosphere of 5% CO2 in air. R115777 inhibited the growth of fresh myeloma cells in all patients examined at 1.0 × 10-7 M and 7 of 10 patients at 1.0 × 10-8 M, but the degree of inhibition differed among the patients. Values represent means ± SDs of quadruplicate cultures. *P < .01.
Discussion
The Ras gene family consists of 3 functional genes, H-Ras, N-Ras, and K-Ras, and encodes M21000 proteins that are intermediates in signal transduction pathways critical for cellular processes such as growth, differentiation, and apoptosis.7-9,14 Activating point mutations in all 3 Ras genes have been detected in a wide variety of human malignancies: Ras gene activation is observed in 30% to 40% of cases and one of the most frequent point mutations in MM.10-14 Ras protein function is dependent on localization to the plasma membrane of the cell, a critical step in which there is a posttranslational modification by the addition of a farnesyl group to the COOH terminus of the protein.7-9,14 Farnesylation is mediated by the enzyme FTase in all Ras proteins, while geranylgeranylation is mediated by the enzymes GGTase type 1 and 2 in K-Ras protein, but farnesylation is the first and most critical step in the posttranslational modification of Ras proteins.7-9,14 Based on these observations, several FTIs have been developed that demonstrated cell growth inhibition and antitumor effects in vitro and in vivo.7-9,14-20,25-30 R115777 is one example of FTIs and acts as a potent nonpeptidomimetic FTI by competitively inhibiting the CAAX peptide-binding site of FTase.7-9,14-20 Recently, R115777 was reported to have significant antitumor effects on several tumor cells and clinical activity in patients with malignancies in phase 1 clinical studies.17-20
In the present study, we demonstrated that R115777 had an inhibitory effect on the growth of human myeloma cell lines U266 and NCI-H929 by inducing apoptosis. The IC50 as detected by Alamar blue assay in the 2 cell lines ranged from 1.0 × 10-7 M to 1.0 × 10-8 M and was similar to the results reported by Gouill et al.16 These concentrations are lower than serum levels of area under the curve (AUC12h) detected when R115777 was given at 500 mg or 600 mg twice a day orally in phase 1 clinical studies.17,18 In the cloned myeloma cells, R115777 did not inhibit cell proliferation and induce cell apoptosis of the RPMI 8226 cell line, which had no Ras gene mutations. While R115777 at 1.0 × 10-7 M inhibited the proliferation of fresh myeloma cells in all patients, these effects were not dependent on the status of N-Ras mutation. Recently, Peters et al27 and Sepp-Lorenzino et al29 demonstrated that the effect of FTIs did not depend on the existence of Ras mutations. Ras protein activates several downstream effectors, including the RhoB, Ral-GEF, Rafs, phosphatidylinositol 3-kinase (PI 3-K), and mitogen-activated protein kinase kinase (MEKK) pathways, and these pathways are a cross-linked complex.7-9,14,31 It is therefore suggested that FTIs inhibit not only Ras protein farnesylation but the farnesylation of other proteins and signaling pathways that stimulate tumor cell proliferation.14-16,25-31 Moreover, Rowley et al32 demonstrated that N-Ras-transfected ANBL6 cells became IL-6 independent, and these observations suggested the relationship between the activation of N-Ras protein and IL-6 in fresh myeloma cells. In our study we supplemented IL-6 to fresh myeloma cells, and it is suggested that the activation of Ras proteins by IL-6 resulted in the more remarkable inhibition by R115777 in all fresh myeloma cells examined compared with cloned myeloma cells. And it is reasonable to speculate that the N-Ras mutation may be less required in MM cells in vivo, because Ras protein could be activated by many cytokines such as IL-6 in the clinical disease state. These observations suggest the possibility of using R115777 as a new therapeutic strategy for hematologic malignancies that have Ras mutations or not. Recently, FTase was reported to be one of the most differentially expressed genes in the global gene expression profiling of MM.33 This also suggests that FTI may be effective in treating patients with a high level of FTase expression.
In conclusion, we showed that R115777 exhibits cytotoxic activity in fresh and cloned myeloma cells in vitro. Apoptosis is the dominant mechanism of R115777-induced cells. The effect of R115777 did not depend on the existence of N-Ras gene mutations in fresh myeloma cells in our study. These observations suggest that R115777 is a potentially important drug in the treatment of multiple myeloma. The data, together with the toxicity and pharmacokinetic profile, indicate that R115777 should be included as a drug in the treatment of MM, and a clinical trial is warranted.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2003-03-0851.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Mr S. Shirahata (SRL, Tokyo, Japan) for pursuing the analysis of N-Ras mutation on fresh myeloma cells.
Supported by research funding from the Johnson & Johnson Pharmaceutical Research and Development Foundation.